Pulmonary hypertension: Molecular aspects of current therapeutic intervention and future direction
Abstract
Pulmonary hypertension (PH) is a life-threatening lung disorder with towering prevalence and risk for future has been gradually rising worldwide. Even, no specific medications are available for pulmonary hypertension; various classes of treatment based upon the origin and magnitude of hypertension are still used for the treatment of PH. Consideration of molecular or signaling modulation is the imperative approach that can offer a new notion for prevalent pharmacotherapeutic agents. Instead of concurrent targets, including endothelin receptor antagonists (ETA/ETB), phosphodiesterase 5 inhibitor (PDF-5), calcium channel blockers, anticoagulants, diuretics, and long acting prostacyclin analog, recent scientific reports revealed the numerous potential alternative therapeutic approaches that can significantly target the pathological signaling alteration associated with PH. Understanding precise molecular cascade involved in PH can be useful for designing preclinical animal experiments and human clinical trials to evaluate target specific novel therapeutic interventions for the treatment of PH. In this review, we discussed the possible molecular signaling involved in the pathogenesis of PH and detailed account of the current status of medications employed for the treatment of PH. Moreover, the newly identified potential target sites and alternative approaches for treating the PH have been discussed.
1 INTRODUCTION
Pulmonary hypertension (PH) is a diverse group of fatal disorders characterized by a sustained increase in pulmonary artery pressure and vascular resistance due to pulmonary artery hyper-constriction (Cogolludo, Moreno, & Villamor, 2007). The elevated blood pressure in the pulmonary artery leads to cardio-muscular stress followed by gradual enlargement of the right ventricle. Similarly, the reduced production of vasodilator including prostacyclin (PGI2) and nitric oxide (NO) or consequent increase in endogenous vasoconstrictors like endothelin-1 (ET-1) and thromboxane A2 in pulmonary circulation leads to pulmonary endothelial dysfunction (Cogolludo et al., 2007). The availability of endothelial derived relaxation factor (EDRF) ‘NO’ was noted to be reduced in the pulmonary circulation due to impaired activation of endothelial nitric oxide synthase (eNOS) (Chester & Yacoub, 2014). Moreover, increased level of serotonin endorses the pulmonary artery smooth muscle cell (PASMC) proliferation, vasoconstriction, and local microthrombosis (Lang & Madani, 2014). Patients with PH have high levels of serotonin in circulation that indicate the influence of serotonin on induction of PH (MacLean, Herve, Eddahibi, & Adnot, 2000). Indeed, several potential sites including prostanoids, endothelin receptor antagonists, phosphodiesterase inhibitors, soluble gunanyl cyclase stimulators, Rho kinase inhibitors, serotonin receptor antagonist, and serotonin transporter blockers, statins, peroxisome proliferator activated receptor agonist, calcium channel blockers, potassium channel openers, tyrosine kinase inhibitors are currently target to impede the vulnerability of PH. Preclinical evidence implicate the role of Rho-kinase in the pathogenesis of pulmonary hypertension (Jasińska-Stroschein, Owczarek, Plichta, & Orszulak-Michalak, 2014). Rho kinase suppresses the myosin phosphatase and augments the contractility of pulmonary artery smooth muscle cells (PASMC) in rats. Transient receptor potential channels (TRPC) has also found to be over expressed in pulmonary hypertension and influence the smooth muscle cell (PASMC) proliferation and pulmonary vascular medial hypertrophy (Malczyk et al., 2017). Certainly, increased oxidative stress contributes to pulmonary vascular cell growth and right ventricular hypertrophy (Sharma, Gourav, Deepa, & Rajput, 2015). Evidence suggests that reactive oxygen species (ROS) exceeds the quenching capacity of antioxidant mechanisms of the cell and protein carbonylation may be involved in the pathology of PH (Sharma et al., 2015; Wong, Bansal, Pavlickova, Marcocci, & Suzuki, 2013).
Clinical evidence confirmed the association of TGF-β with pulmonary hypertensive patients via increasing of interleukin-1, interleukin-6 (Kumar et al., 2017) (Figure 1). Since the molecular signaling cascades involved in the pathogenesis of PH are found to be complex but still unexplored. Recently, various innovative therapeutic interventions have revealed to lower the prevalence of PH such as serotonin antagonists, vasoactive intestinal peptide, stimulators of soluble guanylate cyclase, tyrosine kinase inhibitors, Rho-kinase inhibitors, statins, potassium channel openers are the stochastic pipeline therapeutic agents. The mentioned therapeutic approaches reduced the vulnerability of PH up to some extent but a huge area of research for novel and target specific application still needs a revisit on the molecular or cellular aspects of PH. The present review has been aimed to delineate the pathophysiology and drug therapy of PH with their molecular interaction and explore the signaling cascade involved. Moreover, the numerous alternative approaches with the prevalent hallmark of pivotal strategy have revealed in this context.
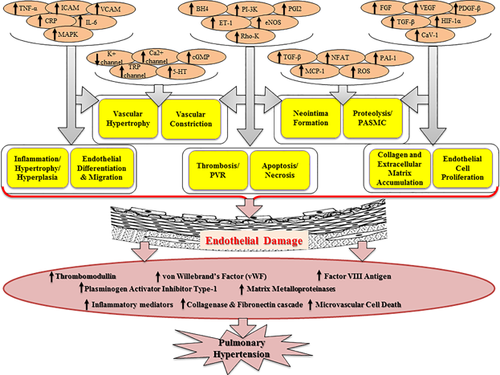
2 PATHOPHYSIOLOGY OF PULMONARY HYPERTENSION
The pathological signaling cascades are the root cause of PH that initiated by vascular endothelial dysfunction at ground level. Endothelial dysfunction act as a precursor for smooth muscle dysfunction of pulmonary arteries and leads to vasoconstriction. Primarily, endothelial dysfunction affects the smaller vessels of lungs followed by the histological alteration in the entire pulmonary vascular tree. The function of endothelium is to maintain blood vessels tone, fibrinolysis, homeostasis, neutrophil recruitment, and production of growth factors (Su, 2015). Endothelial dysfunction endorses by the perturbed level of vasoconstrictor (serotonin and thromboxane) and vasodilator peptides (NO, prostacyclin, EDRF; endothelial derived relaxing factor) (Sharma et al., 2016). Damaged endothelium leads to activation of various coagulation pathways such as factor VIII antigen, von willebrand's factor (vWF), thrombomodulin, and plasminogen activator inhibitor type-1 (Yau, Teoh, & Verma, 2015). Additionally, matrix metalloproteinases (MMP2, MMP9), collagenase, elastase, and fibronectin cascades are also disturbed by endothelial dysfunction (Figure 1). Moreover, pulmonary vascular endothelial dysfunction also leads to microvascular cell death (Tajsic & Morrell, 2011).
3 PHARMACOLOGICAL INTERVENTIONS AND THEIR MOLECULAR ASPECTS
3.1 Prostacyclin analog
Prostacyclins is the common therapeutic approach for the treatment of pulmonary hypertension. Prostacyclin-I2 has potent vasodilatory, anti-coagulatory, anti-inflammatory, and anti-proliferative properties. Prostanoid may accelerate the adenylate cyclase cascade by targeting IP3-DAG pathway through G-Protein coupled receptor activation that can leads to the cyclic adenosine monophosphate (cAMP)-dependent vasodilation. Additionally, prostanoids lead the activation of PPAR δ mediated signaling cascade which triggers the phosphorylation of eNOS/NO. PPAR δ also stimulate angiogenesis, anti-oxidant, anti-inflammatory, and anti-apoptotic pathways (Figure 2), which significantly protect the integrity of endothelium and influence of PH (Li et al., 2012).
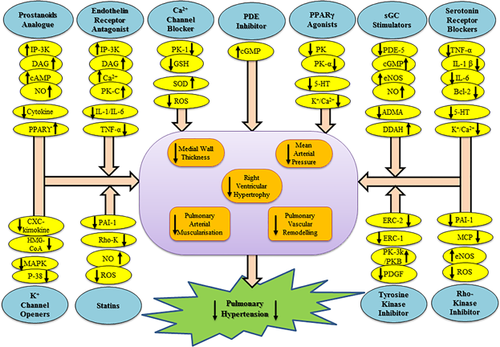
PGI2 analogs have been reported to decrease chemokine secretion, suppress neutrophil adhesion, respiratory burst, and elastase secretion (Ruan, Dixon, Willerson, & Ruan, 2010). Numerous, prostacyclin analogs such as Epoprostenol, Iloprost, Beraprost, Treprosinil, Cicaprost have been studied in the experimental and clinical investigation as promising agents for the treatment of PH. Epoprostenol was the first drug approved by the FDA in 1984 for the management of PH and considered as the first line treatment for pulmonary hypertension (LeVarge, 2015). Whereas, Iloprost which administered by inhalation route has more intrapulmonary selectivity and less systemic side effects. Moreover, Iloprost also prevent the right-to-left cardiac shunt which allows the deoxygenated blood flow from right heart to the left heart (LeVarge, 2015). Beraprost was noted to inhibit the development of pulmonary hypertension through vasodilation, antiplatelet aggregation, and anti-inflammatory effect (LeVarge., 2015). A recent report revealed that intratracheal administration of Beraprost nanoparticle was found to decrease the right ventricular hypertrophy (RVH), right ventricular pressure (RVP) in monocrotaline model (MCT) of rats (Akagi et al., 2016). Moreover, Treprostinil has been noted to reduce the expression of nuclear factor kappa (NFκB), tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), interleukin-6 (IL-6), and granulocyte macrophage colony-stimulating factor in human alveolar macrophages (Pluchart, Khouri, Blaise, Roustit, & Cracowski, 2017). Cicaprost may be effective in preventing PH by protein kinase A-mediated inhibition of adenyl cyclase in rat pulmonary artery smooth muscle cells (Sobolewski, Jourdan, Upton, Long, & Morrell, 2004).
3.2 Endothelin receptor antagonist
Endothelial cells can release potent endogenous vasoconstrictor “ET-1” which has been implicated in the development and progression of vascular endothelial dysfunction (Maneenil, Thatrimontrichai, Janjindamai, & Dissaneevate, 2017). ET-1 binds with two types of receptor including ETA and ETB and upregulate phospholipase C, inositol triphosphate/diacylglycerol (IP3/DAG) cascade that increases intracellular calcium level and leads to vasoconstriction (Figure 2) (Kowalczyk, Kleniewska, Kolodziejczyk, Skibska, & Goraca, 2015). Currently, several potent Endothelin receptor antagonists (Bosentan, Sitaxentan, Ambrisentan) have been used for the treatment of PH. Bosentan was the First endothelin receptor antagonist approved by FDA in 2001 (Maneenil et al., 2017). Right ventricular (RV) dysfunction is the main cause of death in pulmonary arterial hypertension. Whereas, the increase right ventricular mitochondrial capacity in pulmonary hypertensive rats by the treatment of Bosentan and Sildenafil revealed the therapeutic potential of this combined treatment against PH. Additionally, the combined treatment of both also normalizes cardiac output, right ventricular shortening, and protect from pulmonary vascular remodeling (Steinhorn, 2012). Sitaxentan can improve the exercise capacity and, minimize right ventricular systolic pressure and vascular remodeling by inhibiting the elevated level of ET-1 in hypoxia induced pulmonary hypertensive rats (Benza et al., 2015). Sitaxentan can also inhibits the significant level of cytokines (IL-1, TNF-α activation) in PH patients (Benza et al., 2015). Some recent developed endothelin receptor antagonists including CPU0507, CPU0123, LU-135252, BQ123, WS009A, YM598, CI-1020, and C1-1034 have also been reported to overcome pulmonary vasoconstriction and avoid pulmonary vascular remodeling in the animal models (Benza et al., 2015).
3.3 Phosphodiesterase (PDE) inhibitors
Phosphodiesterase level was found to increase in the pulmonary vessel and plays a critical role in the progression of PH (Duarte, Hanson, & Machado, 2013). Phosphodiesterase-1 (PDE-1) has three isoforms which are regulated by calcium-calmodulin and can hydrolyze both cAMP and cGMP (Figure 2). The treatment with PDE1 inhibitor “8-methoxymethyl-isobutyl-1-methylxanthine (8MM-1BMX)” reduced pulmonary vascular remodeling and right ventricular hypertrophy by inhibiting SMC proliferation in rats (Ölmestig, Marlet, Hainsworth, & Kruuse, 2017). Lumefantrine, the PDE-3/4 inhibitor has also reversed small pulmonary arterial muscularization, media hypertrophy in MCT-induced PH in rats (Dony et al., 2008). Sildenafil has proposed a therapeutic tool to treat or prevent pulmonary arterial hypertension in PH patients (Ölmestig et al., 2017). Sildenafil can produce anti-proliferative effect in pulmonary vessel that was observed by improve hypoxia-induced PASMC proliferation and diminished hypoxia-induced enhancement of basal [Ca2+], capacitive calcium entry (CCE) and transient receptor potential gene which inhibits the proliferation of PASMC expression in human pulmonary artery SMCs (Ölmestig et al., 2017). Tadalafil would be more potent therapeutic agent as compare to Sildenafil and Vardenafil as described by significant anti-apoptotic and anti-proliferative action in PASMC (Yamamura et al., 2017). The previous report revealed the individual and combined effect of rosuvastatin and sildenafil that potentially counter PH by decreasing right ventricular pressure, the right ventricular animal model of pulmonary hypertension (Jasińska-Stroschein et al., 2014).
3.4 Soluble guanylyl cyclase stimulator
Soluble guanylyl cyclase is an important target for treatment of PH because that may regulate the level of endogenous NO. Reduction of NO release from endothelial cells can lead the progression of PH through elevated vasoconstriction. NO is a potent vasodilator and inhibitor of vascular SMC proliferation and platelet aggregation, this molecular sequel is provoked by the activation of soluble guanylyl cyclase and cGMP level. The increased level of cGMP leads to pulmonary vasodilation by modulation of Ca2+ homeostasis and sensitivity of contractile apparatus to [Ca2+] (Amirjanians et al., 2017). Soluble guanylyl cyclase stimulator upregulates the coupling of tetrahydrobiopterin and eNOS which subsequently facilitate the biosynthesis of NO (Figure 2). It has been suggested that administration of novel BAY 41-2272 (sGC activator) reduces pulmonary vascular remodeling, right ventricular hypertrophy in neonatal rats (Amirjanians et al., 2017). A recent study revealed that guanylyl cyclase stimulator, Riociguat (BAY 63-2521) is well tolerated and superior to NO therapy. This approach is currently being investigated in phase III clinical trial for the treatment of PH (Amirjanians et al., 2017; Meis & Behr, 2014). Some soluble guanylyl cyclase stimulators such as BAY 41-2272, BAY 41-8543 and Riociguat (BAY 63-2521), CFM-1571, BAY 60-4552, and Vericiguat (BAY 1021189) has been noted to reduce mean pulmonary arterial pressure, vascular remodeling and right ventricle hypertrophy in several experimental models of PH (Benza, Mathai, & Nathan, 2017).
3.5 Rho-kinase inhibitors
Rho kinase signaling pathway is recommended as a novel target for treatment of PH (Lu et al., 2017). Rho associated serine threonine protein kinases (Rho, Ras, Rab, and Ran families) are recommended as a novel potential therapeutic target for treatment of PH (Lu et al., 2017). Rho kinase suppresses myosin phosphatase activity by phosphorylating the myosin binding subunit of enzyme and thus, increase VSMC contraction (Lopez et al., 2016). Rho kinase has supposed to activate various mediators cellular signaling cascade or mediators including monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor-1, and NADPH oxidase, which finally can cause vascular remodeling (Table 1) (Lopez et al., 2016; Lu et al., 2017). Inhibition of Rho kinase cause the diminished expression of eNOS and increase inflammatory mediators in PH (Velayati et al., 2016). Oral treatment with Rho kinase inhibitor including Fasudil is an attractive choice of treatment that inhibits the development of pulmonary arterial hypertension in experimental mice model (Mouchaers et al., 2010). Furthermore, combined treatment of Fasudil with Beraprost (prostacyclin analog) was found to improve the right ventricular hypertrophy and median thickness in pulmonary vessels of MCT-induced pulmonary hypertensive rats (Tawara, Fukumoto, & Shimokawa, 2007). Moreover, Previous studies documented that treatment with Fasudil, Sildenafil individually or their combination significantly reduces the symptoms of PH by decreasing right ventricular pressure and improve exercise capacity (Elias-Al-Mamun et al., 2014). SB-772077-B and Y-27632 (Rho-kinase inhibitor) have shown the ameliorative effect in experimentally induced PH (Chou, Huang, Yeh, & Chen, 2013; Murthy, Nossaman, & Kadowitz, 2010).
| S. no. | Therapeutic interventions | Signaling cascades |
|---|---|---|
| 1 | Calcium channel blockers | Decrease in intracellular Ca2+ in VSMC |
| 2 | Prostacyclin analogs | Increase in IP3/DAG, PPARγ, cAMP, increase in NO, and decrease in chemokine secretion |
| 3 | ETA receptor antagonists | Increase in IP-3/DAG, intracellular Ca2+, decrease in TNFα IL-1, IL-6 |
| 4 | ETA/B receptor antagonists | Increase in IPT-3/DAG, intracellular Ca2+, decrease in TNFα IL-1, IL-6 |
| 5 | Phosphodiesterase V inhibitors | Increase in cAMP and cGMP |
| 6 | Phosphodiesterase ¾ inhibitors | Increase in cAMP and cGMP |
| 7 | Soluble gunanyl cyclase stimulators | Decrease in PDF-5, ADMA, increase in NO, cGMP, eNOS, DDAH |
| 8 | Rho kinase inhibitors | Decrease in eNOS, increase in MCP, PAI-1, NADPH oxidase |
| 9 | PDGF inhibitors | Decrease in PDGF and VEGF |
| 10 | Ligands of PPAR-γ | Decrease in protein kinase, Kv channel, Ca2+channel Down regulation of protein kinase α |
| 11 | Statins | Decrease in inhibition of HMG- CoA-reductase, NADPH oxidase, PAI-1, NO, and Rho Kinase |
| 12 | Multikinase inhibitors | Decrease in PDGF and VEGF |
| 13 | Steroids and immunosuppressive therapy | Decrease in cytokines and chemokines |
| 14 | NFAT (nuclear factor of activated T-cells) inhibitors | Decrease in SOD activity, Ca2+ influx, inflammatory mediators |
| 15 | 5HT2A receptor antagonist | Decrease in 5-HT, entracellularCa2+, Bcl-X, Bcl-2, PAP, TNF-α, IL-1β, IL-6, Kv channels |
| 16 | 5HT transporter inhibitors | Decrease in 5-HT, entracellularCa2+, Bcl-X, Bcl-2, PAP, TNF-α, IL-1β, IL-6, Kv channels |
| 17 | Vasodilator peptides | Increase in NO |
| 18 | Elastase inhibitors | Decrease in elastase activity, Tenascin-C and increase smooth muscle cell apoptosis |
| 19 | Activin receptor -like kinase-5 inhibitors | Decrease in IL-6, TGF-β, basic fibroblast growth factor |
| 20 | Tyrosine Kinase inhibitors | ERK1/2down regulate Raf-1 Pathway |
| 21 | Diuretics | Decrease in electrolytes and fluid retention |
| 22 | Anticoagulants | Decrease in tissue plasminogen activator and Ang-II, increase in fibrinogen, interfere in Vit-K |
| 23 | Inotropic agents | Increase in intracellular Ca2+ activate Src, ERK1/2kinase pathways, PI-3kinase, protein kinase B, NF-κ B, and ROS |
| 24 | κ-opioid receptor agonists | Preservation of eNOS activity and ant-oxidative effect |
| 25 | Potassium channel openers | Decrease in P-38 MAP kinase expiration, restore eNOS, HMG-CoA reductase, CXC chemokine receptor and P-selectin |
| 26 | Vasoactive intestinal peptide | Increase in synthesis of tetrahydrobiopterin |
3.6 Serotonin (5-HT) receptor antagonist and transporter (5-HTT) blockers
Serotonin mediates the pulmonary arterial proliferation, vasoconstriction, and local micro-thrombosis (Sharma et al., 2017). Serotonin inhibitors significantly reduce the inflammatory (TNFα, IL-1β, and IL-6) and proliferative mediators (Table 1). Sarpogrelate has been reported to serve as a protective therapeutic agent against experimental induced PH and pulmonary vascular remodeling through its anti-inflammatory, and antiproliferative properties (Geng et al., 2016). Conversely, Dexfenfluramine/Nordexfenfluramine provoke the release of serotonin which can cause severe PH through activation of 5-HT receptors and increase the influx of extracellular Ca2+ and release of Ca2+ from the sarcoplasmic reticulum in PASMC (Dempsie et al., 2013). A recent report mentioned that administration of Fluoxetine ameliorative the pulmonary arterial hypertension by reducing the expression of Bcl-2, Bcl-x, and increased expression of cleaved caspases-3 and voltage-gated potassium (Kv) channels in rats (Ran, Zhao, Nie, & Chen, 2016). Serotonin receptor modulator RP 5063 also reported for improving pulmonary vascular pathology and right ventricular pressure in MCT-exposed rats by decreasing the molecular mediators including TNFα, IL-1β, and IL-6 (Bhat et al., 2017). Moreover, Citalopram (5-HT inhibitors), GR127935 (5-HT 1B/1D receptor antagonist), Paroxetine, Sertraline, Fluoxetine (selective serotonin reuptake inhibitors) have been reported to reduced pulmonary vascular remodeling in hypoxia-induced pulmonary hypertensive mice (Hood et al., 2017).
3.7 Statins
Clinical and preclinical evidence suggests the statins associated inhibition of Rho-kinase, P38 MAP kinase expression, apoptosis, and improved eNOS expression (Dong et al., 2010; Kilic, Gok, Elibol-Can, Uysal, & Bacaksiz, 2015). Pravastatin (HMG-CoA reductase inhibitor) also down-regulate the expression of stromal-cell derived factor (SDF-1), chemokine receptor 4, and ICAM-1/CD18 pathway against hypoxia induced more PH in rats (Kilic et al., 2015). Furthermore, a placebo-controlled study with Rosuvastatin has revealed the robust fall of P-selectin, tissue plasminogen activator level in PH 9 (Anand, Garg, Duval, & Thenappan, 2016). Simvastatin was shown to retard pulmonary vascular remodeling by inhibiting RhoA/ROCK pathway in cultured PASMCs (Anand et al., 2016). Moreover, Atorvastatin and Fluvastatin were resulted to reduce the right ventricular pressure, ventricular remodeling, and muscularization of the pulmonary artery in rats by increasing the eNOS activity (Lin et al., 2017). Co-administration of the statin with Imatinib or Sildenafil has considered more significant strategy against PH measured by decrease arterial blood pressure, ventricular hypertrophy (Jasińska-Stroschein, Owczarek, Surowiecka, Kącikowska, & Orszulak-Michalak, 2015).
3.8 Peroxisome proliferator activated receptor (PPAR)
Peroxisome proliferator activated receptor belongs to the nuclear family of ligand activated transcriptional factors. PPAR have been implicated in various disorder including cancer, diabetes, cardiomyopathy, and pulmonary hypertension. PPARγ agonist has explored to prevent the development of PH in the preclinical investigation (Maccallini, Mollica, & Amoroso, 2017). This protective approach of PPARγ agonists against PH has carried by significant anti-proliferative, anti-inflammatory, and anti-apoptotic signaling cascades (Idris-Khodja et al., 2017; Maccallini et al., 2017). Moreover, PPARγ agonist has been noted to decrease the expression of NADPH oxidase, plasminogen activator inhibitor-1 production, and stimulates NO production (Chen & Wang., 2017; Idris-Khodja et al., 2017). PPARγ ligands reduce iNOS expression, platelet derived growth factor signaling production, macrophage recruitment, and inflammatory mediator production in PH (Idris-Khodja et al., 2017). PPARγ activation is also responsible for inhibiting Rho/Rho kinase pathway that helps in delaying the progression of disease (Gien, Tseng, Seedorf, Roe, & Abman, 2014). Furthermore, PPARγ agonists have found to reduce the production of ET-1, which is the key signaling mechanism to induce PH. Pioglitazone can majorly reduce proliferation of PVSMC, PVR, and apoptosis in the experimental model of PH (Idris-Khodja et al., 2017; Liu, Tian, et al., 2014; Sharma & Khanna, 2013). Recently, it has been demonstrated PPARγ agonist Rosiglitazone ameliorates endothelin-1-induced vasoconstriction of pulmonary arteries in the rat model (Liu, Tian, Huang, & Wang, 2014). Pioglitazone and Rosiglitazone were noted to alleviate the vascular remodeling and attenuate ET-1, respectively (Behringer et al., 2016). In addition, GW0742 (PPARβ/δ agonist) has been noted to improve the right ventricular pressure against hypoxia-induced PH in rats (Harrington et al., 2010).
3.9 Calcium channel blockers
Since, increased level of intracellular calcium can majorly cause severe vasoconstriction, sliding of myosin and actin filaments in smooth muscle cells and block various vasodilatory pathways (Sharma, Haidarali, & Nagaich, 2014; Sharma, Khanna, & Balakumar, 2014). This over-activation of intracellular calcium in pulmonary endothelial vasculature can certainly lead to hypertension due to smooth muscle contraction. Thus, calcium channel blockers have targeted to minimize the vulnerabilities of PH. Calcium channel blockers are the heterogeneous class of vasodilators and found to be effective for the treatment of PH (Yamamura et al., 2017). Various calcium channel blockers such as Nifedipine, Diltiazem were considered to have therapeutic potential in the management of PH. Nifedipine can cause the sustained reduction in pulmonary artery pressure and pulmonary vascular resistance (Montani et al., 2010). Whereas, Tetrandrine upregulate the expression of protein kinase 1, superoxide dismutase, glutathione in MCT-induced PH in rats (Wang et al., 2016). Moreover, Levosimendan, (Calcium sensitizer and new dual acting non-arrhythmogenic drug) was reported to revert right ventricular failure in PH (Hansen et al., 2017).
3.10 Potassium channel openers
Potassium channels may also play a significant role in the pathogenesis of pulmonary artery hypertension. The decreased expression of voltage gated potassium (Kv) channels in PH promotes pulmonary hypertrophy (Morita & Komuro, 2013). Moreover, Kv channel inhibition results in membrane depolarization, activation of voltage dependent Ca2+ channel in rats which cause PASMC contraction by promoting actin-myosin-interaction and PASMC proliferation (Ke, Wu, Tian, Li, & Du, 2013; Morita & Komuro, 2013). Furthermore, kv channel openers like Dichloroacetate (DCA) have been found to prevent PH in preclinical studies (Velayati et al., 2016). Moreover, Iptaklim and JTV-506 (novel ATP sensitive potassium channel openers), have been considered to reduce right ventricular hypertrophy, pulmonary remodeling in hypoxia induced PH in rats (Zuo et al., 2011). Iptaklim associated reduction in pulmonary artery smooth muscle cell proliferation is also mediated by downregulation of PKC-α (Velayati et al., 2016; Xie, Wang, Wang, & Hu, 2004). In addition, Levcromakalim has been reported to decrease right ventricular pressure in hypoxia-induced PH in piglets (Velayati et al., 2016; Xiao, Cheng, & Chen, 2003). Thus, potassium channel opener may be an attractive target as it plays an important role cell apoptosis, survival and proliferation.
3.11 Tyrosine kinase inhibitors
Vascular adverse events are an evolving dilemma in patients with chronic pulmonary disorders and tyrosine kinase inhibitors can directly cause pulmonary endothelial cell toxicity via the production of mitochondrial reactive oxygen species (Moguillansky, Fakih, & Wingard, 2017). Platelet-derived growth factors (PDGFs) and their receptors (PDGFRs) have served as prototypes for growth factor and receptor tyrosine kinase (Andrae, Gallini, & Betsholtz, 2008). Thus, treatment with Imatinib, (PDGF receptor antagonist) have considered reducing right ventricular pressure, pulmonary proliferation by suppressing activation of downstream signaling pathways in guinea pigs (Maihöfer et al., 2017). Furthermore, the clinical investigations reported that treatment with Imatinib has shown significant improvement in 6 min walk test and hemodynamics in pulmonary hypertensive patients through vasodilation potential (Minami et al., 2017). In addition, treatment with Multikinase inhibitor, Sorafenib reduced right ventricular remodeling and pulmonary arterial muscularization by inhibition of Raf kinase and ERK1/2 signaling pathway in experimental PH (Kimura et al., 2017).
3.12 Miscellaneous
From past couple of years, tremendous efforts have been made to explore the novel target sites or potential future agent that can counteract the induction or progression of PH. In spite of mentioned therapeutic approaches, several other promising targets still need a revisit to explore their inherent perspective. Caveolin-1 is a tyrosine-phosphorylated protein or endocytic structures that help in transportation of molecules across endothelial cells. Caveolin-1 plays a major role in PH (Marsboom et al., 2017). Reduced expression of caveolin-1 and 2 have been observed in the plexiform lesions formed in PH (Han et al., 2016). Cav-1 have been noted to increase cell growth and apoptosis in rats (Han et al., 2016; Marsboom et al., 2017). Moreover, there is an inverse relationship between loss of cav-1 and various pro-mitogenic and anti-apoptotic IL-6/STAT-3 and ERK1/2 signaling (Mathew, 2011). Moreover, cav-1 rescue is associated with the inhibition of STAT-3 activation and attenuation of PH (Mathew, 2011). Estrogens have major role in the activation of endothelial nitric oxide synthetase and SMC growth. Treatment with 2-ethoxyestradiol has considered an anti-mitogenic which can attenuate hypoxia inducible factor-1 and superoxide dismutase in lung tissues of rats (Wang, Zheng, Yuan, Li, & Gong, 2017).
Some recent evidence delineates the fundamental role of P38/MAPK that may consider as an individual approach for the treatment of PH. P38/MAPK mediated stimulation of superoxide production may contribute the endothelial dysfunction and PH. Whereas, P38/MAPK inhibitor “PH797804” has found to be protective against right ventricular hypertrophy and fibrosis formation that is the key factor for inducing PH (Kojonazarov et al., 2017). Moreover, SB-203580, another p38/MAPK inhibitor has significantly reduced PH in the experimental model of rats via upregulation in NO generation and reduced superoxide generation (Takahashi, Mikami, & Yang, 2007). The experimental and clinical study demonstrated the therapeutic perspective of vasoactive intestinal peptide (VIP) for the treatment of PH. VIP can also upregulate the synthesis of tetrahydrobiopterin (Szema et al., 2017) which is a critical cofactor in endothelial nitric oxide production (Goyal et al., 2014). Thus, chronic right ventricular hypertrophy and pulmonary vascular remodeling have observed in VIP knock out (VIP-/-) mice (Hu et al., 2015; Szema et al., 2017). Furthermore, the treatment with inhaled Aviptadil (VIP agonist) was remarked to exhibit pulmonary vasodilating effect and improved oxygenation in patients with PH (Hu et al., 2015; Szema et al., 2017).
Instead of these synthesized therapeutic approaches, numerous herbal interventions can also be targeted for modulation of various signaling alteration involved in PH. Herbal constituents of some phytomedicine are capable of improving the PH by modifying various participated signaling cascades. Resveratrol (Csiszar et al., 2009), Panax ginseng (Araliaceae) (Jiang et al., 2007), Genistein (Loganville) (Homma et al., 2006), Salvia Miltiorrhiza (Lamiaceae) (Liu, Huang et al., 2014), and Chinese herb Naofeikang (Han et al., 2000) have reported significant improvement in eNOS expression and simultaneous increase production of NO that can majorly knock down the endothelial dysfunction and associated PH (Table 2). Indeed, Genistein can also decrease mean arterial pressure of pulmonary vessel and collagen deposition which is the major sign of PH (Homma et al., 2006). Salvia Miltiorrhiza has reported for the significant reduction in heme oxygenase-1 and iNOS level in the pulmonary system (Liu, Tian et al., 2014). Chinese herb Naofeikang and Ruscogenin have additional therapeutic potential against PH by down regulating the inflammatory reaction and macro phase infiltration (Bi et al., 2013; Han et al., 2000). Radix Astragali (Fabaceae) (Chen, Ruan, Xi, Si, & Zhang, 1997), Allium sativum (Amaryllidaceae) (Sun & Ku, 2006), Luteolin (Lamiaceae) (Occhiuto & Limardi, 1994), Rhoifolin (Rutaceae) (Occhiuto & Limardi, 1994), and Punicalagin (Punica granatum) (Shao et al., 2016) have reported for producing the measurable reduction in mean arterial blood pressure, pulmonary vascular remodeling and collagen deposition, which are significantly liable to the pathogenesis of PH. Erigeron breviscapus (Asteraceae) has potent antiproliferative action on smooth muscle cells (Zheng, Xie, Gao, & Zhang, 2012). Traditional chines medicine San-Huang-Xie-Xin-Tang has also reported the potent PDE-5, Rho-Kinase and cyclooxygenase-2 inhibitor which reflect the novel notion for the treatment of PH (Liou et al., 2012). Moreover, widely documented Terminalia Arjuna (Combretaceae) and Punicalagin (Punica granatum) have found to reduce the right ventricular hypertrophy and systolic pressure, that are the root cause of PH (Meghwani et al., 2017). Punicalagin has also been explored for its therapeutic effect on PH through upregulation of No/cGMP signaling pathways and downregulation of oxidative stress (Shao et al., 2016).
| S. no. | Herbal intervention | Targeted signaling cascades |
|---|---|---|
| 1 | Resveratol | Increases expression of eNOS, NADPH oxidase, and improve endothelial function |
| 2 | Panax ginseng | RVH, inhibiting the calcineurin signal transduction pathway, increasing NO release |
| 3 | Genistein | Reduce RVH, medial wall thickness of pulmonary arteries, increasing eNOS |
| 4 | Salvia miltiorrhiza | Decrease the level of heme-oxygenase-1 and iNOS and enhance the level of eNOS |
| 5 | Naofeikang (Chinese herb) | Preserving vessel endothelial cells and lessen the inflammatory reaction and inhibiting PVR |
| 6 | Ruscogenin (traditional Chinese herb) | Decrease inflammatory cytokines, macrophage infiltration |
| 7 | Radix astragali | Decreases mean arterial blood pressure, pulmonary vascular remodeling by inhibiting the type III collagen deposition |
| 8 | Allium sativum | Decrease RVH, right ventricular pressure |
| 9 | Luteolin, Rhoifolin | Decrease PAP, pulmonary artery wedge, and aortic pressure & pulmonary vascular resistance |
| 10 | Punicalagin | Right ventricular hypertrophy and vascular remodeling NO-cGMP signaling and reduced oxidative stress |
| 11 | Erigeron breviscapus | Decreases mPAP, RVH, and smooth muscle cell proliferation |
| 12 | San-Huang- Xie-Xin-Tang | Downregulate the expression of phosphodiesterase type 5, Rho-kinase (ROCK) II, cyclooxygenase-2 (COX-2) [98] |
| 13 | Terminalia arjuna | Decreases right ventricular hypertrophy, right ventricular systolic pressure |
4 CONCLUSION
Molecular interaction of therapeutic interventions to modify the pathological events have been an inimitable method of research to update the endured treatment into advanced. PH is endorsed by several signaling alteration including IP3/DAG, Kv channel, NADPH oxidase, PI3 kinase, Rho kinase, MAP kinase, Ca2+ channel, PDGF, PDE, PPAR, HMG-Co A-reductase, endothelin signaling pathways. Instead of PH, these avenues of abnormality in molecular signaling make it more vulnerable to atherosclerosis, hypertension, coronary heart disease, and pulmonary endothelial damage. The present review demonstrated the several targeted links and molecular aspects of various therapeutic interventions having an association with signaling alteration of PH mediated by vascular endothelial dysfunction. Moreover, various alternative therapeutic interventions including Panax ginseng (Araliaceae), Genistein, Salvia Miltiorrhiza (Lamiaceae), Chinese herb Naofeikang, Radix Astragali (Fabaceae), Allium sativum (Amaryllidaceae), Luteolin (Lamiaceae), Rhoifolin (Rutaceae), Punicalagin (Punica granatum), Erigeron breviscapus (Asteraceae), and Terminalia Arjuna (Combretaceae) also have significant competence to modify these signaling alteration. and may open the new possibility of novel signaling modulator which can restore the pathological events and improve PH.
ACKNOWLEDGMENT
We express our gratitude to Dr. Neena Valecha, Director, NIMR, New Delhi, India for giving inspiration and constant support.
CONFLICT OF INTEREST
The authors declared no conflict of interest.




