Transcription factor HMG box-containing protein 1 (HBP1) modulates mitotic clonal expansion (MCE) during adipocyte differentiation
Abstract
Transcription factor HMG box-containing protein 1 (HBP1) has been found to be up-regulated in rat adipose tissue and differentiated preadipocyte; however, how HBP1 is involved in adipocyte formation remains unclear. In the present study, we demonstrated that under a standard differentiation protocol HBP1 expression fluctuates with down-regulation in the mitotic clonal expansion (MCE) stage followed by up-regulation in the terminal differentiation stage in both 3T3-L1 and MEF cell models. Also, HBP1 knockdown accelerated cell cycle progression in the MCE stage, but it impaired final adipogenesis. To gain further insight into the role of HBP1 in the MCE stage, we found that the HBP1 expression pattern is reciprocal to that of C/EBPβ, and ectopic expression of HBP1suppresses C/EBPβ expression. These data indicate that HBP1 functions as a negative regulator of MCE. In contrast, when HBP1 expression was gradually elevated along with a concomitant induction of C/EBPα at the end of the MCE, HBP1 knockdown leads to a significant reduction of C/EBPα expression, suggesting that HBP1-mediated C/EBPα expression may be needed for the termination of the cell cycle at the end of MCE for terminal differentiation. All told, our findings show that HBP1 is a key transcription factor in the already complicated regulatory cascade during adipocyte differentiation.
Abbreviation
-
- HBP1
-
- HMG box-containing protein 1
1 INTRODUCTION
In vitro adipocyte differentiation is a sequential process that comprises two phases, mitotic clonal expansion (MCE) and terminal differentiation. In the MCE stage, hormonal stimulation triggers growth-arrested preadipocytes to reenter the cell cycle program for proliferation, which is considered a commitment process for differentiation of 3T3-L1 preadipocyte (Tang, Otto, & Lane, 2003b; Yeh, Cao, Classon, & McKnight, 1995). Subsequently, the preadipocytes begin to mature into adipocyte phenotypes with the accumulation of cytoplasmic fat drops during terminal differentiation. The whole process of adipogenesis is coordinately regulated by transcription factors such as CCAAT/enhancer-binding proteins (C/EBPs) and peroxisome proliferator-activated receptor γ (PPARγ).
C/EBPs and PPARγ transcription factors function in a sequential cascade. In response to hormonal stimulation, C/EBPβ expression is up-regulated and this elevated expression of C/EBPβ is crucial for the initiation of the MCE stage when the cells synchronously reenter the cell cycle traversing the G1-S checkpoint (Tang, Otto, & Lane, 2003a; Zhang, Tang, Vinson, & Lane, 2004). Activation of C/EBPβ then transcriptionally induces the PPARγ and C/EBPα genes. C/EBPα is believed to be responsible for cessation of mitotic growth and coordinates with PPARγ to induce the expression of differentiation-specific genes that give rise to adipocyte phenotype (Cao, Umek, & McKnight, 1991).
The transcription factor HMG box-containing protein 1 (HBP1) plays various biological roles, including the regulation of cell cycle control, senescence, and apoptosis (Yee et al., 2004; Zhang et al., 2006). Overexpression of HBP1 often leads to a delayed transition from the G1 to the S phase or senescence in normal cells (Shih et al., 2001; Shih, Tevosian, & Yee, 1998; Tevosian et al., 1997). In cancer cells, ectopic overexpression of HBP1 can result in growth arrest, apoptosis, or differentiation, suggesting HBP1's possible role in tumor suppression (Chen et al., 2010; Kim et al., 2006; Lee et al., 2013; Yao, Works, Romagnoli, & Austin, 2005). Meanwhile, HBP1 also plays a crucial role in tissue differentiation, including muscle (Shih et al., 1998), myeloid (Yao et al., 2005), skin (Borrelli et al., 2010), and nervous cells (Watanabe, Kageyama, & Ohtsuka, 2015). Elevated HBP1 expression has been shown to determine the differentiation timing of erythroid, megakaryocyte and keratinocyte, and cortical neurogenesis (Borrelli et al., 2010; Watanabe et al., 2015). However, the expression of HBP1 blocks muscle cell differentiation (Shih et al., 1998).
The involvement of HBP1 in cell differentiation has also been reported in adipocyte differentiation (Lesage et al., 1994): HBP1 mRNA level has been shown to be abundant in white adipose tissue and differentiated ob1771 preadipocytes (Lesage et al., 1994). However, the underlying mechanism by which HBP1 regulates adipocyte differentiation is still unclear. Therefore, in the current study, we explored the role of HBP1 in the regulation of the MCE stage and subsequent adipocyte differentiation. 3T3-L1, a murine preadipocyte cell line commonly used for adipogenesis research due to its ability to be homogeneously differentiated (Tang et al., 2003b) and mouse embryonic fibroblast (MEF) cells were employed as this study's models. We found HBP1 expression fluctuated during the course of adipocyte differentiation. Suppression of HBP1 expression by HBP1-specific shRNA or siRNA in confluent, growth-arrested cells resulted in an accelerated ushering into proliferative mode yet impaired final differentiation. In an attempt to understand the underlying mechanism through which HBP1 regulates adipocyte differentiation, we ran tests and found that HBP1 may function as a negative regulator of C/EBPβ yet is a positive regulator of C/EBPα during the proceeding of the MCE phase. Thus, our data suggest a novel role of HBP1 in the control of adipocyte differentiation.
2 MATERIALS AND METHODS
2.1 Chemicals, reagents, and antibodies
Dulbecco's modified Eagle's medium (DMEM) and penicillin-streptomycin solution were purchased from Gibco Laboratory (Grand Island, NY). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Antibodies against C/EBPα, C/EBPβ, and PPARγ were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against HBP1 and α-tubulin were purchased from Proteintech Group (Chicago, IL) and GeneTex Inc. (Irvine, CA), respectively. Antibodies against GFP, p16, p21, and p27, and the siRNA for mouse HBP1 were purchased from Santa Cruz Biotech (Santa Cruz, CA).
2.2 Protocol of adipocyte differentiation (Lii et al., 2012)
3T3-L1 preadipocytes and MEF (mouse embryonic fibroblast) cells were obtained from Bioresources Collection and Research Center (BCRC, Hsin-Chu, Taiwan) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 U/ml). Two days after reaching confluence (Day 0), the medium was changed to a differentiation medium (MDI) containing 10 µg/ml insulin, 0.25 mM 3-isobutyl-1-methylxanthin (IBMX), and 0.25 µM dexamethasone (DEX) (Ntambi & Young-Cheul, 2000). Two days later (Day 2), the medium was switched to DMEM containing 10 mg/L insulin and changed every other day till full differentiation.
2.3 Plasmids
Both pLKO-puro and pLKO-puro-HBP1 shRNA vectors were kindly provided by Dr. Amy S. Yee (Tufts University, MA). The pITA-GFP and pITA-HBP1-GFP expressing vectors were obtained from Dr. Xiaowei Zhang (Peking University, China).
2.4 Oil-red O staining
As described previously (Lii et al., 2012), the differentiated preadipocytes were washed twice with PBS and fixed with 10% formaldehyde for 1 hr at room temperature. After washing with distilled water three times, the cells were stained with 1.2 mg/ml Oil-red O/60% isopropanol solution for 10 min. Excess Oil-red O was washed off with distilled water. Stained oil droplets in 3T3-L1 cells were photographed under light microscopy or were extracted with isopropanol and had their absorbance measured at 490 nm.
2.5 Western blotting
Total cell lysates (50 µg) were separated by 10% SDS polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Nonspecific binding was blocked with 5% non-fat milk in TBST buffer (20 mM Tris base, 140 mM NaCl, pH 7.6, 0.1% Tween-20) for 1 hr, followed by incubation with primary antibodies at 4°C overnight. The next day, membranes were then incubated with appropriate secondary antibodies conjugated with horseradish peroxidase. The detection was achieved by measuring the chemiluminescence of the ECL blotting agent, and the images were visualized by densitometric scanning (Alliance 4.7, UVItec, Cambridge, UK).
2.6 Reverse transcription-polymerase chain reaction (RT-PCR) and real-time PCR
Cellular RNA was extracted using a Quick-RNA™ MiniPrep kit (Zymo Research, Irvine, CA). The cDNA was synthesized by an iScript™ cDNA synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was performed using an iQ™ SYBER® Green Supermix (Bio-Rad Laboratories). The primers and the RT-PCR protocol are described as before (Lee et al., 2013). The following primers were used: mouse HBP1, 5′-ACTCCTGTGATGAGCACATGGA-3′(F) and 5′-AGCAGTGCCAAATGGTGGAT-3′(R); C/EBPα, 5′-GAACAGCAACGAGTACCGGGTA-3′(F) and 5′-GCCATGGCCTTGACCAAGGAG-3′(R); C/EBPβ, 5′-GCAAGAGCCGCGACAAG-3′(F) and 5′-GGCTCGGGCAGCTGCTT-3′(R); PPARγ, 5′-TTTTCAAGGGTGCCAGTTTC-3′(F) and 5′-AATCCTTGGCCCTCTGAGAT-3′(R); aP2, 5′-AAGACAGCTCCTCCTCGAAGGTT-3′(F) and 5′-GCGTAAATGGGGATTTGGTCACCA-3′(R); 18S, 5′-GGGAGCCTGAGAAACGGC-3′(F) and 5′-CCGCTCCCAAGATCCAACTAC-3′(R).
2.6 Cell cycle analysis (Lee et al., 2013)
3T3-L1 cells were seeded onto six-well plates and grown until they reached confluence. On day 0, both control and HBP1 knockdown cells were treated with MDI for 6 hr and then the cells were trypsinized, washed with PBS, and fixed in cold 70% ethanol at −20°C. The fixed cells were collected and stained in a solution containing 0.5 ml of 4 µg/ml of PI, 0.5 mg/ml of RNase, and 1% Triton X-100 for 30 min at 4°C. The DNA content of these cells was analyzed using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
2.7 Statistical analysis
Our data are expressed as mean ± SD or mean ± SEM from at least three independent experiments. Statistical significance was analyzed using Student's t-test. Results were considered statistically significant at p < 0.05.
3 RESULTS
3.1 HBP1 expression level fluctuates during adipocyte differentiation process
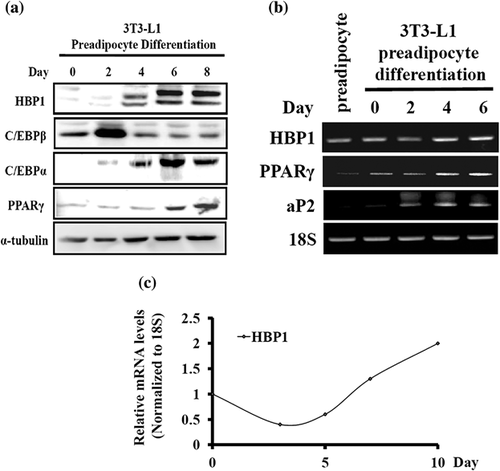
To obtain clues regarding whether or not transcription factor HBP1 is involved in fat tissue formation, we first investigated the HBP1 expression pattern throughout the course of adipose formation in a cell culture model. Following standard protocol of hormonal stimulation for adipocyte differentiation (see section 2, above), 3T3-L1 preadipocyte did not display an obvious increase in HBP1 protein level until day 4, and this elevated HBP1 level was sustained till day 8 when fat drops were well-formed with a concomitant increase in PPARγ and C/EBPα expression (Figure 1a). A similar expression pattern was also observed in the mRNA level: HBP1 mRNA expression was down-regulated after differentiation medium (DMI) stimulation (within days 0 and 2) and then up-regulated gradually till terminal differentiation in 3T3-L1 cells (Figure 1b). This down-and-then-up fluctuation of the expression pattern of HBP1 during the course of adipocyte differentiation was also observed in MEF (mouse embryonic fibroblast) cells (Figure 1c), suggesting HBP1's role in the control of adipocyte differentiation and adipogenesis.
3.2 Reduced HBP1 expression impairs adipocyte differentiation
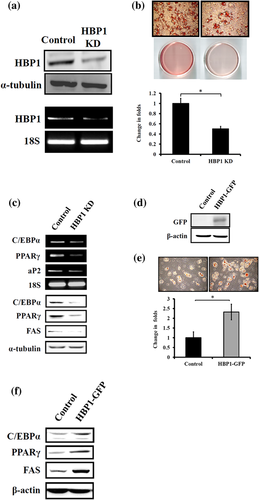
To understand if HBP1 expression level would affect the outcome of adipocyte differentiation, we used shRNA specific to HBP1 mRNA to knock down HBP1 expression in 3T3-L1 preadipocytes and score the fat drop formation following standard differentiation protocol. HBP1 knockdown (KD) (Figure 2a) significantly resulted in the reduced intensity of Oil red O staining (Figure 2b). A similar HBP1 effect was also noted happening in MEF cells (data not shown). The inhibitory effect of HBP1 KD on adipogenesis was further confirmed with reduced expression of the adipocyte markers, C/EBPα, PPARγ, and aP2, on day 8 of the differentiation program (Figure 2c). In contrast, the ectopic overexpression of HBP1 resulted in the increased accumulation of fat depots in MEF cells (with better transfection efficiency than 3T3-L1 cells) following standard differentiation protocol (Figure 2d–f). These data suggest that HBP1 indeed participates in the regulation of adipocyte differentiation process.
3.3 HBP1 regulates mitotic clonal expansion (MCE)
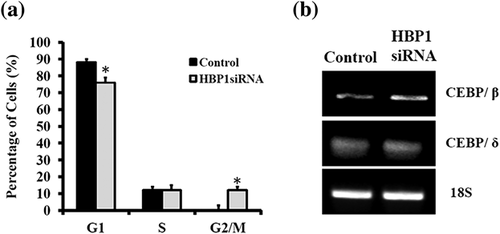
The growth-arrested 3T3-L1 preadipocytes were able to be induced to differentiate via synchronous reentry of the cell cycle with approximately two rounds of division (referred to as MCE) before terminal differentiation (Tang et al., 2003b). This MCE is shown to be a prerequisite for the expression of the adipocyte genes that give rise to the adipocyte phenotype in the culture model, such as 3T3-L1 preadipocyte (Tang et al., 2003b). Since HBP1 is known to be a negative regulator of G1-to-S transition during cell cycle progression (Shih et al., 2001; Tevosian et al., 1997), it is, therefore, rational to speculate as to the effect of HBP1 as a mediator of the MCE progression. Indeed, HBP1 KD hastened cell cycle progression as demonstrated by the earlier entry into the G2/M phase as compared with the none HBP1-KD control (Figure 3a), and it was accompanied by a concomitant increase in C/EBPβ expression (Figure 3b). These data suggest that HBP1 down-regulation and/or C/EBPβ up-regulation may be crucial for the reentry of growth-arrested preadipocytes into cell cycle mode to initiate MCE.
3.4 HBP1 modulates C/EBPβ expression during MCE stage
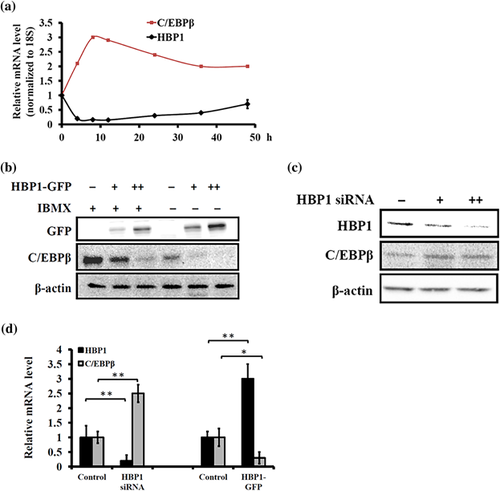
Since both HBP1 and C/EBPβ have been established to be involved in cell cycle regulation, we then examined whether or not C/EBPβ is a target gene of HBP1's modulation of the cell cycle program during the MCE stage. Observing the gene expression pattern of both transcription factors, we found that HBP1 and C/EBPβ expressed themselves reciprocally during the first 48 hr of the MCE stage: HBP1 expression was dramatically down-regulated upon stimulation of a differentiation medium (MDI: IBMX, DEX, and Insulin [Ntambi & Young-Cheul, 2000]) and then increased gradually at the late stage of MCE, whereas C/EBPβ expression was up-regulated following MDI stimulation and then decreased as the MCE stage progressed (Figure 4a). This observation means that C/EBPβ might be a target gene of HBP1 transcription repression. To test this hypothesis, we overexpressed HBP1 by transecting HEK-293T cells with the HBP1-GFP expressing plasmid followed by IBMX treatment, a direct inducer of C/EBPβ expression (Cao et al., 1991; Yeh et al., 1995). As shown in Figure 4b, ectopic overexpression of HBP1 resulted in a dose-dependent suppression of C/EBPβ protein levels regardless of the presence or absence of IBMX. In contrast, HBP1 siRNA led to increased expression of C/EBPβ protein in MEF cells (Figure 4c). A similar HBP1 effect pattern on C/EBPβ mRNA was also found in MEF cells (see Figure 4d), suggesting that HBP1 may function as a transcription repressor of C/EBPβ.
The treatment of MDI allows growth-arrested preadipocytes to enter proliferation mode. With that in mind, we further investigated the effect of MDI on HBP1 activation and found that HBP1 was transcriptionally regulated upon MDI administration; either MDI or IBMX alone suppressed HBP1 expression (Figure 5a) and a 2-kb HBP1 promoter activity (Figure 5b) in a dose-dependent manner. In contrast, DEX, a C/EBPδ activator (Cao et al., 1991), induced HBP1 expression at a high concentration (Figure 5a). These data indicate that HBP1 expression during an adipocyte differentiation program was tightly regulated by hormonal stimulation.
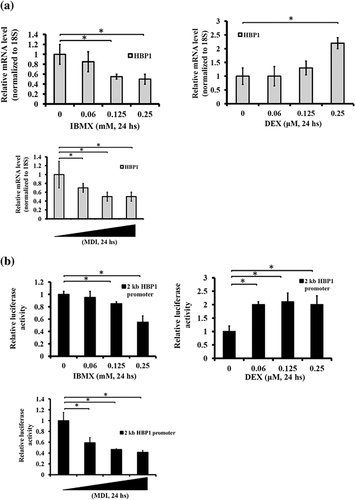
3.5 HBP1 regulates the transition from the cessation of the MCE to the onset of terminal differentiation
In trying to understand the role of HBP1 in adipocyte differentiation, so far, our data indicate that HBP1 KD accelerates the entry of growth-arrested preadipocyte into the cell cycle mode, leading to an impaired adipogenesis at the end. This observation prompted us to hypothesize that HBP1 expression might be required for the termination of the MCE and/or the onset of the terminal differentiation. Indeed, our experimentation showed that HBP1 KD led to delayed termination of the MCE as evidenced by an increased cell count at 48 and 72 hr (referred to as days 2 and 3) following MDI stimulation (Figure 6a). Similar to HBP1, C/EBPα is also antiproliferative (Freytag, Paielli, & Gilbert, 1994). Therefore, we examined the C/EBPα expression pattern during the time course of the MCE following MDI stimulation and found that C/EBPα and HBP1 displayed similar expression patterns. In particular, the expression of both genes increased at the late phase of the MCE (Figure 6b), which suggests both genes have an antiproliferative function. Finally, HBP1 KD resulted in the suppression of C/EBPα expression on day 2 (Figure 6c). These data indicate that HBP1 might regulate C/EBPα expression to terminate the cell cycle mode at the end of the MCE and then commit cells for terminal differentiation.
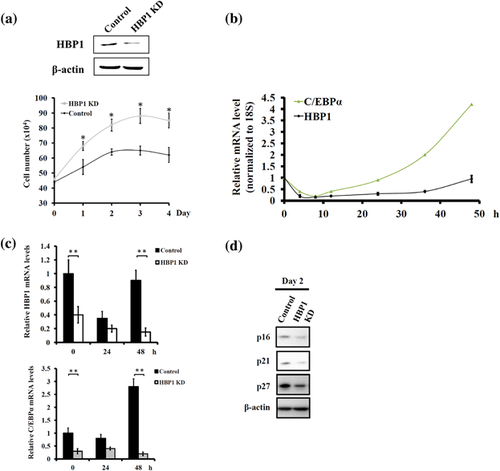
Several cell cycle inhibitors have been widely studied in the control of preadipocyte differentiation (Inoue et al., 2008; Phelps & Xiong, 1998; Reichert & Eick, 1999). HBP1 has been shown to be a positive regulator of cell cycle inhibitors p16 (Li et al., 2010; Wang, Pan, Chen, Huang, & Zhang, 2012) and p21 (Chen et al., 2016); therefore, we also examined the expression pattern of p16 and p21 along with p27 during preadipocyte differentiation. The result indicated that both p16 and p21 exhibited a similar fluctuating pattern with HBP1 (data not shown). In addition, HBP1 KD resulted in decreased expression of p16, p21, and p27 on day 2 of adipocyte differentiation, indicating that HBP1-mediated transcription of p16 and p21 may be also applicable in the context of HBP1 regulation of the MCE during adipocyte differentiation (Figure 6d).
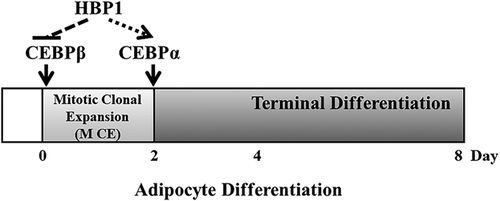
Taken as a whole, our data support the consideration of a novel role of HBP1 in the regulation of adipocyte differentiation (Figure 7). HBP1 expression is down-regulated at the beginning of the MCE to ensure the up-regulation of C/EBPβ and re-entry into postconfluent mitosis. However, HBP1 expression is up-regulated at the end of the MCE to ensure the up-regulation of C/EBPα and the termination of the MCE as well as the onset of terminal differentiation.
4 DISCUSSION
The implication of HBP1's role in adiposity began with the observation by Lesage et al. (1994) that HBP1 expression is found to be high in adipose tissue among rat tissues measured and differentiated Ob1771 cells. In concert with these previous findings, in the current study we found that HBP1 expression level is higher in differentiated adipocyte of both 3T3-L1 and MEF cells. To further understand the mechanisms through which HBP1 regulates adipocyte formation, we proposed and demonstrated that HBP1 at least in part regulates adipocyte differentiation through controlling the progression of the MCE stage via its interplay with C/EBPβ and C/EBPα (Figure 7). To be more precise, the negative regulation of HBP1 on C/EBPβ expression at the beginning of the MCE stage ushers growth-arrested preadipocytes to reenter the cell cycle mode while the positive regulation of HBP1 on C/EBPα expression at the late phase of the MCE stage ensures the cessation of the cell cycle program for terminal differentiation.
The present study provides compelling evidence to support the notion of an obligate role of HBP1 in the regulation of the MCE process during in vitro adipocyte differentiation. The expression level of HBP1 changes along the process of 3T3-L1 adipocyte differentiation. HBP1 was up-regulated in the growth-arrested preadipocyte compared with the non-confluent 3T3-L1 cells (Figure 1b). Upon MDI stimulation to synchronize growth-arrested cells to reenter the cell cycle of the MCE, HBP1 was potently down-regulated during the first 10 hr, followed by a gradual re-induction to that of the growth arrest state (Figure 4a). This re-induction of HBP1 expression reached its peak during the terminal differentiating state (Figure 1a). A similar expression pattern was also observed in the MEF model (Figure 1c). Our observations provide the first clear and detailed picture of the HBP1 expression pattern during the time course of adipocyte differentiation.
C/EBPβ is a crucial transcription factor for MCE and subsequent terminal differentiation (Tang et al., 2003a; Zhang et al., 2004). Its expression is activated and reciprocal to HBP1 upon MDI stimulation (Figure 4a). So far, there is no evidence indicating that both C/EBPβ and HBP1 transcription factors reciprocally suppress each other's expressions. We demonstrated in this study that at least HBP1 alone is a negative regulator of C/EBPβ transcription due to the fact that forced expression of exogenous HBP1 potently suppresses C/EBPβ expression, whereas HBP1 knockdown activates C/EBPβ expression (Figure 4b–d). Nonetheless, we still cannot exclude from consideration the possibility that activation of C/EBPβ upon hormonal stimulation causes repression of HBP1 expression since in a previous study a C/EBPβ mutant failed to down-regulate the expression of p27/kip1, another G1-S checkpoint gene, and thereby disrupted the proceeding of the MCE phase (Zhang et al., 2004). Whether or not C/EBPβ also functions as a transcription repressor of HBP1 is worth further investigation.
C/EBPα has been found to be a master regulator of adipocyte differentiation due to its anti-mitotic activity at the cessation of the MCE stage and its activation of differentiation-specific genes during the terminal differentiation stage (Freytag et al., 1994; Reichert & Eick, 1999). Consistent with such previous findings, we found here that C/EBPα expression was potently down-regulated upon MDI stimulation at the onset of the MCE, followed by a gradual re-induction at the late stage of the MCE (Figure 6b), which coincided with the fluctuation of HBP1 expression. The anti-proliferative activity of C/EBPα has been proposed in another study to be possible through the activation of cell cycle inhibitors, p21, p27, and/or HBP1 (Reichert & Eick, 1999). Whether or not C/EBPα can activate HBP1 remains unclear, but it is obvious that HBP1 can activate C/EBPα expression when HBP1 knockdown results in reduced C/EBPα expression (Figure 6c), a fact which is in agreement with the observation that HBP1 overexpression induces the myeloblast cell line K562 into differentiation lineages of erythroid and megakaryocyte with a concomitantly strong induction of C/EBPα (Yao et al., 2005).
In addition to its interplay with C/EBPβ and C/EBPα, HBP1 may also regulate adipocyte differentiation through its modulation of the Wnt/β-catenin signaling pathway. The activation of the Wnt pathway has been shown before to suppress adipocyte differentiation accompanied by a reduction of C/EBPα and PPARγ as well as an accumulation of free β-catenin (Bennett et al., 2002; Ross et al., 2000). In the canonical Wnt pathway, β-catenin is a crucial downstream effector that functions as a co-activator of the T-cell factor/lymphoid enhancer factor (TCf/LEF) family of transcription factors, leading to the activation of target genes (Eastman & Grosschedl, 1999). Hence, disruption of the β-catenin/TCf/LEF complex via ectopic expression of either a dominant-negative Tcf or a β-catenin antagonist Chibby promotes adipocyte differentiation (Li et al., 2007; Ross et al., 2000). HBP1 has been shown to suppress the Wnt/β-catenin pathway through physical interaction with TCf/LEF, thereby inhibiting its DNA binding (Sampson et al., 2001). Therefore, the possibility that HBP1 may promote adipocyte differentiation through the inhibition of the Wnt/β-catenin/TCf/LEF pathway cannot be dismissed.
ACKNOWLEDGMENTS
This work was supported by the grants NSC 102-2320-B-039-036 and MOST 105-2320-B-039-044-MY2 from the Ministry of Science and Technology, and CMU 103-S-29 from China Medical University, Taiwan, R.O.C. to Chun-Yin Huang.
CONFLICT OF INTEREST
None declared.




