Quercetin protects against radiocontrast medium toxicity in human renal proximal tubular cells
Abstract
Radiocontrast media (RCM)-induced acute kidney injury (CI-AKI) is a major clinical problem whose pathophysiology is not well understood. Direct toxic effects on renal cells, possibly mediated by reactive oxygen species, have been postulated as contributing to CI-AKI. We investigated the effect of quercetin on human renal proximal tubular (HK-2) cells treated with the radiocontrast medium (RCM) sodium diatrizoate. Quercetin is the most widely studied flavonoid, and the most abundant flavonol present in foods. It has been suggested to have many health benefits, including angioprotective properties and anti-cancer effects. These beneficial effects have been attributed to its antioxidant properties and its ability to modulate cell signaling pathways. Incubation of HK-2 cells with 100 μM quercetin caused a decrease in cell viability and pre-treatment of HK-2 cells with 100 μM quercetin followed by incubation with 75 mgI/ml sodium diatrizoate for 2 hr caused a decrease in cell viability which was worse than in cells treated with diatrizoate alone. However, further incubation of the cells (for 22 hr) after removal of the diatrizoate and quercetin caused a recovery in cell viability in those cells previously treated with quercetin + diatrizoate and quercetin alone. Analysis of signaling molecules by Western blotting showed that in RCM-treated cells receiving initial pre-treatment with quercetin, followed by its removal, an increase in phosphorylation of Akt (Ser473), pSTAT3 (Tyr705), and FoxO3a (Thr32) as well as an induction of Pim-1 and decrease in PARP1 cleavage were observed. Quercetin may alleviate the longer-term toxic effects of RCM toxicity and its possible beneficial effects should be further investigated.
1 INTRODUCTION
Radiocontrast media induced-Acute kidney injury (CI-AKI) is still an important consequence of the use of RCM, occurring in up to 30% of patients who receive iodinated contrast media and it is considered to be the third most common cause of hospital acquired AKI (Fahling, Seeliger, Patzak, & Persson, 2017). It has been proposed that the toxic effects of RCM could be due to oxidative stress and subsequent generation of reactive oxygen species (ROS) (Andreucci, Solomon, & Tasanarong, 2014; Pisani et al., 2013) and changes in intracellular signaling pathways, including the upregulation of cell signaling molecules that have been implicated in cell death/inflammation, such as the JNKs (Andreucci, 2011; Andreucci et al., 2011; Andreucci, Faga, Russo et al., 2014; Lee, Sheu, Yen, Lai, & Chang, 2010). The use of the antioxidant N-acetylcysteine to alleviate the effects of RCM has yielded conflicting results (Investigators, 2011; Xu, Tao, Bai, Deng, & Chen, 2016), although other naturally occurring antioxidants, such as α- and γ-tocopherol (vitamin E) have been reported to offer some benefit (Tasanarong, Vohakiat, Hutayanon, & Piyayotai, 2013). In the present study, we set out to investigate the effect of quercetin on the toxic effects of radiocontrast media (RCM) on human proximal tubular renal cells in vitro.
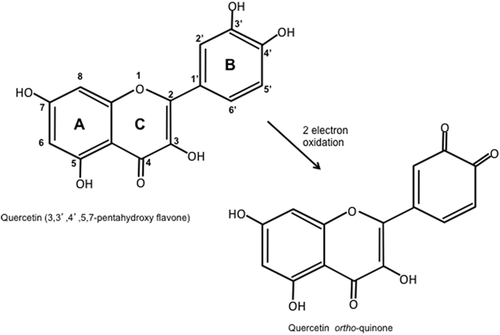
Quercetin is a member of polyphenolic compounds known as flavonoids and is ubiquitous in plants, being found in fruits, vegetables, nuts, seeds, flowers, and bark (Cook & Samman, 1996). Quercetin, in fact, belongs to the flavonol group of flavonoids, its chemical structure consisting of two phenolic rings joined by a heterocyclic oxygen containing pyran ring, with five hydroxyl groups substituted on the three ring structure: hence, its chemical name of 3,3′,4′,5,7-pentahydroxy flavone (Figure 1). It is the most widely studied flavonoid and is the most abundant flavonol present in foods, and has been suggested to have many health benefits including: angioprotective properties, having been linked to decreased mortality due to heart disease and decreased incidence of stroke; anti-cancer effects on a wide variety of cancers including melanoma; anti-inflammatory and anti-obesity effects, as well as reducing the symptoms of osteoarthritis (D'Andrea, 2015). It has been suggested that quercetin imparts its beneficial effects due, in part, to its strong antioxidant properties and is able to lower the levels of radical inflammatory species that have been suggested to be involved in the pathophysiology of many diseases (D'Andrea, 2015; de Oliveira et al., 2016). The catechol group on the B ring and the hydroxyl group at position 3 of the A ring confer the ability to scavenge free radicals via donation of a single electron to the radical cation giving rise to a semi-quinone form of quercetin, which in turn can donate another electron to form the quinone (Figure 1) (Sekher Pannala, Chan, O'Brien, & Rice-Evans, 2001). Furthermore, the catechol group as well as the combination of the carbonyl group on the C ring with the 3- and 5-hydroxyl groups enable quercetin to chelate metal ions especially copper and iron, thus reducing the formation of the hydroxyl radical formed from hydrogen peroxide by the Fenton reaction (Leopoldini, Russo, Chiodo, & Toscano, 2006). But, in addition to its chemical properties, it has also been suggested that quercetin is able to exert its effects in other ways including modulation of redox enzymes, interaction with cell/organelle membranes and modulation of cell signaling pathways (D'Andrea, 2015; de Oliveira et al., 2016). Indeed, quercetin has been reported to have seemingly contrary effects on signaling molecules. On the one hand, it may cause the inhibition of molecules, such as the JNK family of mitogen-activated protein kinases (MAPKs) (Kobuchi, Roy, Sen, Nguyen, & Packer, 1999), that are known to play a role in cell death and inflammation (Kyriakis & Avruch, 2012), while on the other they may also negatively affect molecules associated with cell survival (Williams, Spencer, & Rice-Evans, 2004).
In the present study, we found that incubation of renal cells with quercetin and the RCM sodium diatrizoate together did not alleviate RCM-toxicity in the short-term. However, further incubation of the cells for 22 hr after removal of both quercetin and sodium diatrizoate resulted in a recovery in viability of cells that had been previously treated with quercetin. We therefore set out to characterize the effects of quercetin on signal transduction pathways in the same renal cells and to see how these pathways are modified in both quercetin-treated and non-treated cells upon exposure to the RCM sodium diatrizoate.
2 MATERIALS AND METHODS
2.1 Materials
The radiocontrast agent sodium diatrizoate (denoted as “N,” “NaD,” or “diatrizoate” in parts of this manuscript) was obtained from Sigma–Aldrich Co. (St. Louis, MO) and was dissolved in RPMI cell culture medium and used at a final concentration of 75 mg iodine/ml when incubated with the cells. This concentration chosen for this study is physiologically relevant and calculated based on the dosage commonly used in clinical practice, as mentioned in several previous studies (Andreucci et al., 2006; Andreucci et al., 2011; Andreucci, Faga, Russo et al., 2014; Hardiek, Katholi, Ramkumar, & Deitrick, 2001). Quercetin was also obtained from Sigma–Aldrich Co., Milano, Italy (product no: Q4951) and primary antibodies used in the study were as follows: anti-phospho-ERK1/2 [extracellular signal-regulated kinase] (p44/p42 MAP kinase, cat no:9101, Cell Signaling, Beverly, MA); anti-phospho-Akt (Ser 473, cat no:9271, Cell Signaling); anti-phospho-p38 (cat no:9211, Cell Signaling); anti-phospho-FoxO3a (Thr 32)/anti-phospho-FoxO1 (Thr 24) (cat no: 9464, Cell Signaling); anti-phospho STAT-(signal transducer and activator of transcription)-3 (Tyr705) [cat no:9131 Cell Signaling]; anti PARP(Poly [ADP-ribose] polymerase)-1 (cat no:9532,Cell Signaling); anti-phospho-NF-κB (nuclear factor kappa-light chain-enhancer of activated B cells) [p65 subunit] (Ser276) (cat no:3037, Cell Signaling); anti-Pim [proviral insertion site in Moloney leukemia virus]-1 (cat no:2907, Cell Signaling); anti-phospho JNK(c-jun N-terminal kinase)1/2 (anti-ACTIVE, Promega cat no:V793A); anti-β-actin (cat no:A5441, Sigma).
2.2 Cell culture
The HK-2 cell line, an immortalized human renal proximal tubular epithelial cell line from normal adult human kidney, was used and was obtained from the American Type Culture Collection (ATCC® LGC Standards Srl, Milano, Italy) and grown in 100 mm culture dishes (Corning®, VWR International Srl, Milano, Italy), as previously described (Andreucci et al., 2006). In brief, cells were cultured in DMEM containing Glutamax™ (Gibco Life Technologies Italia, Monza, Italy) supplemented with 10% Fetal Calf Serum and 100 U/ml Penicillin and 100 μg/ml Streptomycin (Sigma–Aldrich) in an atmosphere of 5% CO2 in air at 37°C, up to a confluence of approximately 90–100%. Cells in passages 18–30 were used in our experiments.
2.3 Cell viability
Cell viability was measured by the ability of viable cells to (chemically) reduce MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) (Sigma–Aldrich), as previously reported by us (Andreucci et al., 2010). Cells were grown in six-well plates (Corning, VWR International Srl) and upon reaching a confluence of approximately 90%; the cells were serum-starved overnight, after which subjected to the appropriate treatment with quercetin and NaD: cells were incubated with 10 or 100 μM quercetin only; cells were incubated with 10 or 100 μM quercetin for 30 min (or the appropriate volume of DMSO for those treatments not receiving quercetin) followed by incubation with sodium diatrizoate at a final concentration of 75 mg iodine/ml; or incubation with sodium diatrizoate only (75 mg iodine/ml). After 2 hr, the media was removed and either the cells underwent the MTT assay immediately or were cultured for a further 22 hr in serum-free medium before undergoing the MTT assay. For the MTT assay, cells were washed once with sterile PBS and incubated with 1 mg/ml MTT (in sterile PBS) for 1 h at 37°C; they were then dissolved in dimethyl sulphoxide (DMSO). Measurements of the colored product as a result of MTT reduction were made at 540 nm using a Beckman DU 800 spectrophotometer. Experiments were carried out at least three times.
2.4 Western blot analysis
For analysis of the effect of quercetin on signal transduction molecules in cells incubated with and without sodium diatrizoate, HK-2 cells were grown to 95–100% confluence and then serum-starved 18–20 hr prior to experimentation. Cells were incubated with 100 μM quercetin or with sodium diatrizoate (75 mg iodine/ml) or with both (in which case quercetin was added 30 min prior to addition of sodium diatrizoate). For analysis of signaling molecules over a short time course, cells were harvested at: 15, 30, 60, and 120 min after the addition of sodium diatrizoate (corresponding to 45, 60, 90, and 150 min after the addition of quercetin).
As previously described by us (Andreucci et al., 2015), HK-2 cells, at each time point, were washed with cold PBS and then lyzed in buffer containing: 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM Na4P2O7, 100 mM NaF, 2 mM EGTA, 1 mM DTT, 1 mM NaVO4, 1% (v/v) Triton X-100, 2 µM microcystin, 400 µM PMSF with one protease inhibitor cocktail tablet (Complete Mini™, Roche GmbH, Germany) added for every 10 ml of lysis buffer, as per manufacturers' instructions. Then, the samples were then centrifuged at 10,000g for 10 min and the supernatant was retained (lysate). Part of the supernatant was used to determine the protein content and part utilized for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE). Protein concentrations were determined by using a modified Bradford protein assay (Bradford, 1976) protocol in order to obtain an equal loading (approximately 30 μg of each sample were loaded).
Protein extracts were resolved by SDS–PAGE and transferred to a nitrocellulose membrane (Hybond C® extra, Amersham Biosciences GE Healthcare Life Sciences, Milano, Italy), as previously described (Andreucci et al., 2003). The membrane was incubated for 1 hr at room temperature with 5% (w/v) non-fat powdered milk in a “TBS-Tween buffer” {“TBST”: 20 Mm Tris and 137 mM NaCl, pH 7.6, containing 0.1% (v/v) Tween® 20.The primary antibody, diluted in TBST with 5% (w/v) non-fat powdered milk, was then added to the membrane and incubated overnight at 4°C. The membrane was then washed three times, 5 min each, at room temperature with TBST and incubated for 1 hr with a secondary antibody conjugated with horseradish peroxidase (Dako, Agilent Technologies Italia SpA, Milano, Italy), diluted 1:5,000 TBST with 1% (w/v) non-fat powdered milk at room temperature. It was then washed as above (i.e., three times). The secondary antibodies, conjugated with horseradish peroxidase, were detected by incubation of the membrane with an enhanced chemiluminescence reagent, as described before (Andreucci et al., 2015). These experiments were carried out at least three times.
2.5 Statistics
All results were expressed as mean ± SE. Statistical analysis was performed using ANOVA for unpaired data (GraphPad 4.0 software), as previously reported (Andreucci et al., 2015; Andreucci, Faga, Russo et al., 2014). Statistical significance was defined when p < 0.05.
3 RESULTS
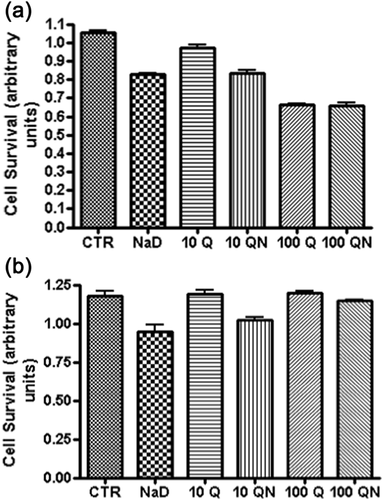
3.1 Viability of HK-2 cells treated with quercetin, diatrizoate, and quercetin/diatrizoate together
Incubation of HK-2 cells with sodium diatrizoate for 2 hr resulted in a loss in cell viability (Figure 2a), as has been previously reported (Andreucci et al., 2006). Cells that were previously pre-incubated with 10 and 100 μM quercetin prior to addition of sodium diatrizoate also suffered a loss in viability with a greater decrease observed with the higher concentration of quercetin. The viability of cells that were incubated with 10 μM quercetin alone remained similar to those of the control group (even though a slight decrease is shown in Figure 2a, this was not statistically significant), but those incubated with 100 μM quercetin showed a decrease in cell viability. Furthermore, cells pre-incubated with quercetin and then exposed to sodium diatrizoate showed a decrease in viability that was less than that observed in cells treated with the corresponding concentration of quercetin alone. Also, cells pre-treated with 100 μM quercetin and then incubated with sodium diatrizoate suffered a greater loss of viability than those cells treated with sodium diatrizoate only or cells treated with 10 μM quercetin + diatrizoate. After incubation of the cells for a further 22 hr in fresh medium, in the absence of quercetin and sodium diatrizoate, the population of cells previously treated with quercetin or quercetin + diatrizoate all had a greater viability than the cells that were previously treated with sodium diatrizoate only (Figure 2b). Furthermore, those previously treated with 100 μM quercetin + diatrizoate had a greater viability than those previously treated with 10 μM quercetin + diatrizoate. The viability of these cells previously treated with 100 μM quercetin + diatrizoate, as well as those previously treated with 100 μM quercetin only was not statistically different to control cells suggesting a full recovery. This remarkable recovery in cell viability prompted us to investigate changes in signaling molecules in cells exposed to the higher concentration of quercetin.
3.2 Incubation of HK-2 cells with quercetin and sodium diatrizoate for a short period of time
These experiments were carried out in order to look at changes in signaling molecules in HK-2 cells as a result of pre-incubation of the cells with 100 μM quercetin for 30 min followed by addition of 75 mg/ml sodium diatrizoate for 2 hr, and also to compare with cells incubated with sodium diatrizoate or quercetin only. Cells were harvested and prepared for western blot analysis at 15, 30, 60, and 120 min after addition of sodium diatrizoate (corresponding to 45, 60, 90, and 150 min after the initial addition of quercetin).
3.2.1 Effects on pAkt (Ser473), pERK 1/2, and pSTAT3 (Tyr705)
Incubation of HK-2 cells with sodium diatrizoate results in a dramatic decrease in the phosphorylation of the kinase Akt (pAkt) at Ser473 (Figure 3a) with only a faint band observed at 15 min. Cells incubated with only quercetin also showed a similar effect on pAkt levels as did cells incubated with both quercetin and sodium diatrizoate. The levels of the phosphorylated forms of the mitogen activated protein kinases ERK 1 and ERK2 (indicated as pERK1 and pERK2, respectively) were also lowered upon incubation with sodium diatrizoate with a recovery in their levels observed during the course of incubation. However, their levels continued to gradually decrease in the presence of quercetin, and while the levels of pERKs in cells treated with both quercetin + diatrizoate paralleled those of diatrizoate-treated cells, nonetheless their levels were always lower than diatrizoate-treated cells. Levels of phospho-STAT3 (Tyr705) also decreased in cells subjected to all three treatments, with those levels in diatrizoate/quercetin-treated cells at 60 and 120 min being lower than the corresponding levels of phosphorylated protein in cells treated with wither quercetin or sodium diatrizoate alone.
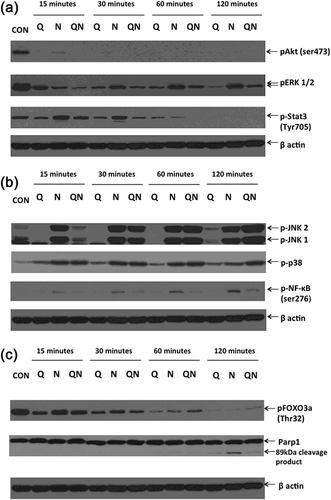
3.2.2 Effects on pJNK 1/2, phospho-p38, and pNF-κB (Ser276)
An increase in phospho-p38 MAPK levels was observed in all three treatments, with both sodium diatrizoate and quercetin/diatrizoate treatment causing a greater increase than quercetin alone (Figure 3b). Furthermore, at the 120 min time point, the phospho-p38 levels appear to be greater in diatrizoate + quercetin treated cells than the corresponding diatrizoate-treated cells. Treatment of cells with sodium diatrizoate also caused a dramatic increase in phosphorylation of the JNK MAP kinases. However, quercetin did not induce JNK phosphorylation (referred to as pJNK), but on the contrary quercetin-treated cells had lower levels compared with control cells. Furthermore, cells pre-treated with quercetin and then incubated with diatrizoate had lower levels of pJNK at the 15 and 30 min time points than the corresponding cells treated with diatrizoate only, but higher levels at 60 and 120 min. Diatrizoate also induced phosphorylation of the p65 NF-κB transcription factor (pNF-κB) at serine 276, whereas quercetin seemed to have a less striking effect, with faint bands of pNF-κB (Ser276) observed at the 15, 30, and 60 min time points. Interestingly, pre-treatment with quercetin and subsequent incubation with sodium diatrizoate resulted in a decrease in pNF-κB (Ser276) levels when compared with the corresponding diatrizoate-only treatment.
3.2.3 Effects on pFOXO3a (Thr32) and PARP-1
Incubation of HK-2 cells with sodium diatrizoate caused cleavage of PARP-1 with an apparent 89 kDa cleavage product visible after 60 min, which became more intense after 120 min (Figure 3c). A cleavage product was also visible in cells treated with quercetin and quercetin + diatrizoate at the 60 min time point, but was less intense than the band observed in cells treated with sodium diatrizoate alone at 120 min. The phosphorylation of the transcription factor FOXO3a (pFOXO3a) at Thr32 decreased with time under all three conditions, with levels of the phosphorylated protein observed to have almost diminished after 120 min.
3.3 Incubation of HK-2 cells for a prolonged period of time after removal of quercetin and diatrizoate
Two hours after addition of sodium diatrizoate, corresponding to 2.5 hr after addition of quercetin, the medium containing these chemicals was removed and fresh medium added, and the cells allowed to incubate for a further 22 hr. At 1, 3, 5, and 22 hr after addition of fresh medium, cells were harvested and again subjected to western blotting.
3.3.1 Changes in Pim-1, pAkt, pERKs, p-STAT3
After removal of quercetin and sodium diatrizoate, Pim-1 kinase were detected in cells that were previously incubated with quercetin at all time points studied with its levels gradually increasing up to 3 hr before decreasing again (Figure 4a). It was also detected, although at lower levels, in diatrizoate + quercetin treated cells at 1, 3, and 5 hr, but was not detected at the 22 hr time point. Notably, Pim-1 was not detected in cells treated with diatrizoate only. While the levels of pSTAT3 (Tyr705) were subdued in quercetin treated cells at the 1 hr time point, its levels dramatically increased at 3 and 5 hr and remained elevated at 22 hr. In diatrizoate-treated cells, the levels of pSTAT (Tyr705) gradually decreased with time, whereas in diatrizoate + quercetin-treated cells they increased gradually with time. In quercetin-treated cells the levels of pAkt (Ser473) increased gradually over the period of incubation from a level below that of the control cells at the 1 hr time point to a level approaching that of the control cells at the 22 hr time point. The levels of pAkt in diatrizoate-treated cells were high initially, but gradually decreased over the time course to below those present in control cells, while in quercetin + diatrizoate-treated cells, the levels of pAkt gradually decrease from 1 to 3 hr, but increased at 5 hr and continued to do so at 22 hr. There were no dramatic changes in levels of pERKs between the three different treatments, with those of diatrizoate-treated cells being slightly more elevated than the other two treatments but all were almost identical at 22 hr.
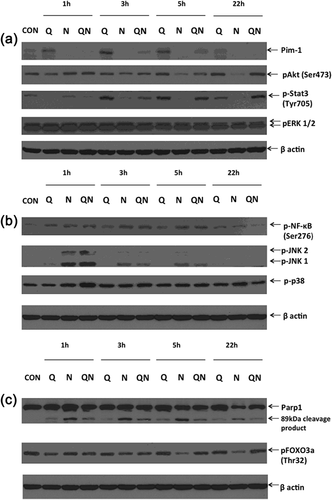
3.3.2 Changes in pJNK, p-p38, and pNF-kB
The levels of pNF-kB in both diatrizoate-treated and quercetin + diatrizoate-treated cells at 1, 3, and 5 hr were higher than in control cells before declining at 22 hr, at which time point the level in quercetin/diatrizoate was lower than in diatrizoate-treated cells (Figure 4b). The phosphorylated isoforms of the JNKs was observed in all three treatments at the 1 hr time course, with much higher levels of pJNKs detected in the diatrizoate-treated and diatrizoate + quercetin-treated than was observed in quercetin-treated cells. These levels declined over the time course, being barely detectable at 22 hr. The levels of phospho-p38 were higher in quercetin + diatrizoate-treated cells than the other two treatments at 1 hr, but gradually decreased so that at 22 hr they were lower.
3.3.3 Changes in PARP-1 and pFOXO3a (Thr32)
The levels of the Parp-1 cleavage product was always higher in diatrizoate-treated cells, gradually decreasing with time, but the level of whole Parp-1 had noticeably decreased in diatrizoate-treated cells at 22 hr (Figure 4c). In quercetin + diatrizoate-treated cells the cleavage product was less and also decreased over the time course, while in quercetin-treated cells the cleavage product was least of the three treatments. Levels of pFOXO3a (Thr32) in diatrizoate-treated cells decreased from 1 to 22 hr, while its levels were maintained in quercetin-treated and quercetin + diatrizoate-treated cells.
4 DISCUSSION
In the study presented, we have observed that pre-incubation of human renal cells with 100 μM quercetin for 30 min followed by exposure of the cells to the RCM sodium diatrizoate for 2 hr, initially caused a greater decrease in cell viability than with the RCM alone, but following a further incubation of 22 hr upon removal of both the diatrizoate and quercetin, resulted in a significant increase in cell viability when compared with cells that were only treated with diatrizoate. In this experimental model, we were trying to mirror a possible scenario in the kidney, whereby human proximal tubule cells may be exposed to a similar concentration of the RCM (75 mg iodine/ml) and for a similar period of time (2 hr) before being filtered out of the kidney (Andreucci et al., 2006; Hardiek et al., 2001). Direct toxic effects of RCM have been suggested as one way that they could damage renal cells and cause contrast-induced acute kidney injury (CI-AKI) (Andreucci, Faga, Pisani, Sabbatini, & Michael, 2014; Sendeski, 2011), and this could be mediated via the generation of reactive oxygen species (ROS) leading to oxidative stress (Andreucci, Solomon et al., 2014; Pisani et al., 2013). However, despite its noted properties as an antioxidant, incubation of cells with quercetin at both concentrations of 10 and 100 μM did not protect these cells from the toxic effects of sodium diatrizoate, when incubated together for up to 2 hr. This may be due to a mechanism of RCM action that entails more than oxidative stress and, indeed, we and others have previously suggested that RCM may modify cell signaling pathways leading to deactivation of prosurvival molecules such as Akt, while activating pro-inflammatory and cell death molecules such as the p38 and JNK family of MAP kinases (Andreucci et al., 2006; Andreucci et al., 2011; Andreucci, Faga, Russo et al., 2014; Gong, Celsi, Carlsson, Norgren, & Chen, 2010; Lee et al., 2010; Michael et al., 2014). Furthermore, we observed that quercetin on its own caused a decrease in cell viability when incubated over a short period of time (Figure 2a) at the higher (100 μM) concentration. Western blot analysis at the higher concentration of quercetin used, showed changes in signaling molecules that would contribute to cell demise such as: causing the dephosphorylation (inactivation) of Akt and phospho-ERK 1 and 2 which have been shown to be important for cell survival and proliferation (Datta, Brunet, & Greenberg, 1999; Roskoski, 2012); dephosphorylation of pSTAT3 at Tyr705, which is critical for its function as a transcription factor in the transcription of genes involved in cell proliferation and differentiation (Xiong, Yang, Shen, Zhou, & Shen, 2014); dephosphorylation (activation) of the transcription factor FOXO3a that may play a role in cell death by up-regulating the expression of pro-apoptotic factors (Fas ligand, tumor-necrosis factor-related apoptosis-inducing ligand) and cell cycle inhibitors (p21WAF/CIP1, p27KIP1) (Zhang, Tang, Hadden, & Rishi, 2011). At the same time, we observed some evidence of the antioxidant properties of quercetin in that phosphorylation of the transcription factor NF-κB at Ser276 was less in quercetin/diatrizoate-treated cells compared with diatrizoate-treated cells, and it has been noted by us and others that phosphorylation of NF-κB at this site may arise as a result of oxidative stress (Andreucci et al., 2009; Andreucci et al., 2015; Morgan & Liu, 2011) and we observed that quercetin added to the cells was able to reduce pNF-κB (Ser276) levels after addition of sodium diatrizoate (Figure 3a). Furthermore, we have also previously noted the phosphorylation of JNKs in HK-2 cells subjected to oxidative stress (Andreucci et al., 2009; Andreucci et al., 2015), and in the present study the levels of pJNKs were also reduced in quercetin/diatrizoate treated cells initially (Figure 3a, 15 and 30 min time points) compared with diatrizoate-treated cells. This again may be due to an antioxidative effect of quercetin, but in this case the effect is short-lived as the pJNK levels increase at 60 and 120 min in contrast to the observation with pNF-kB. In contrast, quercetin seemed to increase the phosphorylation of p38 at 120 min compared with the diatrizoate treated cells. At the 120 min time point, sodium diatrizoate clearly increased the appearance of the PARP-1 89 kDa cleavage product, which has been attributed to the proteolytic actions of caspases and considered a hallmark of apoptosis (Castri et al., 2014). Quercetin reduced the levels of the 89 kDa cleavage product in cells treated with sodium diatrizoate, even if cleaved PARP-1 was also observed in cells treated only with quercetin. This would be expected since both phospho-Akt and phospho-ERK1/2 levels were lowered which would lead to activation of the caspase activating pathways (Datta et al., 1999; Hu, Jiang, Li, & Lu, 2005) including those mediated by FOXO3a which, as already mentioned, was itself activated (dephosphorylated).
Removal of both the quercetin and sodium diatrizoate from the cells resulted in a dramatic increase in cell viability of cells that were previously incubated with quercetin only or quercetin/diatrizoate compared with cells incubated with diatrizoate alone. Granado-Serrano et al reported that incubation of human hepatoma cells with quercetin for 4 and 18 hr caused a dramatic decrease in cell viability that was dose dependent, with 100 μM quercetin causing approximately 70% decrease in cell viability after 18 hr incubation (Granado-Serrano, Martin, Bravo, Goya, & Ramos, 2006). Furthermore, other studies have shown that incubation of neuronal cells with quercetin caused an initial (within 30 min) dephosphorylation (inactivation) of Akt (Ser473), which then recovered after a longer period (within 6 hr) of incubation (Spencer, Rice-Evans, & Williams, 2003), although this was observed for a concentration of quercetin lower (10 μM) than the one we used to investigate changes in signaling molecules. Another study also showed recovery of pAkt levels within 18 hr of continuous incubation of human hepatoma cells with 50 μM quercetin, after pAkt levels steadily declined for the first 4 hr (Granado-Serrano, Angeles Martin, Bravo, Goya, & Ramos, 2008). However, in our case the renal cells were exposed to quercetin for 2.5 hr before it was removed and the cells were then incubated for a longer period of time in its absence and so clearly the situation is not quite the same, and therefore differences would be expected considering also the differences in the types of cells used.
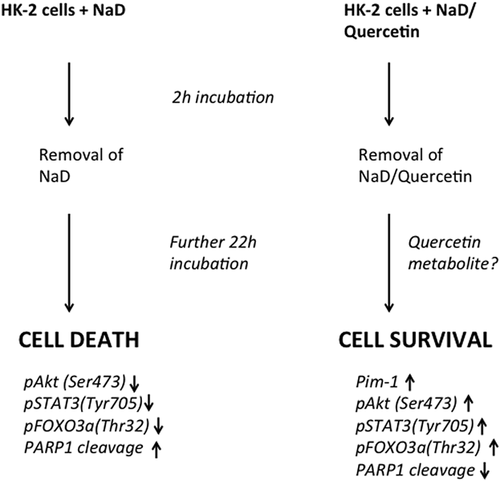
Nonetheless, the recovery of cells after removal of quercetin is remarkable, especially in those cells that were first pre-treated with quercetin and then subjected to sodium diatrizoate. These cells recovered viability compared with the sodium diatrizoate-treated cells and our analysis of signaling molecules showed some dramatic differences that could account for this. We observed that cells previously treated with quercetin only demonstrated a recovery in pAkt (Ser473) and pSTAT3 (Tyr705) levels as well as in pFOXO3a (Thr 32) levels. An increase in the expression of the serine/threonine kinase Pim-1 was also observed. This kinase is known for its pro-proliferative actions, having many substrates involved in cell cycle progression and apoptosis (Bachmann & Moroy, 2005; Blanco-Aparicio & Carnero, 2013), and has been reported to protect cells against certain stresses (e.g., airway epithelial cells against cell death caused by exposure to cigarette smoke [de Vries et al., 2014]). Pim-1 has been reported to be translated in an active configuration and, so far, there have not been any reports to suggest the existence of any post-translational modifications that alter its activity, such as phosphorylation (Blanco-Aparicio & Carnero, 2013). It is worth noting that the transcription factor STAT3 has been implicated in regulating Pim1 expression (Bachmann & Moroy, 2005), although in our study we observe Pim1 expression before recovery in the phosphorylation status of STAT3 (Tyr705). We are not sure of the actual mechanisms leading up to the changes in signaling molecules observed. Certainly, we report that quercetin causes a reduction in activation of molecules known to play a role in cell survival, even though, as mentioned, others have observed a biphasic response when some cells are incubated for longer periods of time with this flavonol. Hence, one might suggest that quercetin metabolites may be generated that may confer a different outcome than quercetin itself. However, some have reported that certain metabolites of quercetin, such as ortho (o)-quinone, may be toxic to cells (Metodiewa, Jaiswal, Cenas, Dickancaite, & Segura-Aguilar, 1999), as quinones are Michael acceptors reacting with nucleophilic species, including the sulphur atom of thiol groups in proteins and the nitrogen atom also present in proteins and nucleic acids, although others have suggested that the ortho-quinone, formed from oxidation of quercetin, is rapidly hydrolyzed and therefore not likely to damage important biological molecules (Bolton & Dunlap, 2017). Furthermore, it has been suggested that these o-quinone metabolites may be involved in the induction of an antioxidant defence mechanism via the electrophilic responsive element (EpRE) also known as the antioxidant responsive element (ARE), leading to the expression of antioxidant enzymes such as NAD(P)H/quinone oxidoreductase 1 (NQO1) and glutathione-S-transferases (Rietjens, Al Huseiny, & Boersma, 2011).
Baral, Pariyar, Kim, Lee, and Seo (2017) observed opposite effects of quercetin and one of its metabolites, quercetin-3-O-glucuronide (Q3GA) in neural stem cells: while quercetin caused a decrease in Akt phosphorylation and decrease in cell viability, Q3GA promoted Akt phosphorylation and cell proliferation which was shown to be Akt-dependent. We have also previously demonstrated that a white grape juice extract caused an increase in Akt phosphorylation when incubated with the HK-2 cells used in the present study (Andreucci et al., 2015). The major phenolic component of this extract was in fact quercetin-3-glucuronide and was calculated to be present at a concentration of approximately 8 μM. However, there were other polyphenolic compounds present in this extract and it would not be possible to conclude that Akt phosphorylation was due solely to the quercetin glucuronide. Furthermore, in the present study one would have to assume that there was glucuronidation of the quercetin once it was taken up in the cell, which is possible as human proximal tubule epithelial cells possess the necessary UDP-glucuronosyltransferase enzymes (Mutsaers et al., 2013). Certainly, quercetin has been reported to be taken up rapidly within minutes or even seconds when added to cells in an in vitro culture (Fiorani et al., 2010; Walle, Vincent, & Walle, 2003) and it is feasible that quercetin would be metabolized within the time frame of its incubation with the HK-2 cells, and those metabolites could still remain inside the cell even after the medium is replaced. Fiorani et al. (2010) observed a rapid uptake and intracellular accumulation of quercetin in Jurkat cells, and its release from the cell was slow, since 60% of the original amount of quercetin was still associated with the cell 2 hr after incubation in fresh medium. Furthermore, they observed that quercetin was relatively more concentrated in the mitochondria and was also in an active form, thus being able to suppress membrane lipid peroxidation by the potent peroxynitrite oxidant, during which quercetin was reduced and also redistributed to other cellular compartments. However, we observed that in the short-term incubation quercetin was unable to alleviate the toxic effects of sodium diatrizoate. It is therefore possible that in our case, once the RCM (and hence oxidative) stimulus is removed, any oxidative species are neutralized by the quercetin that still persist within the cells, and at the same time influence activation/deactivation/regulation of signaling molecules by itself or through one of its metabolites. Another issue to consider is that we do not know the exact mechanism of RCM toxicity, in that we do not know for sure whether reactive oxygen species themselves are the cause of cell death or are a secondary effect of RCM action, an issue that is still a matter of contention (Pisani et al., 2013). One group, using an ex vivo model of rat kidney thick ascending limbs, has suggested the latter in that they observed direct cell damage caused by RCM with consequent oxidative stress and the formation of ROS (Liu et al., 2014). This would fit in with our observations that quercetin was unable to ameliorate the toxic effects of sodium diatrizoate in the short term, and in fact making it worse at the higher concentration used. But, once the sodium diatrizoate was removed, the intracellular pool of quercetin (or a quercetin metabolite) would have acted to quench the ROS that would have arisen due to the action of the sodium diatrizoate. Furthermore, our observations that an initial pre-treatment with quercetin also caused, in the long run, an activation of signaling molecules, such as Akt and STAT3, as well as an induction of Pim-1, would further strengthen the proliferative processes leading to cell survival (summarized in Figure 5). However, we must stress that quercetin was also toxic to the renal cells and any potential benefits against the toxic effects of RCM would need to be verified in further in vivo studies. Given that nowadays CI-AKI is still the third most common cause of all hospital-acquired kidney failure (Andreucci, 2014; Mamoulakis, Tsarouhas, Fragkiadoulaki, & Heretis, 2017; Nash, Hafeez, & Hou, 2002), although several preventive measures have been suggested (Andreucci, Faga, Pisani et al., 2014; Andreucci, Solomon et al., 2014), the ultimate objective, after the aforementioned in vivo studies, would be to trial the use of quercetin in clinical studies, as there exist very few feasible options (Andreucci, Faga, Serra, De Sarro, & Michael, 2017) to lessen the known side-effects of RCM (Andreucci, Solomon et al., 2014) and any novel pharmaceutical or nutraceutical intervention would be welcome in order to significantly decrease the high incidence of CI-AKI.
ACKNOWLEDGMENTS
Dr. Ashour Michael is currently recipient of an “Assegno di ricerca” from “Magna Graecia” University of Catanzaro (Italy). We would also like to acknowledge: the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA), Regione Calabria Project “Rete Regionale di informazione sul farmaco: informazione, formazione, e farmacovigilanza.”
DECLARATION
The results presented in this paper have not been published previously in whole or part, except in abstract form.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest




