Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases
Abstract
Mesenchymal stem cells (MSCs) can differentiate into not only cells of mesodermal lineages, but also into endodermal and ectodermal derived elements, including neurons and glial cells. For this reason, MSCs have been extensively investigated to develop cell-based therapeutic strategies, especially in pathologies whose pharmacological treatments give poor results, if any. As in the case of irreversible neurological disorders characterized by progressive neuronal death, in which behavioral and cognitive functions of patients inexorably decline as the disease progresses. In this review, we focus on the possible functional role exerted by MSCs in the treatment of some disabling neurodegenerative disorders such as Alzheimer's Disease, Amyotrophic Lateral Sclerosis, Huntington's Disease, and Parkinson's Disease. Investigations have been mainly performed in vitro and in animal models by using MSCs generally originated from umbilical cord, bone marrow, or adipose tissue. Positive results obtained have prompted several clinical trials, the number of which is progressively increasing worldwide. To date, many of them have been primarily addressed to verify the safety of the procedures but some improvements have already been reported, fortunately. Although the exact mechanisms of MSC-induced beneficial activities are not entirely defined, they include neurogenesis and angiogenesis stimulation, antiapoptotic, immunomodulatory, and anti-inflammatory actions. Most effects would be exerted through their paracrine expression of neurotrophic factors and cytokines, mainly delivered at damaged regions, given the innate propensity of MSCs to home to injured sites. Hopefully, in the near future more efficacious cell-replacement therapies will be developed to substantially restore disease-disrupted brain circuitry.
1 INTRODUCTION
Stem cells have the ability to renew themselves continuously or differentiate into many cell types. In adult life, stem cells have been identified in several tissues where, by the continuous production of new cells, they guarantee physiological conditions and repair mechanisms after injuries or diseases. In many organs, such as the blood, skin, or gastrointestinal tract, stem cells extensively work throughout the organism's entire life, contributing to rapid replacement of dead or worn out cells. Other tissues, in which repair mechanisms are not so efficient, do not easily recover after injuries or extensive degenerative events.
This is the case of the central nervous system (CNS), where for a long time it was believed that only microglia, astrocytes, and oligodendrocytes proliferate in the adult organism, whereas, neurons were considered unable to divide. Indeed, this limitation has to be partially revised, since it has been demonstrated that in at least two areas of the brain, the dentate gyrus of the hippocampus and the subventricular zone adjacent to the lateral ventricles (SVZ), neural stem cells (NSCs) can continuously generate new functional neurons (Gage, 2002). However, they likely account for neurogenesis only within limited regions of the brain and are not able to counteract diffuse neuronal death as it occurs in neurodegenerative diseases (Lie, Song, Colamarino, Ming, & Gage, 2004). In these cases, the gradual and progressive loss of neural cells severely impairs the quality of life of patients, mostly because of cognitive and behavioral dysfunctions.
As pharmacological approaches have induced in many instances poor effects, stem cell-based brain transplantation therapies have been extensively investigated (Chan et al., 2014). Different lines of stem cells have been explored (Blundell & Shah, 2015). Embryonic stem cells have been widely studied for their pluripotency and very high proliferative potential. However, they raise ethical/religious issues and involve the risk of easily producing tumors. Induced pluripotent stem cells, reprogrammed from autologous somatic cells could also differentiate to neural cells, but their safe use has not yet been properly assessed. Considering their nature, NSCs would appear the most suitable tool (Kim, Lee, & Kim, 2013). In fact, they already give origin to the three main cell types of the mammalian CNS: neurons, astrocytes, and oligodendrocytes. Unfortunately, having to be extracted from the nervous tissue, autologous NSCs are hardly available in sufficient amounts without significant harm for the patient (Chan et al., 2014). In addition, data on NSC transplantation are controversial, since when injected in vivo, they may remain in the undifferentiated form. These cells are also susceptible to immune responses upon allogeneic transplantation (Rossignol et al., 2014).
The use of MSCs appears more suitable since they are available in satisfactory amounts, feature low immunogenicity, can differentiate into multiple cell lineages (Arutyunyan, Elchaninov, Makarov, & Fatkhudinov, 2016; Li & Ikehara, 2013; Lo Furno, Mannino, Cardile, Parenti, & Giuffrida, 2016), including neurons and glial cells when cultured under specific conditions (Lo Furno et al., 2013). Moreover, they can be used in clinical treatments without raising significant ethical or religious issues (Tanna & Sachan, 2014). Among many available tissues, the most studied MSCs are those from umbilical cord (UC-MSCs), bone marrow (BMSCs) and adipose tissue (ASCs). Some other sources have also been examined, such as dental pulp (Xiao & Tsutsui, 2013) or even menstrual blood (Zemel'ko et al., 2013).
In the present review, we focus on results obtained using MSCs as a potential therapeutic tool for the treatment of some disabling neurodegenerative disorders such as Alzheimer's disease (AD), Amyotrophic Lateral Sclerosis (ALS), Huntington's disease (HD), Parkinson's disease (PD). The large number of positive effects reported, both at histological and behavioral levels, have encouraged a progressively increasing number of therapeutic approaches in humans.
2 ALZHEIMER'S DISEASE
Alzheimer's Disease, the most common type of dementia, is characterized by degeneration and death of neurons throughout the brain, mainly in the basal forebrain, amygdala, hippocampus, and cerebral cortex. AD is considered a multifactorial and heterogeneous disease and is generally known as a late-onset disease, accompanied by cognitive dysfunction and associated with the accumulation of amyloid β (Aβ) plaques and neurofibrillary tangles. As the disease progresses, memory and cognitive functions progressively decline, patients become demented and prematurely die (Chan et al., 2014; Chang, Lee, & Suh, 2014; Kim et al., 2013). Although the pathogenesis of the disease is not clearly defined, genetic factors such as rare mutations in amyloid precursor protein (APP), apolipoprotein E, and presenilins (PS) seem to be involved in about 5% of cases.
Dysfunction of the presynaptic cholinergic system is considered one of the causes of cognitive disorders. Thus, pharmacological therapy has been mainly based on molecules aimed at increasing the acetylcholine concentration, by inhibiting acetylcholinesterase. In some people, these treatments can temporarily improve symptoms or slow down their progression, but they are mostly palliative, without real protection against progressive cell degeneration (Amemori, Jendelova, Ruzicka, Urdzikova, & Sykova, 2015). Searching for more effective treatments, a cell-based therapy could represent a promising tool to ameliorate neuropathological deficits.
Most preclinical studies have been carried out in double transgenic animal models characterized by expression of mutated APP and PS1 (APP/PS1). Otherwise, the animal model was generated by intracerebral/intracerebroventricular injections of Aβ aggregates. Less frequently, some other animal models have been adopted. The main findings are summarized in Table 1.
| Authors | Investigation | Administration route—type of MSCs | Main findings |
|---|---|---|---|
| Li et al. (2008) | Rat model by β-amyloid injection into the hippocampus. | Hippocampus transplantation of gene-modified BMSCs to overexpress NGF. | Cell engraftment and migration in surrounding tissue. Tendency to express ChAT-like immunopositivity. Reduced hippocampal neuronal loss. Significant improvement in learning and memory. |
| Lee, Jin et al. (2009) | Mouse model by β-amyloid injection into the hippocampus. | Bilateral injections of BMSCs into the hippocampus. | Reductions of Aβ deposits, likely due to activation of microglia. Change of microglial morphology from ramified to ameboid, indicative of phagocytosis. |
| Lee et al. (2010) | Double transgenic APP/PS1 mouse model. | Bilateral injections of BMSCs into the hippocampus. | Attenuation of the cognitive impairment of spatial memory. Reductions of Aβ deposits, likely due to restoration of microglial function. |
| Kim et al. (2012) | Transgenic Tg2576 mouse model. | Intracerebral (hippocampus) or intravenous injection of ASCs. | Significant improvement in learning and memory. Reduction of Aβ deposition. Increasing of endogenous neurogenesis and synaptic and dendritic stability. ASCs were able to migrate into the brain through the blood brain barrier. |
| Lee et al. (2012) | Double transgenic APP/PS1 mouse model. | Hippocampal transplantation of UC-MSCs. | Significant improvements in cognitive functions. Reduction of Aβ deposits in the hippocampus and frontal cortex. Positive immunomodulatory effects associated to microglia changes from classic phenotype to the “alternatively activated” form. |
| Yang et al. (2013) | Double transgenic APP/PS1 mouse model. | Hippocampal transplantation of UC-MSCs after previous neuron-like differentiation. | Significant improvements in cognitive functions. Reduction of Aβ depositsassociated with “alternatively activated” microglia. |
| Ma et al. (2013) | Double transgenic APP/PS1 mouse model. | Hippocampal transplantation of ASCs. | Microglial activation. Enhanced Aβ clearing capacity in the hippocampus and the cortex. |
| Yan et al. (2014) | Double transgenic APP/PS1 mouse model. | Hippocampal transplantation of ASCs. | Reduction of oxidative stress, less cognitive impairment and enhanced of neurogenic activity in subgranular and subventricular zones of the brain. |
| Panchenko et al. (2014) | Bulbectomized mouse model. | Intravenous injection of BMSCs. | BMSCs were able to migrate into the brain through the blood brain barrier. Some engrafted cells showed astrocyte markers. None revealed neural differentiation. |
| Shin et al. (2014) | In vitro study. | Co-culture of MSCs and Aβ-treated neuronal cells. | Significant enhancement of autolysosome formation and clearance of Aβ both in vitro and in vivo models. Increased neuronal survival against Aβ toxicity. |
| Mouse model by intraventricular β-amyloid injection | Intravenous administration of MSCs. | ||
| Salem et al. (2014) | AlCl3-induced rat model. | Intravenous injections of BMSCs. | BMSCs were able to home to the injured nervous tissue. Better preserved histological structure in the hippocampus, showing increased number of ChAT-positive cells. |
| Yamazaki et al. (2015) | Transgenic 5xFAD mouse model. | Injection of ASC-CM. | Improvements of antidepression-related behavior. |
| Oh, Kim et al. (2015) | In vitro study. | Co-cultures of MSCs and NPCs. | Improvement of hippocampal neurogenesis. Enhancement of NSC differentiation into mature neurons, possibly by stimulation of the Wnt signaling pathway. |
| Mouse model by intraventricular injections of Aβ. | MSCs. | ||
| Naaldijk et al. (2016) | Double transgenic APP/PS1 mouse model. | Intravenous injections of BMSCs. | Engrafted BMSC in hippocampus and cerebral cortex. Modification of size and number of microglial cells. Reduced dimensions of pyroglutamate-beta amyloid plaques at the hippocampal level. |
| Matchynski-Franks et al. (2016) | Transgenic 5xFAD mouse model. | Transplantation of BMSCs into the lateral ventricles, the hippocampus, or both regions. | Reduction of cognitive deficit. Attenuation of Aβ42 in the hippocampus and other cerebral regions. |
| Ruzicka et al. (2016) | Triple transgenic (PS1/tau/APP) mouse model. | Transplantation of BMSCs into the lateral ventricle. | Better preservation of working memory. Downregulation of potentially toxic Aβ*56 levels in the entorhinal cortex. Improved neurogenesis in SVZ. |
| Boutajangout et al. (2016) | Double transgenic APP/PS1 mouse model. | Intracarotid injections of UC-MSCs. | Reduction of the cognitive loss. Reduction of Aβ deposits in the cerebral cortex and the hippocampus. |
| Kim et al. (2015) | Phase I clinical trial. | Stereotactic injection of UC-MSCs into the hippocampus and precuneus. | Although no improvement in the pathology is described, stereotactic administration of hUCB-MSCs seems feasible, safe, and well tolerated. |
2.1 MSC treatments
Bilateral delivery of murine BMSCs into the dentate gyrus of the hippocampus was tested on an acute mouse model of AD, induced by injection of Aβ aggregates (Lee, Jin, & Bae, 2009). As a result, Aβ deposits were reduced, likely due to activation of surrounding microglia. A signal of microglial phagocytosis was indicated by the change of cell morphology from ramified to amoeboid. In a later work of the same research group, improvements were also described after bilateral injection of BMSCs in the hippocampus of APP/PS1 mice (Lee et al., 2010). In this study, attenuated cognitive impairment of spatial memory was found associated with significant reductions of Aβ deposition and restoration of microglial function.
Therapeutic potential of intracerebral (bilateral transplantation into the dentate gyrus of the hippocampus) or intravenous injections of human ASCs (hASCs) was investigated on a transgenic (Tg2576) mouse model (Kim et al., 2012). The intracerebral injection was aimed at examining hASCs influences in the early stage of the disease, while the intravenous injection was designed to prevent or delay the onset of the disease. Learning and memory of animals significantly improved and Aβ deposition was reduced using both methods. In addition, synaptic and dendritic stability were increased, as well as endogenous neurogenesis. Since intravenous injected hASCs were able to pass through the blood brain barrier (BBB) and migrate into the brain, the authors conclude that this strategy can represent a convenient and safe way of hASC administration, useful for both the prevention and treatment of AD. The ability of human BMSC (hBMSC) to pass the BBB and engraft into the brain has also been reported by Panchenko et al. (2014) after intravenous administration in bulbectomized mice characterized by the development of Alzheimer-type neurodegeneration. However, only some of the engrafted cells showed astrocyte markers and none revealed neural differentiation.
Positive effects have been reported by delivering human UC-MSCs (hUC-MSCs) into the hippocampus of transgenic APP/PS1 mouse model (Lee et al., 2012). Significant improvements in cognitive functions, amelioration of the disease pathophysiology and positive immunomodulatory activities have been described, together with a significant reduction of Aβ deposits in the hippocampus and frontal cortex. Decreased Aβ deposition was associated with microglia changes from the classic phenotype to the “alternatively activated” form. Even more efficacious results were reported by Yang et al. (2013) in the same animal model, after single injections in the hippocampus of hUCB-MSC previously differentiated into neuron-like cells.
Hippocampal transplantation of ASCs in a transgenic APP/PS1 mouse model promoted microglial activation, with enhanced Aβ clearing capacity in the hippocampus and the cortex (Ma et al., 2013). Although ASC-induced mechanisms on microglial activation are not clear, they are able to prevent Aβ deposition. In the same mouse model, a reduced oxidative stress and less cognitive impairment have been reported following hippocampal ASC transplantation. In addition, enhanced neurogenic activity was detected in the hippocampal dentate gyrus and SVZ (Yan et al., 2014). Significantly improved hippocampal neurogenesis and enhancement of the differentiation of NSCs into mature neurons were also reported by Oh, Kim, Park, Shin, and Lee (2015) by in vitro and in vivo (Aβ-treated mice) studies, likely through the Wnt signaling pathway.
A neuroprotective effect against Aβ toxicity was reported by Shin et al. (2014), using human MSC treatments, both in vitro and in vivo experiments. Reduced neuronal death was observed in Aβ-treated neuronal cultures and in the hippocampal region of mice in which Aβ was injected into cerebral ventricles and MSCs in the tail vein. Increased neuronal survival was correlated to enhanced autolysosome formation and clearance of Aβ.
By single intravenous injections, BMSCs were delivered into rats in which AD was induced by oral administration of AlCl3 (Salem, Ahmed, Atta, Ghazy, & Aglan, 2014). Results obtained were compared with those from untreated AD animals or animals treated with rivastigmine and cerebrolysin, administered daily. It has been shown that BMSCs were able to home to the injured nervous tissue and produce significant increases in the number of positive cells for choline acetyltransferase, survivin, seladin-1, and nestin gene expression. Overall, BMSC-induced effects were similar to or even better than drug intake. Furthermore, a well preserved histological structure was detected in the hippocampus of these rats.
Intravenous injections of ASC conditioned medium (ASC-CM) in 5xFAD transgenic mice restored the shortened immobility time, as evaluated by the tail suspension and forced swim tests (Yamazaki, Jin, Tsuchiya, Kanno, & Nishizaki, 2015). The authors also report improvements of antidepression-related behavior, at least in part by modulating Akt and GSK-3β activity at the hypothalamic level. It is supposed that the use of ASC-CM might also be extended for psychological disorders.
After injection in the tail vein of APP/PS1 mice, BMSCs were found in the hippocampus and cerebral cortex, mainly around Aβ plaques (Naaldijk et al., 2016). A reduction was induced in the dimensions of pyroglutamate-beta amyloid plaque at the hippocampal level. This appears of interest since these plaques would play a major role in AD progression because of their aggregation propensity, their role in the initial seeding event, and their resistance against degradation. These findings would be promoted by immunomodulatory influences, as also indicated by modifications of size and number of microglial cells.
Transplantation of BMSCs into the lateral ventricles, the hippocampus, or both regions was performed in the 5xFAD transgenic mouse model (Matchynski-Franks et al., 2016). A reduction of cognitive deficits was described as well as an attenuation of Aβ42 in the hippocampus and other cerebral regions. Therefore, the authors recommend the intracerebroventricular administration of BMSCs since it can provide comparable or better outcomes using safer, less invasive brain surgery than intracerebral injections.
Intracerebroventricular transplantation of hBMSCs was also performed in a triple transgenic (PS1/tau/APP) mouse model at the age of 8 months (Ruzicka, Kulijewicz-Nawrot, Rodrigez-Arellano, Jendelova, & Sykova, 2016). After 6 months from implantation, a noticeable preservation of working memory was observed. It is suggested that such preservation might be due to the protective effect of MSCs on glutamine synthetase levels and considerable downregulation of potentially toxic Aβ*56 levels in the entorhinal cortex. In addition, although none of the implanted cells was still present, improved neurogenesis was revealed in SVZ.
Direct injection of hUC-MSCs into the carotid artery of APP/PS1 mice was able to reduce cognitive loss and Aβ deposits in the cerebral cortex and the hippocampus (Boutajangout, Noorwali, Atta, & Wisniewski, 2016).
3 AMYOTROPHIC LATERAL SCLEROSIS
Amyotrophic lateral sclerosis (ALS) is a severe, persistent neurodegenerative disease. It is characterized by the progressive loss of motor neurons in the cerebral cortex, brain stem, and spinal cord. Clinical symptoms consist of a gradual weakening and atrophy of muscles. Death usually occurs within 5 years from diagnosis and is mostly due to paralysis of respiratory muscles (Kim et al., 2013). Although the pathogenesis is still unknown, probable mechanisms involved in this multifactorial disease include glutamate-mediated excitotoxicity, oxidative damage, protein aggregation, mitochondrial dysfunction, altered axonal transport, and insufficient production of neurotrophic factors (Boillée, Vande Velde, & Cleveland, 2006). Most cases are sporadic (90%), whereas, the remaining cases are familial (FALS). In 15–20% of FALS cases, mutations of the gene encoding superoxide-dismutase 1 (SOD1) have been detected. This genetic basis has permitted the development of transgenic rodent models featuring clinical symptoms and histopathological features of both familial and sporadic human ALS (Bendotti & Carri, 2004). Among various mutant human SOD1 genes (G37R, G85R, G93A), G93A is the most commonly used. The main findings are summarized in Table 2.
| Authors | Investigation | Administration route—type of MSCs | Main findings |
|---|---|---|---|
| Rizvanov et al. (2008) | Transgenic SOD1 G93A mouse model. | Retro-orbital injection of UC-MSCs engineered to express human VEGF and mouse neural L1 cell adhesion molecule (L1CAM). | Increased homing ability and proliferation at the site of neurodegeneration in the spinal cord parenchyma. Preferential differentiation into endothelial cells forming new blood vessels. |
| Gu et al. (2010) | In vitro study. | Co-culture of ASCs and spinal cord astrocytes from SOD1 G93A mice. | Neuroprotective effects possibly related to reduced glutamate excitotoxicity. |
| Corti et al. (2010) | Transgenic SOD1 G93A mouse model. | Intravenous injection of BMSCs. | Preservation of motor neurons. Improvement of neuromuscular function. |
| Marconi et al. (2013) | Transgenic SOD1 G93A mouse model. | Intravenous injection of ASCs. | Neuroprotective and neuroregenerative effects. Presence of ASCs in the spinal cord, not expressing neuronal, or glial markers. Upregulation of GDNF and bFGF. |
| Kim et al. (2014) | Transgenic SOD1 G93A mouse model. | Intravenous or intracerebroventricular injection of ASCs. | Significant increase of animal survival. Delay in the onset of clinical symptoms. A very small fraction of engrafted ASCs in the lumbar spinal cord were positive for neuronal markers, none for GFAP. |
| Fontanilla et al. (2015) | Transgenic SOD1 G93A mouse model. | intraperitoneal injection of ASC-CM. | Neuroprotective effects. Preservation of motor neurons. Inhibition of inflammatory pathways in the spinal cord. |
| Jeon et al. (2016) | Transgenic SOD1 G93A mouse model. | Intraperitoneal injection of ASC extract. | Reduction of apoptotic cell death. Recovering of mitochondrial dysfunction. Improvement of motor functions. Delayed symptoms onset, rotarod test failure, and death. |
| Deda et al. (2009) | Phase II clinical trial. | Transplantation in brain stem and cervical spinal cord of Autologous BMSCs. | Improvement of general conditions with no adverse effects after 1 year of follow-up. |
| Karussis et al. (2010) | Phase I/II clinical trial. | Intrathecal and intravenous delivery of autologous BMSCs. | Possible propagation of MSCs from the lumbar site of inoculation to the nervous tissue. Stabilization or even improvement of clinical symptoms, with no adverse effects during a follow-up of 6–25 months. |
| Mazzini et al. (2010) | Phase I clinical trial. | Spinal cord injection of autologous BMSCs, previously expanded in autologous CSF. | Safe procedure to be further developed for future clinical applications. |
| Blanquer et al. (2012) | Clinical trial on hospitalized patients. | Intraspinal infusion of autologous bone marrow mononuclear cells. | No serious transplant-related adverse events. No acceleration of disease-related decline. Higher number of motoneurons in the anterior horns at the level of the transplanted spinal segments. |
| Oh, Moon et al. (2015) | Phase I clinical trial. | Two repeated intrathecal injections of autologous BMSCs. | Using this procedure, assumed safe and feasible, MSC therapeutic effects would be reinforced and maximized. |
| Rushkevich et al. (2015) | Clinical trial on hospitalized patients. | Combined intravenous administration of autologous undifferentiated BMSCs and injection of neural-induced BMSCs via lumbar puncture. | No serious side effects or complications. Slowdown of the disease progression and improvement of functional status of patients. |
| Syková et al. (2017) | Phase I/IIa clinical trial. | Intrathecal injection of previously expanded autologous BMSCs. | No serious adverse reactions after an 18-month follow-up period. Slowdown of the disease progression. |
| Petrou et al. (2016) | Phase I/II and IIa clinical trial. | Intramuscular and/or intrathecal injections of BMSCs, previously induced to secrete neurotrophic factors. | No serious treatment-related adverse effects in 6 months of follow-up. Improvements in the forced vital capacity and the ALSFR-R score, especially in patients receiving both injections. |
3.1 MSC treatments
Intravascular (tail vein) administration of BMSCs promoted the survival of motor neurons and improved neuromuscular function in SOD1 G93A mice (Corti et al., 2010). Moreover, the duration of the disease and lifespan was extended. Consistently, intravenous administration of ASCs in the same mouse model was able to promote neuroprotective and neuroregenerative actions (Marconi et al., 2013). A small amount of transplanted ASCs were found in the spinal cord, though they did not show neuronal or glial markers. In the authors' opinion, beneficial influences could be due to ASC increased paracrine activity rather than their neural differentiation. In fact, in the spinal cord of these mice, some neurotrophic factors, such as glial-derived neurotrophic factor (GDNF) and basic fibroblast growth factor (bFGF) were found significantly upregulated. While secretion of bFGF could be due to a direct action of ASCs, increased levels of GDNF might be related to enhanced, ASC-induced, production from host glial cells. Such neuroprotection would yield a strong and long lasting effect on motor performance.
Human ASCs were administered in SOD1 G93A mice (Kim et al., 2014). Rotarod test, paw grip endurance, and reflex index were used to evaluate motor behavior. Overall, the best results were obtained in ALS mice intracerebroventricularly transplanted with ASCs, although improvements also occurred after intravenous administration. Moreover, a significant increase of animal survival and delay in the onset of clinical symptoms was observed. At the histological level, a considerable number of transplanted hASCs were found engrafted in the lumbar spinal cord, but only a very small fraction of these cells was positive for neuronal markers, and none for GFAP. It is concluded that neuroprotective activities are probably due to the production of cytokines/growth factors from transplanted hASCs, rather than a replacement of lost neurons or glia. In agreement with these results, a neuroprotective role has been reported following intraperitoneal injection of ASC-CM in SOD1 G93A mice (Fontanilla et al., 2015). According to the authors, nerve growth factor (NGF) might be responsible for the preservation of motor neurons and inflammatory pathway inhibition in the spinal cord. In fact, NGF neutralized ASC-CM did not extend the survival time after disease onset.
Intraperitoneal administration of hASC extract in SOD1 G93A mice was able to reduce apoptotic cell death and recover mitochondrial dysfunction (Jeon et al., 2016). Western blot analysis of spinal cord tissue showed a significantly increased expression of p-Akt, p-CREB, and PGC-1α. In addition, choline acetyltransferase immunostaining in the ventral horn of the lumbar spinal cord showed a large number of motor neurons, featuring normal morphology. Improved motor function, delayed onset of symptoms, rotarod test failure, and death were also noticed. MSC paracrine neuroprotective activity has also been supported by in vitro studies in co-culture of hASCs and spinal cord astrocytes from SOD1 G93A mice (Gu et al., 2010). Soluble factors responsible for these effects include vascular endothelial growth factor (VEGF), hepatocyte growth factor, and insulin-like growth factor-1. These soluble factors might also enhance glutamate uptake by upregulating the expression of glutamate transporter 1 (GLT1), likely due to a reduced activation of caspase-3 (the enzyme responsible for GLT1 cleavage). This finding seems of great interest for preventing extracellular glutamate accumulation, whose neuro-excitotoxicity would contribute to ALS progression.
4 HUNTINGTON'S DISEASE
Huntington's disease (HD) is an autosomal dominant, late-onset neurodegenerative disease. Although it may occur before 20 years of age, in more than 90% of cases it develops at an age of 35–40 years (Fink et al., 2015). Characterized by progressive neuronal cell death, HD symptoms consist of a cognitive decline, behavioral changes, involuntary choreic movements, and psychiatric disturbances. HD is associated with an expanded and unstable CAG trinucleotide repeat in the huntingtin gene (htt), which yields abnormal accumulation of the Htt protein and cellular toxicity. As a result, a significant loss of neurons occurs, particularly GABAergic medium-sized spiny neurons in the caudate nucleus and putamen. Various other brain regions may, however, be involved, especially in later stages of the disease. In HD patients, a substantial reduction (about 19%) of total brain volume can be found, associated with an enlargement of lateral ventricles. Currently, available pharmacological treatments can only relieve symptoms to improve the quality of life in HD patients. The disease constantly progresses and culminates in death within 15–20 years after the onset of motor symptoms.
Preclinical investigations to develop cell-based therapeutic approaches have been mainly carried out in two categories of animal models (Kerkis, Haddad, Valverde, & Glosman, 2015; Lescaudron, Naveilhan, & Neveu, 2012; Maucksch, Vazey, Gordon, & Connor, 2013). In the first type, the disease is simulated by inducing intrastriatal neuronal death by excitotoxic lesions caused by injections of kainic acid, quinolinic acid (QA), or 3-Nitroproprionic acid (3-NP). The second class includes genetically modified animal models overexpressing Htt protein (N1T1-82Q2, R6/2, R6/2-J2, N171-82Q, YAC128). The main findings are summarized in Table 3.
| Authors | Investigation | Administration route—type of MSCs | Main findings |
|---|---|---|---|
| Lee, Chu et al. (2009) | QA-induced rat model. | Intrastriatal injections of ASCs. | Reduced apoptosis of striatal cells. Reduced enlargement of lateral ventricles. Significant improvements in the apomorphine-induced rotation tests. |
| Transgenic R6/2 mouse model. | Intrastriatal injections of ASCs. | Attenuated loss of striatal neurons. Reduced huntingtin aggregates. Increased survival, rotarod performance, and limb clasping. | |
| In vitro study. | Cultures of human cerebral neuronal cells transfected with mutant huntingtin gene in ASC-CM. | Reduced apoptosis. Decreased levels of N-terminal fragments of mutant Htt. Increased expression of PGC-1α. | |
| Dey et al. (2010) | Transgenic YAC128 mouse model. | Striatal transplantation of BMSCs, engineered to overexpress NGF and/or BDNF. | Restoration of NeuN positive cell counts to those of wild-type animals. |
| Im et al. (2010) | Transgenic YAC128 mouse model. | Striatal transplantation of ASCs derived from healthy donors or HD patients. | Reduction of striatal atrophy only for treatment with normal ASCs. HD-derived ASCs might be useful for therapeutic applications after optimizing their paracrine activities. |
| Lin et al. (2011) | QA-induced mouse model. Transgenic R6/2-J2 mouse model. | Intrastriatal transplantation of hBMSCs. | Some engrafted cells that express typical neuronal or glial markers. Increased survival rate. Significant reduction in motor function impairment only in QA mice. |
| Rossignol et al. (2011) | 3NP-induced rat model. | Intrastriatal transplantation of different amounts of BMSCs. | Preservation of the anterior part of the striatum. Less enlargement of lateral ventricle. More evident behavioral improvements after injection of a lower number of cells. Beneficial effects are mainly related to BDNF release. |
| Im et al. (2013) | Transgenic R6/2 mouse model. | Intraperitoneal injection of ASC cell-free extracts. | Reduced striatal atrophy and mHtt aggregation. Slower progression of the disease. Less weight loss. Improvement of rotarod performance. |
| Fink et al. (2013) | Transgenic R6/2 mouse model. | Intrastriatal transplantation UC-MSC after low or high number of passages in vitro. | Considerable attenuation of brain atrophy. Significant preservation of metabolic activity in striatal tissue, only if injecting high-passaged UCB-MSCs. No evident reductions in motor deficits, except for transient sparing in a spatial memory task. |
| Rossignol et al. (2014) | Transgenic 51 CAG rat model. | Intrastriatal transplantation BMSCs, NSCs, or both. | Short-term behavioral benefits after NSC transplantation compared to BMSC, inducing a lower immune response. Best results after injections of both cell types, possibly due to a protective immunomodulatory actions of BMSCs. |
| Lee et al. (2016) | In vitro study. | Cultures of R6/2 mice-derived neuronal cells with ASC derived exosome. | Significant decrease of mutant huntingtin aggregates. Reduction of mitochondrial dysfunction and cell apoptosis by enhancing the p-CREB-PGC1a pathway. |
| Linares et al. (2016) | Transgenic N171-82Q mouse model. | Intranasal administration of BMSCs pretreated with lithium and valproic acid. | Significant improvement of ambulatory distance and mean velocity. Increased animal survival. Reduction of striatal neuronal loss and huntingtin aggregates. |
| Pollock et al. (2016) | Transgenic YAC128 mouse model. Transgenic R6/2 mouse model. | Striatal injection of BMSCs engineered to overexpress BDNF. | Decreased striatal atrophy and reduced anxiety in YAC128 mice. Significant increase in neurogenesis-like activity and the mean lifespan in R6/2 mice |
4.1 MSC treatments
Intrastriatal injections of hASCs were tested in a QA-induced rat model and R6/2 transgenic mice (Lee, Chu et al., 2009). Compared to controls, hASCs transplantation in the QA-model induced a lower number of apoptotic striatal cells and a reduction of lateral ventricle enlargement, likely due to attenuated brain atrophy. In addition, significant improvements in the apomorphine-induced rotation tests were observed. In R6/2 transgenic mice, attenuated loss of striatal neurons, and reduced huntingtin aggregates were found, together with increases of rotarod performance, limb clasping, and survival. In vitro experiments, designed to evaluate influences of soluble factors, mutant htt-transfected cerebral neurons were cultured with ASC-CM. The authors report decreased apoptosis, decreased levels of N-terminal fragments of mutant Htt, and increased expression of PGC-1 α. Corroborating results were found in a later in vitro study, where the neuroprotective effects of ASC exosomes were confirmed on R6/2 mice-derived neuronal cultures (Lee, Liu, Im, & Kim, 2016). Presumably, by upregulating the p-CREB-PGC1α pathway, ASC exosomes significantly decrease mutant Htt levels in HD neuronal cells, reduce mitochondrial dysfunction and cell apoptosis. Although the specific factors responsible for these actions need to elucidated, the authors believe that treatments with ASC exosomes may represent a safe and effective therapeutic strategy, compared to stem cell transplantation.
To evaluate the possibility of autologous ASC transplantation in HD patients, hASCs derived from healthy donors were compared with those from HD patients in YAC128 transgenic mouse model (Im et al., 2010). Bilateral striatal transplantation of normal hASCs in 8-month-old mice reduced striatal atrophy, as evaluated 4 months later, whereas, the same treatment with HD-derived ASCs failed to prevent it. Moreover, injection of normal ASCs at 12 months of age maintained the rotarod performance for 4 weeks. In vitro cultures showed that, although both types of hASCs were characterized by the same phenotype, a reduced NGF production was revealed for HD-ASCs, if not cultured under appropriate conditions. The authors conclude that autologous transplantation of ASCs in HD patients can be useful, but after enhancing their paracrine activity. The beneficial paracrine actions were verified upon intraperitoneal injection of cell-free extracts of ASCs in an R6/2 mouse model (Im et al., 2013). Results show a lower striatal atrophy, a reduced mutant Htt aggregation, a slower progression of the disease, less weight loss, and a better rotarod performance.
Intrastriatal transplantation of hBMSCs was investigated in two HD mouse models (Lin et al., 2011). In QA mice, an increased survival rate and a significant reduction in motor function impairment were reported. Similar findings were described in the R6/2-J2 animal model, except for the improvement of motor functions. The authors conclude that transplanted hBMSCs might be capable of survival and integration with the host cells, increasing neural proliferation and differentiation, neurotrophic support, and anti-apoptotic effect. However, only a few engrafted cells expressed typical neuronal or glial markers.
Different amounts of BMSCs were injected into the striatum of a 3NP rat model for comparative analysis (Rossignol et al., 2011). In all treated animals, a better preservation of the anterior part of the striatum occurred, as well as a reduced enlargement of lateral ventricle size. It is pointed out that more evident behavioral progress was obtained by injecting a lower number of cells, as if an excessive amount of cells might alter striatal neuronal networks. As none of the transplanted BMSCs expressed neural phenotypes, it is suggested that improvements could be mediated by the release of trophic factors, namely BDNF.
After a low (3–8) or high (40–50) number of passages in culture, UC-MSCs were transplanted in the striatum of an R6/2 mouse model (Fink et al., 2013). No evident reductions in motor deficits were observed in comparison with untreated R6/2 mice, except for a transient sparing in spatial memory task. However, R6/2 mice receiving high-passage UC-MSCs considerably attenuated brain atrophy and significantly preserved metabolic activity in striatal tissue.
In a comparative study, BMSCs, NSCs, or both types of stem cells were transplanted into the striatum of a transgenic rat model (51 CAG rat model of HD; von Hörsten et al., 2003) to verify their capability to reduce disease-related deficits (Rossignol et al., 2014). Inducing a strong immune response, transplantation of NSCs conferred only short-term behavioral progress, whereas, transplantation of BMSCs, evoking a lower immune response, was able to provide longer term benefits. The best results were achieved after injections of both BMSCs and NSCs, which provided long-term benefits and increased survival of the transplanted NSCs. It is concluded that BMSCs can prolong NSC survival by producing a more suitable microenvironment in the host nervous tissue.
Intranasal administration of BMSCs preconditioned with lithium and valproic acid (VPA) was investigated in N171-82Q transgenic mice (Linares et al., 2016). Compared to control HD mice, open-field motor functions revealed that ambulatory distance and mean velocity were significantly improved in HD mice treated with preconditioned MSCs, better than those transplanted with untreated BMSCs. Moreover, preconditioned BMSCs increased animal survival, reduced striatal neuronal loss, and decreased Htt aggregates. Preconditioning with these drugs was considered useful because of their neuroprotective/neurotrophic features (Chiu, Liu, Leeds, & Chuang, 2011). They activate multiple pro-survival signaling pathways, and induce substantial beneficial effects on animal models of neurological and psychiatric disorders. In fact, oral administration of lithium and VPA may alleviate spontaneous locomotor deficits and depressive-like behaviors in N171-82Q and YAC128 mouse models.
5 PARKINSON'S DISEASE
Parkinson's disease (PD) is a common neurodegenerative disorder. It is characterized by a progressive and extensive degeneration of dopamine-producing neurons in the pars compacta of Substantia Nigra (SNpc) at mesencephalic level, as well as their terminals in the striatum. However, other brain regions, such as the cerebral cortex and lower brain stem, can also be affected. Because of the functional role of nigrostriatal projections, typical motor symptoms of PD include resting tremor, muscle rigidity, bradykinesia, postural instability, and impaired ability to control voluntary movements. Other symptoms include cognitive dysfunctions, sleep disturbances and hyposmia. PD is commonly considered a non-genetic disorder since viral infections, programmed cell death, or environmental factors may play a critical role in the etiology. However, in about 5% of cases, PD seems associated with specific gene mutations. In most of these cases, mutations of the α-synuclein gene is responsible for intracytoplasmic eosinophilic inclusions (Lewy bodies), and spontaneous cell death (Kalia, Kalia, McLean, Lozano, & Lang, 2013). The main findings are summarized in Table 4.
| Authors | Investigation | Administration route—type of MSCs | Main findings |
|---|---|---|---|
| Bouchez et al. (2008) | 6-OHDA-induced rat model. | Intrastriatal grafting of rat BMSCs. | Significant reduction of amphetamine-induced rotations. Partial restoration of density of dopaminergic markers in nerve terminals and nigral cell bodies. |
| Glavaski-Joksimovic et al. (2010) | 6-OHDA-induced rat model. | Striatal implantation of BMSCs engineered to overexpress GDNF. | About 2 weeks of cell survival after implantation. Significant decrease in the amphetamine-induced rotation at 4 weeks. Numerous rejuvenated TH-positive fibers at 5 weeks. |
| Blandini et al. (2010) | 6-OHDA-induced rat model. | Striatal transplantation of BMSCs. | Significant reduction of apomorphine-induced rotations. Engraftment and survival of BMSCs, expressing typical glial markers, but not neuronal. |
| Ren et al. (2013) | MPTP monkey model. | Unilateral injection of BMSCs engineered to overexpress GDNF into the striatum and SN. | Increased dopamine levels and dopamine uptake in the grafted striatum. More numerous TH-positive fibers between SN and striatum. Better preservation of motor functions in the contralateral limbs. |
| Danielyan et al. (2014) | Transgenic [(Thy1)h[A30P] αS] mouse model. | Intranasal administration of BMSCs. | BMSCs were able to migrate to olfactory bulb and several brain regions. |
| Hoban et al. (2015) | Lipopolysaccharide rat model. | Striatal injection of BMSCs engineered to overexpress GDNF. | Transplanted cells died within 1 week. Protection of nigrostriatal terminals was still effective at 21 days from transplantation. |
| Schwerk et al. (2015) | 6-OHDA-induced rat model. | Transplantation of autologous ASCs in SN. | Early increase of neurogenesis in SVZ. Most grafted ASCs were in the region of the SN and in the surrounding arachnoid mater, expressing BDNF and S100b. Some ASCs were also found around blood vessels, showing endothelial phenotypes. |
| Schwerk et al. (2015) | 6-OHDA-induced rat model. | Transplantation of autologous ASCs in SN. | After 6 months from injections, ASCs were found around the SN and the arachnoid mater, expressing pericyte and endothelial markers. Preserved dopamine levels and up-regulation of anti-inflammatory cytokines. Increased neurogenesis in the hippocampus and SVZ. Improvement of memory functioning. No improvement of motor behavior. |
| Cerri et al. (2015) | 6-OHDA-induced rat model. | Intracarotid administration of BMSCs. | Appreciable migration of BMSCs to the brain only after transient permeabilization of the BBB. No significant modifications in the development of lesion-induced damage or motor impairment. |
| Suzuki et al. (2015) | 6-OHDA-induced rat model. | Intravenous administration of BMSCs. | Significant preservation of tyrosine hydroxylase (TH) positive neurons in the SNpc, likely due to microglia-mediated anti-inflammatory mechanisms. Inhibition of methamphetamine-stimulated rotational behavior. |
| Choi et al. (2015) | 6-OHDA-induced rat model. | Intravenous administration of ASCs. | Better preservation of nigral dopaminergic neurons. Decreased number of altered mitochondria. Improvement of behavioral performance at 3 weeks. |
| Ahmed et al. (2016) | Rotenone-induced rat model after ovariectomy. | Intravenous administration of BMSCs. | BMSCs were able to home to the injured brain. Significant decrease of serum TGF-β1 levels and significant increase of brain TH and nestin gene expression. Increased levels of serum BDNF and brain DA. |
| Salama et al. (2017) | Rotenone-induced mouse model. | Intranasal administration of BMSCs. | Successful migration of BMSCs into different brain regions. Significant preservation of nigral dopaminergic neurons and striatal TH-positive fibers. Mitigation of the progressive rotenone-induced deterioration of locomotor functions. |
| Venkataramana et al. (2010) | Clinical trial. | Stereotactic injection of autologous BMSSs into SVZ. | Improvement of clinical conditions in a follow-up of 10–36 months. Improvements in facial expression, gait, and freezing episodes. No clinical worsening, parenchymal changes, or evidence of tumor formation. |
| Canesi et al. (2016) | Phase-I study in patients affected by progressive supranuclear palsy. | Infusion of autologous BMSCs into the cerebral arteries. | Stabilization of motor functions for at least 6 months was observed during 1 year of follow-up. |
Effective treatments for PD are not available at the present. Since the disease is associated with the lack of nigrostriatal dopaminergic projections, dopamine replacement is commonly used to relieve PD symptoms. However, long-term treatment with L-DOPA induces severe side effects, including drug-induced motor complications, and above all, abnormal, uncontrollable movements, known as dyskinesia (Lescaudron et al., 2012). For these reasons, alternative approaches have been designed.
Transplantation of human fetal brain tissue into the striatum of patients with advanced PD has been able to improve their cognitive and motor behavior (Kim et al., 2013). However, these strategies imply some limitations because of the difficulty to obtain enough fetal tissue and for ethical and religious issues. These difficulties may be overcome by stem cell-based therapies, especially using MSCs. Experiments were mostly carried out on animal models, in which PD-like symptoms can be induced by intracerebral injections of 6-hydroxydopamine (6-OHDA; especially for rat models) or after intravenous/intraperitoneal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; more suitable for mouse and non-human primate models). Another animal model featuring PD symptoms can be generated by intraperitoneal/subcutaneous administration of rotenone, which replicates many aspects of the human disease.
5.1 MSC treatments
In adult rats, lesioned by unilateral striatal injection of 6-OHDA, rat BMSCs were delivered to the same region (Bouchez et al., 2008). Compared to controls, in transplanted animals the number of amphetamine-induced rotations was significantly decreased and the density of dopaminergic markers in nerve terminals and cell bodies was partially restored. The authors attribute this finding to the rescue of dopaminergic neurons and/or sprouting from the surviving nigrostriatal fibers. Similar result were also observed by Blandini et al. (2010) using hBMSCs in the same animal model. In this study, the development of neuroprotective effects was still indicated by a significant reduction of apomorphine-induced rotation. Histological examination confirmed the engraftment and survival of BMSCs, which showed typical glial, but not neuronal markers. It is hypothesized that neuroprotection might be due to the increased production of neurotrophic factors such as GDNF. The retrograde transportation of this neurotrophin from striatal terminals to mesencephalic cell bodies would, in turn, favour survival of dopaminergic nigrostriatal neurons and their axonal terminals.
Autologous ASCs were transplanted into the SN of rats, 1 week after the medial forebrain bundle was fully lesioned by injections of 6-OHDA (Schwerk et al., 2015). As early as 3 days after transplantation, an increased SVZ neurogenesis was detected, as compared to non-transplanted rats. This finding seems of interest because damage of the SVZ-olfactory bulb axis is likely responsible for the hyposmia in PD patients. Grafted ASCs were mostly localized in the region of the SN and in the surrounding arachnoid mater. They express BDNF and S100β, an astrocyte marker known to promote cell proliferation and neuronal survival. Some cells were also found around blood vessels, showing endothelial phenotypes. This line of differentiation might be useful in repairing lesion-induced vasculature alterations. The same research group also describes effects evaluated after 6 months (Schwerk et al., 2015). ASCs were also localized around the SN and the arachnoid mater, expressing pericyte and endothelial markers. ASC-induced influences included improved memory functioning, up-regulation of peripheral anti-inflammatory cytokines, preserved dopamine levels, and increased neurogenesis in hippocampal and SVZ regions. However, enhanced cognitive functions were not paralleled by improved motor functions. The authors also suggest that monolayer cultured ASCs might be more efficacious than spheroid ASCs, and are therefore more suitable candidates for transplantation (Berg et al., 2015).
Intracarotid administration of rat BMSCs was evaluated in a 6-OHDA rat model (Cerri et al., 2015). Appreciable delivery of BMSCs to the brain of lesioned animals was obtained after mannitol pretreatment to transiently permeabilize the BBB. While the development of 6-OHDA-induced damage or motor impairment were not modified, progressive normalization of the pathological response to apomorphine administration was observed. The authors conclude that arterial infusion of BMSCs may lead to functional compensatory changes in the nigrostriatal system, although neuroprotective/neurorestorative activity remains to be ascertained, at least in these experimental conditions.
Intravenous administration of hBMSCs was analyzed in a rat model with unilateral striatal lesions induced by 6-OHDA (Suzuki et al., 2015). As a result, methamphetamine stimulated rotational behavior was inhibited and the number of tyrosine hydroxylase (TH) positive neurons (i.e., dopaminergic neurons) in the SNpc was significantly preserved, likely due to anti-inflammatory mechanisms related to modulation of microglial activation. Improvements of the behavioural performance were confirmed also after 3 weeks from intravenous administration of hASCs in the same rat model (Choi et al., 2015). Moreover, nigral dopaminergic neurons were better preserved and the number of altered mitochondria was decreased. Since mitochondrial dysfunction likely plays a role in the loss of dopaminergic neurons in PD patients and in mouse models, it is suggested that reduced mitochondrial damage may account for decreased cell death of nigrostriatal neurons.
Rat BMSC intravenous administration was tested in another rat model of PD, generated by ovariectomy and subcutaneous injections of rotenone (Ahmed et al., 2016). BMSCs were able to home to the injured brain and elicited a significant decrease in serum TGF-β1 levels associated with a significant increase of brain TH and nestin gene expression. Serum BDNF and brain DA levels were also increased. It is concluded that the normalization of these biomarkers can possibly be attributed to immunomodulatory, anti-inflammatory and neurotrophic effects of BMSCs. An intact histological structure of the striatum was also observed in the brain sections.
Other administration routes and other MSC lines have also been investigated. A non invasive successful method of murine BMSC administration was reported by Danielyan et al. (2014) in a transgenic [(Thy1)h[A30P] αS] mouse model. After intranasal application, BMSCs were detected in the olfactory bulb and brainstem, other than the cortex, amygdala, striatum, hippocampus, and cerebellum. In future studies, the optimal dosage and the administration rate will be better defined to obtain functional improvements. Intranasal delivery of murine BMSCs were also evaluated in a mouse model generated by intraperitoneal injections of rotenone (Salama et al., 2017). Successful delivery of MSCs was verified by histological analysis in different brain regions. Striatal TH-positive fibers and nigral dopaminergic neurons were significantly preserved. In addition, neurobehavioral assessment showed that MSC administration was able to mitigate the progressive rotenone-induced deterioration of locomotor functions. The authors emphasize this easy, cheap and potentially safe alternative route for stem cell delivery in neurodegenerative disorders.
Promising results have also been obtained exploring human dental pulp stem cells (Chun, Soker, Jang, Kwon, & Yoo, 2016). Studies in vitro show that, when cultured in particular conditions, they can differentiate towards neural lines. In a portion of these cells, an increased TH expression was also detected.
6 GENETICALLY ENGINEERED MESENCHYMAL STEM CELLS
A large body of evidence demonstrates that MSCs transplanted into the brain promote functional recovery mainly by producing trophic factors that favour survival and regeneration of host neurons. Their typical trophic support can be enhanced using genetically engineered MSCs as delivery vehicles. For treatment of chronic neurodegenerative diseases, MSCs have been most commonly genetically modified to overexpress neurotrophic factors (mainly NGF, BDNF, GDNF) whose neuroprotective/neuroregenerative actions are widely acknowledged. (Deng et al., 2016; Huang, Tabata, & Gao, 2012; Wyse, Dunbar, & Rossignol, 2014). In addition, surface receptor expression can be similarly manipulated to enhance MSC migration toward injured sites. Different strategies have been investigated for engineering MSCs (Muroski, Levenson, & Strouse, 2014). Though efficient, electroporation techniques imply significant loss of viable cells; viral vectors are commonly used, despite considerable biosafety restrictions, such as risks of secondary infection, immunogenic responses, excessive secretion of neurotrophins; non-viral approach are also available, for example by using solid gold nanoparticles. The main findings are summarized in Tables 1-4, for related diseases.
Gene-modified BMSCs to overexpress NGF were transplanted into the hippocampus of an AD rat model, in which the β-amyloid protein was injected bilaterally into the hippocampus (Li et al., 2008). Transplanted cells survived and migrated into surrounding tissue and other sites (corpus striatum and other hippocampal structures) acquiring a tendency to express choline acetyltransferase-like immunopositivity. Compared to controls, a reduced neuronal loss was observed in the hippocampus of these animals, showing significant progress in learning and memory.
Retro-orbital injections of genetically modified hUC-MSCs were carried out in a pre-symptomatic G93A mouse model of ALS (Rizvanov et al., 2008). UC-MSCs were transiently transfected by electroporation to express human VEGF and the mouse neural L1 cell adhesion molecule (L1CAM). As a result, UC-MSCs successfully grafted into nervous tissue of ALS mice and survived for over 3 months. According to the authors, the expression of L1CAM was responsible for increased homing ability and proliferation of transplanted cells at the site of neurodegeneration in the spinal cord parenchyma. However, rather than neural, they differentiate into endothelial cells forming new blood vessels, likely because of increased VEGF expression. This might, however, enhance neuroprotective/neuroregenerative effects because more neurotrophic factors would be delivered to the damaged regions from newly formed blood vessels.
Positive data were reported by injecting BMSCs, genetically modified using a retrovirus to overexpress NGF and/or BDNF, into the striatum of a YAC128 mice model of HD (Dey et al., 2010). Compared to controls, transplanted animals exhibited reduced clasping and improvements on the rotarod task. In addition, striatal neuronal nuclei (NeuN) positive cell counts were restored almost to those of wild-type mice.
By lentiviral transduction, hBMSCs, engineered to overexpress BDNF, were transplanted into the striatum of immune-suppressed HD transgenic mouse models (Pollock et al., 2016). Decreased striatal atrophy and reduced anxiety were detected in YAC128 mice. A significant increase in neurogenesis-like activity and the mean lifespan were observed in R6/2 mice.
Genetically, modified hBMSCs were implanted into the striatum of 6OHDA rats. Cells were first transfected with the intracellular domain of the Notch1 gene to generate SB623 cells; then, lentiviral transduction was performed to enhance their expression of GDNF (Glavaski-Joksimovic et al., 2010). After 1 week, histological analysis showed that numerous engrafted cells were surviving. At 2 weeks, their number was significantly reduced, and only cell debris was found at 4 weeks. However, although no surviving SB623 cell was present, numerous rejuvenated TH-positive fibers were observed after 5 weeks. The authors suggest that, even if GDNF was secreted only shortly after the grafting, it was able to exert longer functional recovery in this rat PD model. This conclusion is supported by statistically significant decreases in the amphetamine-induced rotation, evaluated at 4 weeks post implantation. They also hypothesize that GDNF secreted into the striatum could be uptaken by nigrostriatal fibers and retrogradely transported to neuronal cell bodies in the SN. Similar conclusions were drawn by Hoban, Howard, and Dowd (2015) in an inflammatory rat model of PD (the lipopolysaccharide model). After intrastriatal injection of BMSCs, genetically engineered to overexpress GDNF, dense areas of TH-immunoreactivity were revealed in proximity to the transplant site. It is concluded that, although most transplanted cells die within 1 week, GDNF secretion is able to provide a longer-lasting protection of nigrostriatal terminals, as evaluated 21 days after transplantation.
Promising results have also been described in an MPTP monkey model of PD, using autologous BMSCs, genetically modified to overexpress GDNF (Ren et al., 2013). In particular, 2 weeks before intravenous MPTP administration, unilateral injections of GDNF-MSC were made into the striatum and SN. In these monkeys, motor functions were better preserved in the contralateral limbs. Moreover, higher dopamine levels and greater dopamine uptake were found in the striatum of the grafted hemisphere, along with more numerous TH-positive fibers between the SN and striatum. It is suggested that this procedure represents a safe vehicle to deliver GDNF, able to preserve nigral and striatal functions, although the loss of SN dopaminergic neurons was not prevented in this work. The authors admit that, for a closer simulation of human therapeutic applications, further studies should be differently designed. More precisely, MPTP induction of the disease should precede GDNF-MSC transplantation. In fact, therapeutic approaches occur in patients already at a late PD stage.
7 CLINICAL TRIALS
Based on positive outcomes from animal models, some clinical trials have already been performed and many others are currently in progress (clinicaltrials.gov). Many of them have been designed to assess the safety of treatments. The main findings are summarized in Tables 1, 2, and 4 for related diseases.
For AD patients, various types of MSCs and different administration routes have been explored worldwide (Duncan & Valenzuela, 2017). In a Korean phase 1 clinical trial, hUC-MSCs were stereotactically injected into the hippocampus and precuneus of nine patients with mild-to-moderate AD (Kim et al., 2015). Although no attenuations of the pathology are described, no serious adverse events are reported. Therefore, the authors conclude that stereotactic administration of hUCB-MSCs into the brain of patients is feasible, safe, and well tolerated.
Numerous clinical trials are being made in ALS patients, especially using BMSCs. Most of them (phase I/II) have been designed to demonstrate the safety of transplantation procedures, but some progress has already been observed (Czarzasta et al., 2017; Mazzini, Vescovi, Cantello, Gelati, & Vercelli, 2016). The functional status of patients was generally assessed by the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALS-FRS), the most widely used scale to evaluate disease progression. This scale analyzes 12 parameters: speech, salivation, swallowing, writing, eating and utensils use, dressing and hygiene, turning in bed, walking, stairs climbing, breathing, orthopnea, and respiratory insufficiency.
In a phase II clinical study, early improvements of symptoms were described after injection of autologous BMSCs directly into the brainstem and cervical spinal cord of 13 patients in Turkey (Deda et al., 2009). After a follow-up period of 1 year, although three patients died of lung infection and myocardial infarct, nine of them were in better conditions than the preoperative period, even though with some degree of decline. One patient remained stable. According to the authors, these effects would be due both to implanted BMSCs and activation of endogenous stem cells.
A phase I/II clinical trial was carried out in Israel by Karussis et al. (2010). In patients with ALS and multiple sclerosis, intrathecal and intravenous delivery of autologous BMSCs was designed to maximize their migration to the nervous system through the cerebrospinal fluid (CSF) and blood circulation. Magnetic resonance imaging showed a possible propagation of BMSCs from the lumbar site of inoculation to the occipital horns, meninges, spinal roots, and spinal cord parenchyma. In addition, beneficial immunomodulatory influences were also obtained as early as 4 hr after BMSC transplantation. The ALS-FRS score showed a slight deterioration in the 2 month interval before BMSC administration, whereas a clinical stabilization or even amelioration was observed in some patients during a follow-up of 6–25 months, without any significant immediate or late adverse effects.
In a phase I clinical trial carried out in Italy on 10 ALS patients (Mazzini et al., 2010), autologous BMSCs were injected into the spinal cord at a high thoracic level, after in vitro expansion and suspension in autologous CSF. As no serious adverse effects were noted in treated patients, the authors assume that this procedure is safe enough to be further used and developed for future approaches.
The safety of intraspinal (posterior spinal cord funiculus) infusion of autologous bone marrow mononuclear cells was proven on ALS patients in Spain (Blanquer et al., 2012). Four patients died for reasons unrelated to the procedure, whereas no serious transplant-related adverse events were observed in the others. Although no significant clinical improvement is reported, no acceleration was noticed in the rate of decline of forced vital capacity, ALS-FRS, and other specific scales. Notably, autopsy samples showed a higher number of motoneurons in the anterior horns at the level of the transplanted spinal segments.
Two repeated intrathecal autologous BMSC injections were performed in a Korean phase I clinical study on seven ALS patients (Oh, Kim et al., 2015). The authors believe that this procedure is safe and feasible, as no serious adverse events occurred during the 12-month follow-up period. They also claim that this procedure would maximize BMSC migration and strengthen therapeutic outcomes in brain and spinal cord parenchyma.
Autologous BMSCs were transplanted in ALS patients in Belarus by a combined administration method (Rushkevich et al., 2015). After 6–7 days from intravenous delivery of undifferentiated BMSCs, neural-induced BMSCs were injected via lumbar puncture. The authors assume that this cell therapy is safe since no serious side effects or complications occurred after a 12-month follow-up period. They also report a slowed disease progression and an improvement of the functional status, as evaluated by the ALSFRS score.
In a phase I/IIa clinical trial, autologous BMSCs were isolated, expanded, and injected via lumbar puncture into the CSF of 23 ALS patients in the Czech Republic (Syková et al., 2017). Results indicate that this intrathecal application of BMSCs was safe since no serious adverse reactions were observed after an 18-month follow-up period. The authors also report a slowing down in the progression of the disease since a reduction in the ALSFRS score decline was revealed at 3 months and, in some cases, for 6 months after BMSC administration. Forced vital capacity remained stable or above 70% for 9 months.
Autologous BMSCs, in vitro induced to secrete neurotrophic factors, were transplanted in patients with ALS in Israel (Petrou et al., 2016). In this phase I/II clinical study, intramuscular (six patients at early stage of ALS) or intrathecal (six patients at more advanced stages) injections were made. In phase IIa, 14 patients with early stage ALS received a combined intramuscular and intrathecal transplantation. Overall, the forced vital capacity and the ALSFR score were improved, compared to the expected decline, especially in patients receiving intrathecal or both injections. Since only mild and transient treatment-related adverse effects were noticed over the 6 months of follow-up, the procedure was considered safe and well tolerated, to be further used in clinical trials for patient benefit.
Clinical trials on PD patients have also been already started. In an Indian clinical trial, autologous BMSCs were transplanted stereotactically into the SVZ of seven patients with advanced PD (Venkataramana et al., 2010). This brain region was chosen since it physiologically contains the largest number of NSCs involved in neurogenesis. In a follow-up period of 10–36 months, amelioration of clinical conditions was observed by using the Unified Parkinson's Disease Rating Scale (UPDRS), Hoehn and Yahr (H&Y), and Schwab and England (S&E) scales. Subjective improvements were also reported for symptoms such as facial expression, gait, and freezing episodes. Moreover, the dosage of L-DOPA was significantly reduced in two patients. Being an open-label study, the authors do not entirely exclude a placebo effect, although the persistence of clinical improvements over 20–26 months makes it unlikely. Even if the exact mechanisms are not clearly understood, this trial has confirmed the safety of the procedure, as none of the patients showed clinical worsening until the end of the observation period. In addition, cranial magnetic resonance imaging did not show any abnormal parenchymal changes.
In an Italian pilot phase I study, infusion of BMSCs into the cerebral arteries was tested in five patients affected by progressive supranuclear palsy (PSP), a rare, severe, and no-option form of Parkinsonism (Canesi et al., 2016). Most patients were alive after 1 year from the administration, except one who died for reasons not associate with the treatment (accidental fall). Clinical conditions of all patients were assessed using specific rating scales (UPDRS, H&Y, PSP rating scale) before cell administration and after 1, 3, 6, and 12 months. Rating scale scores indicate that motor functions remained stable for at least 6 months during the 1-year follow-up. These findings are considered encouraging since in these patients motor function deterioration would have been normally faster. The next phase II study will definitively provide information on the efficacy of this approach, easily applicable to other neurodegenerative disorders.
Altogether, although no serious adverse effects have been reported, the safety of treatments has to be carefully evaluated when using cell-based therapies in humans. In fact, it is not entirely known whether surviving cells may give rise to abnormal nervous tissue, maintain their stemness features, or even evolve into different cell lines and induce unexpected events. Another potential risk could be related to their angiogenetic activity and their tendency to home to the hypoxic regions near tumors (Joyce et al., 2010). As these features could became extremely dangerous, a careful screening must be done in selecting patients for stem cell implantations. Cell-free strategies, using exosomes or conditioned media, are perhaps closer to widespread clinical applications as ideal carriers to delivery therapeutic agents to nervous tissue that is otherwise difficult to reach (Lee et al., 2016). Notably, the potential benefit-to-risk ratio is another major factor to be considered. Particularly for those patients in advanced stages of disabling diseases, when no effective treatments are available.
8 CONCLUSIONS
Data from the studies above described show that the use of MSCs may induce appreciable amelioration in neurodegenerative diseases, where only poor results can be achieved by pharmacological approaches or other therapeutic strategies. Beneficial MSC-induced effects primarily rely on their particular tendency to home to injured areas, in particular to hypoxic, apoptotic, or inflamed areas (Figure 1). Notably, their ability to pass the BBB has permitted different administration routes (Kerkis et al., 2015). In fact, MSC engrafting at the injured nervous tissue has been reported not only upon intracerebral injection, but also after intracerebroventricular, intracarotid, intravenous, or even intranasal administration.
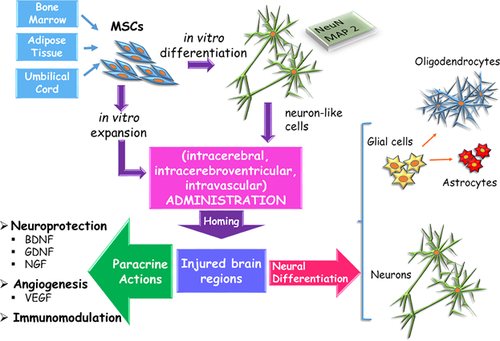
However, it should be pointed out that, although less invasive, systemic administrations have some limitations. After intravenous injections, many cells are trapped in the lungs and fail to reach target tissues. Intra-arterial delivery implies more invasive procedures and may lead to microthrombi formation, thus, compromising tissue microcirculation. The wide dispersion of cells in the blood stream would require a strong homing ability, which may be affected by the loss of expression of homing molecules during in vitro expansion. In this regard, different strategies have been developed. For example, by expanding MSCs under hypoxic conditions, by adding various chemicals or cytokines to the culture medium or by genetic engineering to overexpress homing molecules. These issues have been extensively addressed in a recent review by De Becker and Van Riet (2016).
Different mechanisms can be taken into account to explain MSC-mediated improvements. From in vitro studies, it has been reported that they can differentiate into neural elements, expressing typical neuronal and glial markers (Lo Furno et al., 2013). On this basis, the optimistic aim of MSC transplantation into the brain is to replace lost neurons and restore damaged nervous networks. Data obtained in vivo weakly corroborate this hypothesis. In most studies, only a small fraction of transplanted cells has been verified as stably engrafted, and only in some cases have they shown neuronal or glial differentiation. In addition, the number of grafted cells rapidly decreases, and often none of them can be detected after a few weeks from transplantation. On this basis, one must conclude that most beneficial actions are exerted rather by mechanisms that are different from MSC neural differentiation.
Most authors agree that the neuroprotective/neurotrophic effects observed upon MSC transplantation are mainly promoted by their paracrine activity. Indeed, the production of an impressive amount of neurotrophic growth factors, chemokines and cytokines has been demonstrated (Drago et al., 2013; Lee et al., 2016; Paul and Anisimov, 2013). By these paracrine actions, the local immune system can be suppressed, and apoptosis and free radicals levels reduced (Joyce et al., 2010). MSC paracrine activities seem improved by pro-inflammatory and hypoxic stimuli, by the exposure to apoptotic cell environment, and by activated microglia. Moreover, recruitment, differentiation and paracrine function of resident progenitor cells, neurons or glial cells can be enhanced. Additional positive mechanisms include angiogenesis stimulation, enhanced glutamate uptake, or production of extracellular matrix molecules. Stimulation of angiogenesis would help the diffusion of soluble factors along newly formed vessels in the injured tissue. By enhancing glutamate uptake, its neuro excitotoxicity would be decreased. The extracellular matrix molecules may stimulate growth and axonal extension. As modifications of cytokines and neurotrophic factors are also associated with various mental illness, MSC based therapies have also been suggested for psychiatric disorders (Colpo et al., 2015).
Some particular features of UC-MSCs, BMSCs, or ASCs deserve to be highlighted (Momin, Mohyeldin, Zaidi, Vela, & Quiñones-Hinojosa, 2010). UC-MSCs can be derived with no discomfort for the newborn or the mother, from the already detached umbilical cord. However, their use has some limitations. They can only be used for allogeneic transplantation and the cell yield might be limited. BMSCs were the first to be identified and have been the most studied and, especially for in vivo studies and clinical trials, are currently widely tested. However, this source of MSCs may not be the easiest one, since BMSC harvesting is characterized by limited cell yield and painful invasive procedures. In many respects, the use of ASCs seems more suitable for translational medicine. First, their availability is virtually unlimited from subcutaneous deposits of adipose tissue, which is easily harvested with minimally invasive procedures (Yeh et al., 2015). Second, in comparison with BMSCs, ASCs have often shown higher proliferation and differentiation ability for mesodermal and neural lineages (Calabrese et al., 2015; Zhang, Liu, Yao, Yang, & Xu, 2012). Not only can ASCs be quickly obtained for autologous use but, thanks to their low immunogenicity, they are also suitable for allogeneic transplantations.
It is reasonable to expect that more studies and clinical trials will be carried out in the near future using MSCs, either as naïve stem cells, or after neural induction or genetically engineered to enhance their innate paracrine and chemotactic potential, at least until more efficacious cell-replacement therapies are available to restore disease-disrupted brain circuitry.
ACKNOWLEDGMENT
We wish to thank Dr. Antony Bridgewood of the Scientific Bureau of the University of Catania for language support.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




