Fatty acid synthase knockout impairs early embryonic development via induction of endoplasmic reticulum stress in pigs
Abstract
Fatty acid synthase (FAS) is an important enzyme involved in the de novo synthesis of long-chain fatty acids. During development, the function of FAS in growth is greater than that in energy storage pathways; therefore, we hypothesized that knockout of FAS would affect early embryonic development owing to the induction of endoplasmic reticulum (ER) stress. In the present study, the function of FAS was studied using the CRISPR (clustered regularly interspaced short palindromic repeats)/ CRISPR-associated protein 9 (Cas9) system. Cas9 and single-guide RNA (sgRNA) were injected into parthenotes to decrease the number of FAS-positive embryos. The efficiency of knockout was assayed by DNA sequencing. We found that FAS knockout caused excessive production of reactive oxygen species (ROS). Excess ROS induced ER stress, resulting in activation of the adaptive unfolded protein response (UPR). FAS knockout caused splicing of the X-box binding protein 1 gene (XBP1) and expression of spliced XBP1 mRNA. In addition, FAS knockout caused phosphorylation of PKR-like ER kinase (PERK), and an increase in the mRNA expression of the ER stress-regulated genes, activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP). Finally, Ca2+ was released from the ER and taken up by the mitochondria. As the ER stress became intolerable, apoptosis was initiated. These results demonstrate that FAS knockout induced ROS generation, which mediated the activation of UPR via the ER stress, ultimately leading to apoptosis induction.
1 INTRODUCTION
Fatty acid synthase (FAS) is an important enzyme that is involved in the de novo synthesis of long-chain fatty acids. FAS synthesizes long-chain fatty acids from substrates such as acetyl-CoA, malonyl-CoA, and NADPH (Maier, Jenni, & Ban, 2006). FAS is critical for energy homeostasis because it converts redundant food into lipids for storage and provides energy via fatty acid oxidation, when needed. FAS is involved in many biological processes, including generation of milk lipids and membrane biogenesis (Alim et al., 2014; Chirala et al., 2003; Morris et al., 2007). The importance of FAS was demonstrated in FAS knockout mice, which showed embryonic death before implantation (Chirala et al., 2003).
The endoplasmic reticulum (ER) is a membrane-bound organelle that is responsible for protein folding, modification, and trafficking, as well as cellular homeostasis. It ensures proper folding and assembling of proteins, and prevents misfolding. The ER also plays important roles in lipid synthesis and energy metabolism (Cox, Chapman, & Walter, 1997; Sriburi, Jackowski, Mori, & Brewer, 2004). Therefore, there is a relationship between the ER stress response and the pathways that regulate lipid synthesis. FAS-driven synthesis of phospholipids occurs in the ER, and FAS inhibition induces ER stress (Little, Wheeler, Fels, Koumenis, & Kridel, 2007; Swinnen et al., 2003). Therefore, previous studies established a critical link between FAS activity and ER function. However, the relationship between FAS and ER function is limited to early development.
The ER is very sensitive to changes in intracellular homeostasis and extracellular stimuli (Cao & Kaufman, 2014). The accumulation of misfolded proteins in the ER activates the unfolded protein response (UPR), which is a pro-survival response that decreases the accumulation of unfolded and misfolded proteins to recover normal ER function (Shen, Zhang, & Kaufman, 2004). The UPR comprises three sensors: protein kinase RNA-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6). Activated IRE1 causes the deletion of a 26-nucleotide intron from the unspliced transcript of XBP1 (XBP1-u), the gene encoding X-box binding protein 1, to produce spliced XBP1 (XBP1s). As PERK is one of the sensors, its trans-autophosphorylation leads to activation of eukaryotic initiation factor 2α (eIF2α) when the UPR is activated (Hao et al., 2009). The subsequent activation of signaling pathways by ER stress plays a critical role in early embryonic development.
To detect the effect of FAS on ER function, the present study used the CRISPR (clustered regularly interspaced short palindromic repeats)/CRISPR-associated protein 9 (Cas9) system, which is an efficient gene editing technology for the study of gene function (Mout et al., 2017; Wang et al., 2013). The system comprises Cas9 and a single-guide RNA (sgRNA). CRISPR/Cas9 relies on the sgRNA that directs DNA breaks at the desired location, which are then repaired by the DNA double-strand break repair mechanism. Thus, the system allows the removal or addition of genes at will (Cong et al., 2013; Hendel et al., 2015). The CRISPR/Cas9 system has been used to efficiently generate knockout or knockin models via zygotic injections of Cas9 and sgRNA in many organisms, including mice (Wang et al., 2013), rats (Li et al., 2013), zebrafish (Jao, Wente, & Chen, 2013), and pigs (Kwon, Namgoong, & Kim, 2015). Therefore, the CRISPR/Cas9 system was used in this study to knockout the pig FAS gene and thus study the function of FAS.
The results demonstrated a link between FAS and ER stress. FAS knockout induced ER stress, which in turn activated the UPR. In addition, FAS knockout caused splicing of XBP1 and phosphorylation of PERK. FAS knockout also led to changes in Ca2+ homeostasis. Therefore, we hypothesized that FAS knockout impairs porcine pre-implantation development via the induction of ER stress.
2 METHODS AND MATERIALS
All chemicals used in this study were purchased from Sigma–Aldrich (St. Louis, MO), unless otherwise indicated.
2.1 Oocyte collection, in vitro maturation, and embryo culture
Ovaries were collected from prepubertal gilts at a local slaughterhouse and transported to the laboratory in saline at 37°C. Cumulus-oocyte complexes (COCs) were isolated and collected as described previously (Liang, Zhao, Choi, Kim, & Cui, 2015). COCs were washed three times in Tyrode's Lactate- 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (TL-HEPES), and then cultured in M199 supplemented with 10% porcine follicular fluid, 0.1 g/L sodium pyruvate, 0.6 mM l-cysteine, 10 ng/ml epidermal growth factor, 10 IU/ml luteinizing hormone, and 10 IU/ml follicle stimulating hormone at 38.5°C for 44 hr in a humidified atmosphere of 5% CO2. After maturation, cumulus cells were removed by treatment with 0.1% hyaluronidase and repeated pipetting. To activate parthenogenesis, oocytes with polar bodies were selected, activated by two direct current pulses of 1.1 kV/cm for 60 μs, and then incubated in porcine zygote medium (PZM-5) containing 7.5 µg/ml of cytochalasin B for 3 hr at 38.5°C. Finally, embryos were maintained in PZM-5 for 7 d at 38.5°C in a humidified atmosphere with 5% CO2. On the 5th day, fetal bovine serum was added to the medium to a final concentration of 10%.
2.2 Generation and injection of Cas9 and sgRNA into parthenotes
The sgRNA sequences were designed using the CRISPR design tool (http://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design), corresponding to the exons of FAS. The generation of Cas9 and sgRNAs was described in a previous study (Kwon et al., 2015). Briefly, a fragment containing the FAS exon (exon 16 or exon 19) was amplified by PCR. The PCR product was purified by gel extraction and used for in vitro transcription using T7 MEGA shortscript kits (Life Technologies; Foster City, CA), and purified by phenol/chloroform extraction.
After 4 hr of parthenogenetic activation, one-cell-stage parthenotes were microinjected with a mixture of Cas9 mRNA (600 ng/µl) and the sgRNAs (200 ng/µl) under a Nikon TE2000-U inverted microscope (Nikon Corporation; Tokyo, Japan), using a FemtoJet microinjector (Eppendorf; Hamburg, Germany). As a control, Cas9 mRNA alone was microinjected into one-cell-stage parthenotes. After microinjection, the parthenotes were cultured in PZM5 medium.
2.3 Isolation of genomic DNA from blastocysts and PCR amplification
To prepare genomic DNA, blastocysts were washed in phosphate-buffered saline (PBS)/polyvinyl alcohol (PVA), followed by freezing in liquid nitrogen and subsequent thawing. Portions of genomic DNA containing guide sequences for sgRNA1 (exon 19 of porcine FAS) and sgRNA2 (exon 16 of porcine FAS) were amplified by PCR using the PCR primers listed in S1 Table and Pfu-X DNA polymerase (Solgent; Daejun, Korea). The resulting PCR products were purified by gel purification, and sequenced directly using the PCR primers used for amplification.
2.4 Reactive oxygen species (ROS) staining
Blastocysts (n = 30, three repeats) were incubated for 15 min in in vitro embryo culture (IVC) medium containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) at 37°C. After incubation, the blastocysts were washed three times in IVC medium and transferred to PBS drops in a polystyrene culture dish. The fluorescent signals were captured using an epifluorescence microscope. The fluorescence intensity of the control group was arbitrarily set at 1, and that of the knockout group was measured and expressed as a relative value with respect to the control group.
2.5 Terminal deoxynucleotidyltransferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP) nick-end labeling (TUNEL) assay
The blastocysts (n = 31, three repeats) were collected, fixed in 3.7% paraformaldehyde for 15 min at room temperature, and permeabilized in 0.5% Triton X-100 for 30 min at 37°C. The blastocysts were incubated with fluorescein-conjugated dUTP and terminal deoxynucleotidyltransferase enzyme (In Situ Cell Death Detection Kit, Roche; Mannheim, Germany) for 1 hr at 37°C. The embryos were washed three times in PBS/PVA, treated with Hoechst 33342 for 5 min, and mounted onto glass slides. Images were captured using a confocal microscope (Zeiss LSM 710 META, Jena, Germany).
2.6 Immunofluorescence staining and confocal microscopy
For immunostaining, blastocysts (n = 28, three repeats) were fixed in 3.7% paraformaldehyde dissolved in PBS/PVA for 20 min at room temperature, and then permeabilized with PBS/PVA containing 0.5% Triton X–100 at 37°C for 1 hr. After incubation with blocking solution (PBS containing 1% bovine serum albumin), blastocysts were incubated with the first antibody, anti-XBP1 (1:200; 7160, Santa Cruz, CA; Rabbit polyclonal IgG), and antibody recognizing phosphorylated PERK (P-PERK) (1:200; 3719, Cell Signaling Technology Inc., Danvers, MA; rabbit monoclonal IgG) overnight at 4°C. After washing three times in PBS/PVA, blastocysts were incubated with the appropriate secondary antibody and washed three times in PBS/PVA. The blastocysts were then stained with Hoechst 33342 for 5 min, washed three times in PBS/PVA, mounted onto slides, and examined using a confocal microscope (Zeiss LSM 710 META, Jena, Germany). Images were processed using Zen software (version 8.0, Zeiss, Jena, Germany).
2.7 Measurement of Ca2+
On the 7th day, the embryos (n = 35, three repeats) were cultured in medium containing Fluo-4-AM (5 µM, Invitrogen) for 1 hr at 38.5°C. The embryos were then washed three times in the medium and then cultured in medium containing Rhod-2-AM (2 µM, Invitrogen) for 1 hr at 38.5°C. To assess the effect of FAS on release of Ca2+ from ER, embryos were treated with Ru360, and then washed in culture medium without Ru360. The embryos were then incubated with Fluo-4-AM and Rhod-2-AM as before. Fluorescent signals were captured using an epifluorescence microscope.
2.8 Reverse transcriptase quantitative real-time PCR (RT-qPCR)
mRNA was extracted from 10 blastocysts per group, using a DynaBeads mRNA Direct Kit (Dynal Asa, Oslo, Norway), according to the manufacturer's instructions. cDNA was obtained via reverse transcription of mRNA, using the Oligo (dT)12-18 primer and SuperScript III Reverse Transcriptase (Invitrogen). The amplification conditions were as follows: 95°C for 3 min; followed by 40 cycles of 95°C for 15 sec, 60°C for 20 sec, and 72°C for 10 sec; with a final extension at 72°C for 7 min. The primers were shown in S1 Table (Guo, Kim, & Cui, 2017; Kwon et al., 2015; Zhang et al., 2011). Relative gene expression was normalized to internal porcine GAPDH mRNA level using the 2−ΔΔCt method.
2.9 Statistical analysis
All data were analyzed by one-way analysis of variance (ANOVA) and Chi-square test implemented in the Statistical Package for the Social Sciences (SPSS). Differences among treatments were examined by Duncan multiple range test. Data are expressed as mean ± standard error of the mean. Each experiment was performed in triplicate and differences were considered significant if P < 0.05.
3 RESULTS
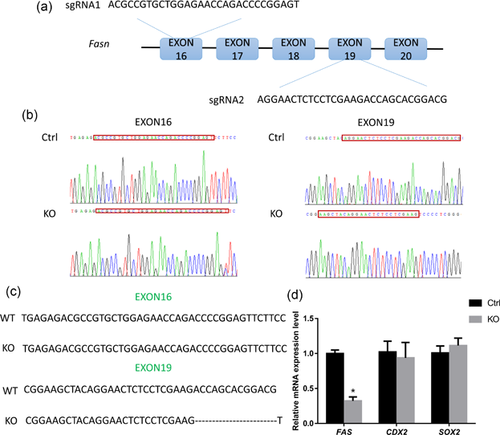
3.1 CRISPR/Cas9-mediated targeting of FAS in porcine parthenotes
To test the targeting efficiency, exons 16 and 19 were selected as the CRISPR target sites (Figure 1a). The parthenotes were microinjected with Cas9 mRNA and sgRNAs for exon 16 or 19, and with Cas9 mRNA alone as the control. We assessed the targeting efficiency by PCR and DNA sequencing using embryonic DNA flanking the guide sequence (Figure 1b). Among the clones, 33.3% displayed a deletion of exon 19 (Figures 1b and 1c); however, no mutations or deletions occurred in exon 16. Therefore, the sgRNA for exon 19 was used in subsequent experiments. To test for off-target effects, RT-qPCR was performed. The products of the CDX2 and SOX2 genes (caudal type homeobox 2 and sex determining region Y-Box 2, respectively) play important roles in early embryonic development. CDX2 is expressed in the trophectoderm and affects the blastocyst hatching process in porcine embryonic development (Bou et al., 2017). SOX2 is a sensitive marker and maintainer of pluripotency (Liu et al., 2015). We observed that only the mRNA expression of FAS decreased significantly, not that of CDX2 or SOX2 (Figure 1d). These results showed that microinjection of Cas9 and sgRNA successfully targeted and deleted FAS with an efficiency of 33.3%.
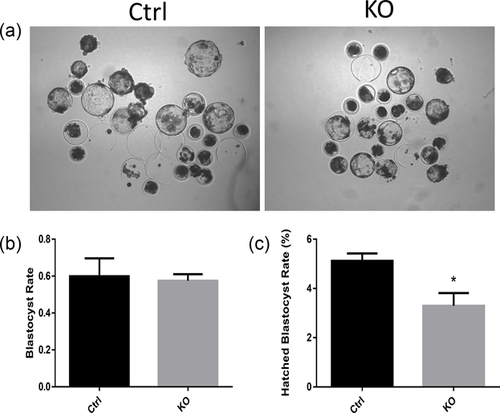
3.2 FAS knockout impairs blastocyst hatching
As shown in Figures 2a and 2b, FAS knockout showed no significant effect on blastocyst formation (59.93 ± 5.58% vs. 57.53 ± 2.01%). It did, however, reduce blastocyst hatching (5.12 ± 0.30% vs. 3.30 ± 0.52%). These results demonstrated that FAS plays an important role in porcine embryogenesis, particularly in blastocyst hatching.
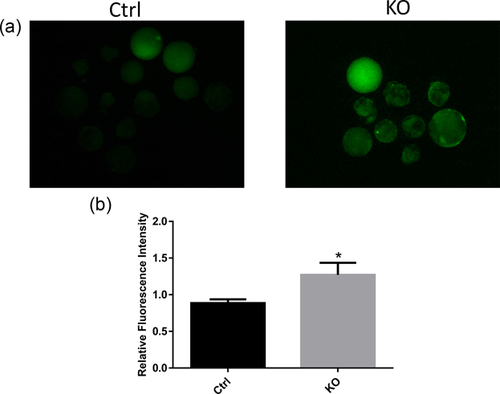
3.3 FAS knockout leads to ROS generation
Embryos are vulnerable to ROS. In embryonic development, many enzymes and pathways can produce ROS (Goto, Noda, Mori, & Nakano, 1993; Johnson & Nasresfahani, 1994). As shown in Figures 3a and 3b, FAS knockout increased the production of ROS, as indicated by the increased fluorescence intensity. The fluorescence intensity of the control group was arbitrarily set at 1, and that of the knockout group was found to be significantly higher. Therefore, FAS may regulate embryonic development via ROS-mediated pathways.
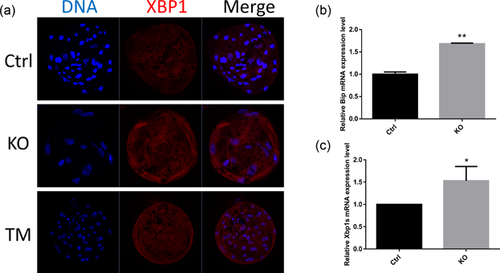
3.4 FAS knockout induces ER stress resulting in splicing of XBP1
ER stress caused by FAS knockout was implicated by the detection of spliced XBP1, identified by immunostaining and RT-PCR. As a positive control, tunicamycin (TM), an ER stress inducer, was used. As shown in Figure 4a, the XBP1 protein level increased after knockout of FAS and treatment with TM. The XBP1 mRNA expression level also increased (Figure 4b). Therefore, FAS knockout resulted in splicing of XBP-1. The level of binding immunoglobulin protein (BiP), an ER stress-inducible chaperone, also increased (Figure 4c). These results suggested that FAS knockout causes ER stress.
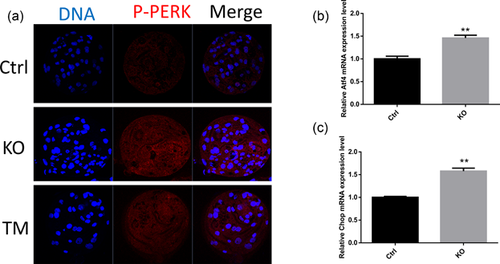
3.5 FAS knockout induces phosphorylation of PERK
To detect ER stress, phosphorylation of PERK was examined. FAS knockout induced the phosphorylation of PERK to a greater extent than that in the control group (Figure 5a), which is similar to the results of TM treatment. ATF4, whose translation is mediated by PERK, was also examined. The mRNA expression level of ATF4 increased significantly after FAS knockout (Figure 5b). The ATF4 downstream mediator, C/EBP homologous protein (CHOP), was also probed and the results suggested that the mRNA expression level of CHOP also increased in comparison with that in the control group (Figure 5c).
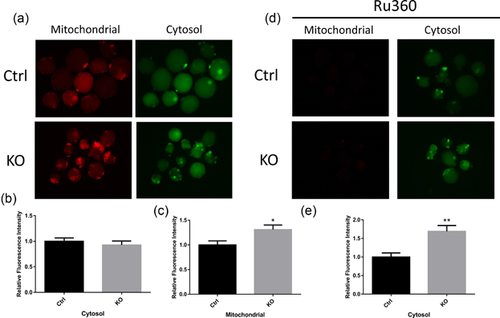
3.6 FAS knockout leads to Ca2+ release from the ER
ER stress leads to Ca2+ release from ER, emphasizing the important function of ER in Ca2+ homeostasis. First, the Ca2+ content in the cytosol was examined using Fluo-4-AM, and in the mitochondria using Rhod-2-AM. There was no significant effect on Ca2+ in cytosol between the control and knockout groups (Figure 6a and 6b). However, the Ca2+ content in the mitochondria increased after FAS knockout (Figures 6a and 6c). To confirm the function of FAS in Ca2+ homeostasis, embryos were treated with Ru360 to prevent movement of Ca2+ into the mitochondria. Consequently, the Ca2+ content in the cytosol increased significantly (Figures 6d and 6e). These results revealed that via induction of ER stress, FAS affects Ca2+ homeostasis.
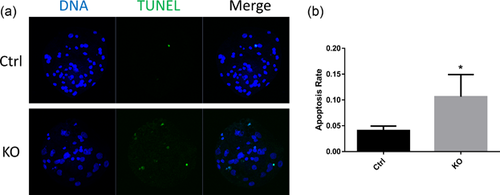
3.7 FAS knockout induces apoptosis
Excessive accumulation of ROS induces apoptosis. Apoptosis occurs when ER dysfunction exceeds the endurance of the ER (Breckenridge, Germain, Mathai, Nguyen, & Shore, 2003). We found that FAS knockout induced apoptosis in blastocysts, whereas this was not seen in the control (Figure 7a). The apoptotic rate, which was determined by the ratio of TUNEL-positive to total cells, also increased (Figure 7b). These results demonstrated that FAS knockout could induce apoptosis.
4 DISCUSSION
The ER is a multifunctional organelle important for protein folding, processing, and delivery to the target location (Tabas & Ron, 2011). ER dysfunction can cause the perturbation of lipid synthesis, energy metabolism, and embryonic development. Many stimuli, including ROS, result in the accumulation of misfolded and unfolded proteins in the ER, which is termed ER stress. In response to ER stress, an adaptive response, the UPR, is activated to maintain ER homeostasis (Oslowski & Urano, 2011). ER stress is a series of precise responses that result in the disruption of ER function. Previous studies have revealed an association between lipid synthesis and the ER stress response (Sriburi et al., 2004). FAS acts as a critical enzyme in the synthesis of fatty acids, which is associated with ER stress (Little et al., 2007). In the present study, we investigated the function of FAS by FAS gene knockout using the Crispr/Cas9 system. The results demonstrated that FAS knockout results in ROS-mediated ER stress.
Exposure of embryos to oxidative stress is one of the reasons for reduction in their developmental capacity (Lane & Gardner, 2005). When ROS production exceeds the tolerance capacity of the embryos, oxidative damage occurs (Guerin, El Mouatassim, & Menezo, 2001). In early embryonic development, many factors and metabolic pathways, such as metabolism disorders and the embryonic environment, can lead to ROS generation, and hence to oxidative stress. Excessive ROS production can cause developmental arrest and damage to embryos produced in vitro. Therefore, excessive ROS production has a negative effect on embryonic development (Bain, Madan, & Betts, 2011; Cebrian-Serrano, Salvador, Raga, Dinnyes, & Silvestre, 2013). Previous studies have shown that inhibition of FAS can lead to ROS production, resulting in embryo arrest (Guo et al., 2017). In the present study, the results showed that FAS knockout could impair blastocyst hatching, but not blastocyst formation, via the generation of ROS. Thus, FAS knockout, via excessive ROS production, displayed a negative effect on early embryonic development.
In response to ER stress, the UPR, which involves three ER transmembrane signal transducers, is activated (Malhotra & Kaufman, 2007). In embryos, this results in embryonic death and low developmental competency of blastocysts (Basar et al., 2014; Hao et al., 2009). Alterations in two previously described signaling pathways were detected in this study, which demonstrated that FAS inhibition induces phosphorylation of PERK and splicing of XBP1. First, spliced XBP1 was detected by immunostaining and RT-PCR. The results demonstrated that FAS knockout can increase the expression level of spliced XBP1 in response to ER stress. Under these conditions, XBP1 is cleaved to remove a 26-nucleotide intron from the full-length mRNA by conventional splicing. This results in a translational frameshift to create a longer XBP1 protein. XBP1 is an important factor in protein folding, ER translocation, and phospholipid biosynthesis, which functions by activating many UPR-related genes. (Cao & Kaufman, 2014; Ron & Walter, 2007). In addition, inhibition of XBP1 splicing positively affects porcine embryo development, which is associated with cell death(Zhang et al., 2011). Similar results were observed in mouse preimplantation embryos, wherein inhibition of XBP1 splicing improved embryo development and decreased apoptosis (Zhang, Diao, Kim, & Jin, 2012a; Zhang, Diao, Oqani, Han, & Jin, 2012b). In addition, ER transmembrane protein sensors, such as IRE1, PERK, and ATF6, are also essential regulators that are always found in association with the ER stress-inducible chaperone BiP (Jolly & Morimoto, 2000). Under stress conditions, the release of BiP from ER sensors activates the UPR to maintain proper protein folding (Bertolotti, Zhang, Hendershot, Harding, & Ron, 2000). The active form of BiP causes arrest of embryonic development (Basar et al., 2014). In the present study, we also observed that FAS knockout increased BiP expression. FAS knockout, therefore, prevented embryonic development via an increase in BiP expression, which activated the UPR. In response to the UPR, XBP1 was spliced.
PERK is a critical ER transmembrane sensor protein that binds to BiP. In response to ER stress, PERK is released from BiP and undergoes trans-autophosphorylation (Hao et al., 2009; Malhotra & Kaufman, 2007). Activation of PERK induces the maximum transcription UPR-dependent genes (Harding, Zhang, Bertolotti, Zeng, & Ron, 2000). ATF4 is one of the targets activated by phosphorylation of PERK (Malhotra & Kaufman, 2007). Several antioxidative stress response- and apoptosis-related genes are also activated, such as CHOP (Marciniak et al., 2004). Although all three UPR transcription factors can induce the transcription of CHOP, the PERK-ATF4 complex is mainly required for CHOP regulation (Harding et al., 2000; Rutkowski et al., 2006). Therefore, FAS knockout can induce ER stress to regulate the transcriptional expression of the pro-apoptotic gene, CHOP. In this study, FAS knockout led to increased levels of p-PERK in response to ER stress, resulting in the increased mRNA expression levels of ATF4 and CHOP. Thus, FAS knockout may induce apoptosis by increasing the expression of ATF4 and CHOP, which is mediated by increased p-PERK levels after activation of the UPR.
The ER is also the primary Ca2+ storage organelle in the cell, and protein folding reactions require high levels of ER intraluminal Ca2+. Excessive unfolded protein accumulation in the ER may release Ca2+ into the cytosol resulting in the generation of ROS (Berridge, 2002). Ca2+ is an important regulator in the feedback relationship between the ER and mitochondria. During ER stress, the release of Ca2+ is an inducer of apoptosis via BAX/BAK-regulated Ca2+ efflux into the cytosol to activate caspase cascades (Scorrano et al., 2003). In addition, the entrance of Ca2+ into the mitochondria can cause cytochrome c release to activate apoptosis (Scorrano et al., 2003). In this study, FAS knockout resulted in Ca2+ entering the mitochondria from the ER. Ru360 treatment, which prevents Ca2+ from entering the mitochondria, confirmed the effect of FAS knockout on Ca2+ efflux (more Ca2+ was observed in the cytosol when FAS was knocked out). We found that FAS knockout-induced Ca2+ efflux from the ER contributed to the induction of apoptosis. Subsequently, apoptosis induction would disrupt embryonic development. Therefore, FAS knockout-induced apoptosis leads to disrupted embryonic development, which is caused by increased entry of Ca2+ into the mitochondria from the ER.
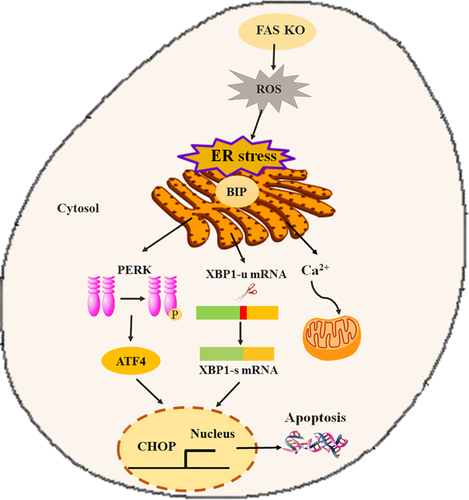
In this study, we have shown that FAS knockout can induce an ER stress response that activates the apoptosis pathway (Figure 8), which might explain the negative effect of FAS knockout on embryonic development. Our results also established a mechanistic relationship between FAS and ER function in early embryonic development.
AUTHORS' CONTRIBUTIONS
J.G. and N.-H.K. designed and conceived the experiments; J.G. wrote the manuscript and all authors reviewed it. Y.-J.N., K.-T.S., and J.-W.K. provided assistance with the experiment procedures. X.-S.C. provided professional advice on the experimental design and paper writing.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2015R1D1A1A01057629), Republic of Korea. We thank Professor Dong Il Jin from Chungnam National University for providing the anti-XBP1 antibody.
COMPETING INTERESTS
The authors declare no competing financial interests.




