Adult-onset brain tumors and neurodegeneration: Are polyphenols protective?
Abstract
Aging is a primary risk factor for both neurodegenerative disorders (NDs) and tumors such as adult-onset brain tumors. Since NDs and tumors are severe, disabling, progressive and often incurable conditions, they represent a pressing problem in terms of human suffering and economic costs to the healthcare systems. The current challenge for physicians and researchers is to develop new therapeutic strategies in both areas to improve the patients’ quality of life. In addition to genetics and environmental stressors, the increase in cellular oxidative stress as one of the potential common etiologies has been reported for both disorders. Recently, the scientific community has focused on the beneficial effects of dietary antioxidant classes, known as nutraceuticals, such as carotenoids, vitamins, and polyphenols. Among these compounds, polyphenols are considered to be one of the most bioactive agents in neurodegeneration and tumor prevention. Despite the beneficial activity of polyphenols, their poor bioavailability and inefficient delivery systems are the main factors limiting their use in medicine and functional food. The development of polymeric nanoparticle-based delivery systems able to encapsulate and preserve polyphenolic compounds may represent a promising tool to enhance their stability, solubility, and cell membrane permeation. In the present review we provide an overview of the main polyphenolic compounds used for ND and brain tumor prevention and treatment that explores their mechanisms of action, recent clinical findings and principal factors limiting their application in medicine.
1 INTRODUCTION
Aging is the progressive decline in intrinsic physiological function over time. It is a primary risk factor for both neurodegenerative disorders (NDs) and tumors, such as adult-onset brain tumors (particularly, glioblastoma and neurofibromatosis, type 2) (Stoll, Horner, & Rostomily, 2013).
NDs, such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD) and amyotrophic lateral sclerosis (ALS), result in the gradual dysfunction and/or slowly progressive loss of post-mitotic neuronal cells in the brain and spinal cord (Plun-Favreau, Lewis, Hardy, Martins, & Wood, 2010). Tumors are the result of uncontrolled proliferation of cells characterized by a heightened resistance to cell death. Although tumor and neurodegeneration seem to share few common features, the molecular genetics and cell biology show a greater overlap between these age-related disorders (Plun-Favreau et al., 2010). Since NDs and tumors are severe, disabling, progressive and, particularly for NDs, incurable conditions, they represent a pressing problem in terms of human suffering and economic cost to the health care systems (Gustavsson et al., 2011). In addition, the incidence of age-related disorders can be expected to increase, given that prospective studies show the number of people aged 65 or older is forecasted to grow to 20% of the population by the year 2050 (Marr, Thomas, & Peterson, 2010). A large number of studies suggest the need to develop new therapeutic strategies in both areas in order to improve the patient's quality of life (D'Angelo et al., 2017; Plun-Favreau et al., 2010).
Although the etiology of ND and tumor has not yet been fully elucidated, risk factors such as genetics and environmental stressors (cigarette smoking, diet, exposures to chemicals, and radiation) seem to play key roles (Anand et al., 2008; Gilbert, 2009; Durazzo, Mattsson, Weiner, & Alzheimer's Disease Neuroimaging I, 2014; Spagnuolo et al., 2016).
Over last 2 decades a large body of literature data has reported the increase in cellular oxidative stress as one of the potential common etiologies in both disorders (Spagnuolo et al., 2016). Oxidative stress, that is, the imbalance between production of free radicals and reactive metabolites (known as reactive oxygen species − ROS), leads to cell damage, impairment of the DNA repair mechanisms, mitochondrial dysfunction and genome instability (Gandhi & Abramov, 2012). This evidence suggests the potential role that anti-oxidant compounds play in the prevention and treatment of aging-related disorders, such as NDs and tumors (Rodriguez-Morato et al., 2015).
Recently, a considerable number of natural compounds present in the diet have been proposed as possible candidates in neuroprotection and tumor prevention (Ebrahimi & Schluesener, 2012; Spagnuolo et al., 2016). Physicians and researchers have focused on the beneficial effects of dietary anti-oxidant classes, known as nutraceuticals, such as carotenoids, vitamins, and polyphenols. These heterogeneous class of molecules are present in fruits, vegetables, olives, dry legumes, cereals, beverages (such as tea, wine, beer, coffee, and chocolate) and in other natural products (Barone et al., 2017; D'Archivio et al., 2007; D'Archivio, Filesi, Vari, Scazzocchio, & Masella, 2010; Russo, Spagnuolo, Tedesco, & Russo, 2010). Given their potent anti-oxidant and anti-inflammatory activities, polyphenols are considered to be one of the most bioactive agents in neurodegeneration and tumor prevention (Zhou et al., 2016). Although a growing body of experimental evidence shows the beneficial effects of polyphenols for several diseases, a strong proof of their efficacy is needed for each particular health claim (Gupta, Patchva, & Aggarwal, 2013; Pandey & Rizvi, 2009). Factors such as the right dosages and safety profile have to be better addressed for these natural molecules to be introduced in routine treatments. In addition, poor bioavailability and inefficient delivery systems of dietary polyphenols are the main factors that limit their use in medicine, functional food, and supplements (D'Archivio et al., 2007, 2010). Unfortunately, a large part of ingested polyphenols persists in the colon, where they are undergone metabolic transformations which, in turn, determine a substantial modification in their structure and biological activity (Lewandowska, Szewczyk, Hrabec, Janecka, & Gorlach, 2013; Spagnuolo et al., 2016). Given the promising therapeutic effects of polyphenolic compounds, the challenge of clinicians and researchers is to develop delivery systems able to preserve and transport them to target organs.
In the present review we provide a comprehensive overview of the main polyphenolic compounds used for ND and brain tumor prevention and treatment and analyze their mechanism of action, the recent clinical findings and the principal factors limiting their application in medicine.
2 POLYPHENOLIC COMPOUNDS
In the last years a growing number of experimental and epidemiological studies have supported the beneficial effects of polyphenols in preventing several age-related disorders including tumors and NDs. Among the known natural bioactive compounds, polyphenols are popular for their enhanced anti-oxidant activity, safety, and absence of side-effects (Squillaro, Peluso, & Melone, 2017).
- Flavonoids—Polyphenolic compounds comprising 15 carbons with two aromatic rings connected by a three-carbon bridge. Although the basic flavonoid skeleton can present numerous substituents, the majority of flavonoids come about naturally as glycosides rather than aglycones. These compounds comprise six main subclasses: flavanols, flavones, isoflavones, flavanones, anthocyanidins, and flavan-3-ols (Del Rio et al., 2013; Tsao, 2010).
- Flavanols—They are widely present throughout the plant kingdom with the exception of fungi and algae. The most common flavonols are kaempferol, quercetin, isorhamnetin, and myricetin. Onion bulbs (Allium cepa) are among the richest sources of quercetin-4′-O-glucoside and quercetin-3,4′-O-diglucoside. The disaccharide quercetin-3-O-rutinoside is found in several vegetables and fruits, such as apples, buckwheat, most citrus fruits, figs, and both black and green tea (Del Rio et al., 2013; Slimestad, Fossen, & Vagen, 2007).
- Flavones—This subclass of flavonoids is not widely distributed; the most common flavones are apigenin, luteolin, wogonin, and baicalein. Celery (Apium graveolens), parsley (Petrosilinum hortense) and some herbs contain large amount of flavones (Del Rio et al., 2013).
- Isoflavones—They are found almost exclusively in leguminous plants. Substantial amounts of isoflavones (e.g., daidzein and genistein) are present in soybeans (Glycine max) and their food products, such as tofu. Other dietary sources of isoflavones include chick pea (rich in biochanin A) and peanut (rich in genistein). Given their structural similarity to estrogen, isoflavones are classified as phytoestrogens (Del Rio et al., 2013; Gupta et al., 2013).
- Flavanones—They are present in high concentrations in the flavedo of citrus fruits. The most common flavanone is the hesperetin-7-O-rutinoside, also known as hesperidin, which is also found in apricots, plums and bilberries (Del Rio et al., 2013).
- Anthocyanidins—They are common plant pigments; these molecules form conjugates with sugars and organic acids to generate a multitude of anthocyanins of differing colors, and as a consequence, they are visible in fruits and flowers. The most common anthocyanidins are pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin (Del Rio et al., 2013).
- Flavan-3-ols—The most complex subclass of flavonoids, they range in size from simple monomers (i.e., catechin and its isomer epicatechin) to the oligomeric and polymeric proanthocyanidins, also known as condensed tannins. Green tea (Camellia sinensis) contains very high levels of (epigallocatechin-3-gallate (EGCG) and (−)-epicatechin-3-gallate (Del Rio et al., 2013).
-
Non-flavonoids: The C6–C1 phenolic acids are non flavonoids of dietary significance. Gallic acid, the most common phenolic acid, is widely found in fruit and plants. The related ellagic acid-based ellagitannins are found in large amounts in raspberries (Rubus idaeus), strawberries (Fragaria ananassa), blackberries (Rubus spp.), pomegranates (Punica granatum), and persimmon (Diospyros kaki), walnuts (Juglans regia), and hazelnuts (Corylus avellana) (Del Rio et al., 2013).
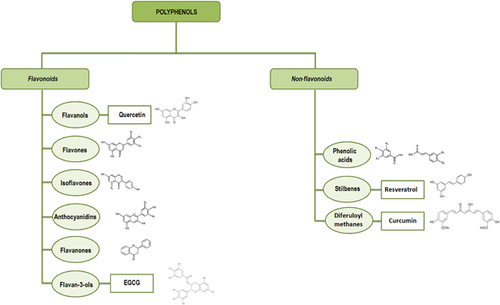
Among the non-flavonoid molecules, the following phenolic compounds are worthy of mention: i) secoiridoids and the ligstroside, found in olive oil (Tasioula-Margari & Tsabolatidou, 2015); ii) stilbenes, present in a number of plant families (e.g., Vitaceae), whose main exponent is resveratrol, detected in wine (Bavaresco, Fregoni, Cantu, & Trevisan, 1999; Del Rio et al., 2013); iii) diferuloylmethanes, among which curcumin, the principal curcuminoid of turmeric (Curcuma longa) (Malik & Mukherjee, 2014).
3 POLYPHENOLS AND NEURODEGENERATION
NDs are a heterogeneous group of disorders characterized by the gradual dysfunction and progressive loss of post-mitotic cells in the central or peripheral nervous system (Spagnuolo et al., 2016). NDs, including AD, PD, HD, and ALS, share common cellular and molecular mechanisms such as the deposition of abnormal misfolded and/or aggregated proteins, oxidative stress accumulation, mitochondrial dysfunction, defect in neuronal transport mechanisms, impairment in the autophagic flux, alteration in proteasome activity and inflammation (Ciechanover & Kwon, 2015; Kiriyama & Nochi, 2015; Sweeney et al., 2017). Because of the lack of clinically effective drugs for ND treatment, the challenge for physicians is to discover new therapeutic approaches to slow down neurodegeneration (Melone, Jori, & Peluso, 2005). It is widely demonstrated that nutritional intervention for subjects at risk of developing NDs is strongly required. A dietary intake of polyphenols is known to limit cellular oxidative stress (Bhullar & Rupasinghe, 2013). Polyphenols act by modulating several therapeutic targets; these natural molecules show strong anti-oxidant activity since they decrease oxidative damage by neutralizing free radicals and modulate the expression of free radical-generating enzymes and/or the expression of enzymes involved in intracellular anti-oxidant defense (Spagnuolo et al., 2016). Because the brain is one of the most metabolically active organs, it is extremely susceptible to ROS injury; this is due to high oxygen consumption necessary to sustain high energy needs, to low levels of anti-oxidant enzymes and the presence of high level of polyunsaturated fatty acids in neuronal membranes that make them more prone to oxidation (Chen, Guo, & Kong, 2012; Wang & Michaelis, 2010). Much evidence indicates that oxidative stress also plays a pathogenic role in neuro-inflammation. The high ROS levels produced in brain tissues modulate synaptic and non-synaptic communication between neurons and glia and give rise to neuro-inflammation and cell death, which, in turn, determine neurodegeneration (Hussain et al., 2016).
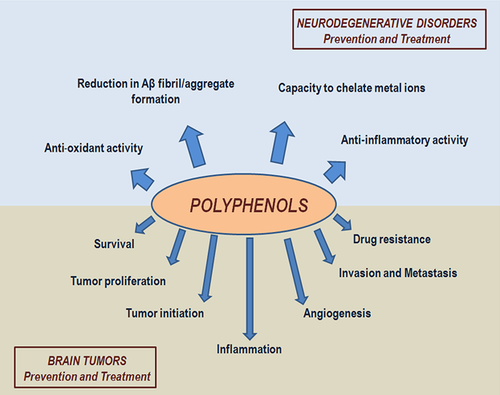
In addition to their anti-oxidant activity, the neuroprotective action of dietary polyphenols involves other mechanisms such as a reduction in amyloid-beta (Aβ) fibril/aggregate formation (a neuropathological hallmark of AD), the chelation of accumulated metal ions in specific brain regions of AD, PD, HD, and ALS patients and the high anti-inflammatory activity exerted by inhibiting the expression of pro-inflammatory genes such as cyclo-oxygenase, nitric oxide synthesis, and several cytokines (Figure 2) (Jayasena et al., 2013; Spagnuolo et al., 2016).
Several studies have shown that polyphenols interact with cellular signaling pathways which are directly or indirectly involved in neurodegenerative processes. In particular, they interact with important signaling cascades involved in cell growth, survival, differentiation, and programmed cell death (Ebrahimi & Schluesener, 2012; Moosavi, Hosseini, Saso, & Firuzi, 2016). Polyphenols affect these cellular functions by modulating the phosphorylation status and the expression levels of key protein involved in extracellular signal-regulated kinase (ERK), phosphoinositide 3-kinase (PI3 K), Akt/protein kinase B (Akt/PKB), tyrosine kinases, protein kinase C (PKC), and mitogen-activated protein kinase (MAPK) pathways (Claude et al., 2014; Ebrahimi & Schluesener, 2012; Moosavi et al., 2016).
Studies performed on PC12 cells showed that some polyphenol compounds have a direct agonistic effect on tropomyosin receptor kinase (Trk) receptors that regulate important pro-survival neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) (Moosavi et al., 2016; Zhang et al., 2012). It has been also demonstrated that flavonoids such as daidzein and genistein act by increasing the expression levels of neurotrophic factors. Other findings show that ferulic acid increases the phosphorylation of cyclic adenosine monophosphate response element-binding protein (CREB) in the hippocampus of corticosterone-treated mice (Leuti et al., 2013; Yabe et al., 2010). It is widely reported that flavonoids bind to the ATP-binding sites of several proteins involved in cellular signaling pathways, such as mitochondrial ATPase and to benzodiazapine binding sites of GABA-A adenosine receptors, thus altering their activity (Dekermendjian et al., 1999; Ebrahimi & Schluesener, 2012).
4 POLYPHENOL NEUROPROTECTIVE POTENTIAL
Curcumin, resveratrol and the green tea polyphenol, epigallocatechin-3-gallate (EGCG) are the most widely investigated polyphenols for their therapeutic potential in several diseases, including NDs (Del Rio et al., 2013). Studies in the past few years have also highlighted the role of quercetin in preventing risks of NDs (Elumalai & Lakshmi, 2016).
4.1 Curcumin
Curcumin is the main bioactive component in turmeric, an important ingredient of traditional Indian and Asian food and medicine. In foods, turmeric is also used in curry, a powder made up of a blend of spices (Hamaguchi, Ono, & Yamada, 2010). The regular curry consumption in a population of elderly non-demented Asians has been reported to be related to better cognitive functions (Ng et al., 2006). In vivo and in vitro evidence suggests that curcumin can counteract neurodegeneration due to its anti-oxidant, anti-fibrilogenic, and anti-inflammatory proprieties (Chongtham & Agrawal, 2016; Garcia-Alloza, Borrelli, Rozkalne, Hyman, & Bacskai, 2007). An in vitro model of nucleation-dependent polymerization showed that curcumin inhibited the Aβ fibril formation and destabilized preformed Aβ fibrils in a dose-dependent manner (Ono, Hasegawa, Naiki, & Yamada, 2004). Recently, Wang and Michaelis (2010) demonstrated that curcumin inhibited the growth of Aβ fibrils using a quartz crystal microbalance with dissipation monitoring (QCM-D) study. The authors showed that the inhibition process is due to the structural conversion of the growing Aβ fibrils, which can hinder the formation of long Aβ aggregates. Park et al. (2008) reported that pretreatment of PC12 cells with curcumin significantly decreases DNA damage and oxidative stress by reducing elevated intracellular calcium levels and tau hyperphosphorylation induced after the exposure to Aβ. An intriguing in vitro study demonstrated that curcumin significantly reduced the microglial proinflammatory markers, inducible nitric oxide synthase (iNOS), and tumor necrosis factor alpha (TNF-α), in rat glial cells exposed to oxidative and proinflammatory stimuli. In addition, the authors reported that the protective curcumin neuroantiinflammatory effect is promoted by the induction of heme oxygenase 1 (HO-1), an anti-oxidative protein (Parada et al., 2015). In 2015 Banji, Banji, & Kalpana (2014) evaluated the effect of curcumin in rats treated with D-galactose, a reducing sugar that induces oxidative stress and causes mitochondrial alteration and neuron apoptosis. The authors demonstrated that curcumin reduces the oxidized lipids, mitochondrial enzymes and cleaved caspase-3 expression levels. In the same year Chongtham and Agrawal (2016) provided evidence that curcumin significantly alleviated the symptomatology in a Drosophila model of HD. The study showed that curcumin treatment reduced neuronal loss and ameliorate motor neuronal dysfunction. The effectiveness of curcumin in ameliorating cognitive deficit in a rat model of sporadic dementia of Alzheimer's type (SDAT) has also been reported. This study suggests that curcumin supplementation may be useful for SDAT treatment (Ishrat et al., 2009). Harish and colleagues demonstrated the beneficial effect of three bioconjugates of curcumin in contrasting oxidative stress induced by gluthatione (GSH) depletion in N27 dopaminergic neuronal cells. Given that oxidative stress elicited by GSH depletion is considered an early event in PD, the authors suggest that curcumin derivatives could represent new strategies for the treatment of PD (Harish et al., 2010).
As reported in the literature data, curcumin treatment shows positive outcomes in animal models of ND.
As regards human studies, in 2016 Rainey-Smith and collaborators conducted a randomized, placebo-controlled, double-blind study of older adult to evaluate the effectiveness of a curcumin formulation (Biocurcumax™) in improving the cognitive outcome. Clinical and cognitive analyses were made at baseline and at 6 and 12 months. The results showed that curcumin had a limited influence on cognitive function and/or quality of life. The authors suggested that additional biological markers involved in AD pathogenesis should be analyzed (Rainey-Smith et al., 2016). The study was registered with the Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/).
To date, according to data reported by the US National Institute of Health (http://www.clinicaltrial.gov/; searching for: “Neurodegenerative disorders” and “Curcumin”) 5 clinical trials concerning the effect of curcumin in ND have been conducted (Table 1). The results of the clinical trial entitled “Curcumin in Patients with Mild to Moderate Alzheimer's Disease” were published by Ringman and collaborators. In this study 36 subjects with mild-to-moderate AD were enrolled and treated with oral administration of curcumin (CurcuminC3 Complex®) for 24 week with an open-label extension to 48 weeks. The authors demonstrated that curcumin was generally well tolerated but were unable to evidence the efficacy of curcumin complex in AD. A possible explanation for these results could be related to the low bioavailability of this specific curcumin formulation (Ringman et al., 2012). Experimental findings suggest curcumin may be a promising compound for ND treatment, but additional clinical trials involving a larger number of patients and longer duration of treatment, as well as new formulation of curcumin are still lacking.
| Polyphenol compound | Study Title | Condition | Status | Clinical trial ID |
|---|---|---|---|---|
| Curcumin | A pilot study of curcumin and ginkgo for treating Alzheimer's disease | Alzheimer's disease | Completed | NCT00164749 |
| Curcumin | Efficacy and safety of curcumin formulation in Alzheimer's disease | Alzheimer disease | Unknown | NCT01001637 |
| Curcumin | Curcumin and yoga therapy for those at risk for Alzheimer's disease | Mild cognitive impairment | Recruiting | NCT01811381 |
| Curcumin | Curcumin in patients with mild to moderate Alzheimer's disease | Alzheimer's disease | Completed | NCT00099710 |
| Curcumin | Short term efficacy and safety of perispinal administration of etanercept in mild to moderate Alzheimer's disease | Alzheimer's disease | Completed | NCT01716637 |
| Resveratrol | Resveratrol and Huntington disease | Huntington disease | Recruiting | NCT02336633 |
| Resveratrol | Resveratrol for Alzheimer's disease | Alzheimer's disease | Completed | NCT01504854 |
| Resveratrol | Pilot study of the effects of resveratrol supplement in mild-to-moderate Alzheimer's disease | Alzheimer disease | Withdrawn | NCT00743743 |
| Resveratrol | Randomized Trial of a Nutritional Supplement in Alzheimer's Disease | Alzheimer's disease | Completed | NCT00678431 |
| Resveratrol | Effect of food on BIA 6–512 (Trans-resveratrol) | Parkinson disease | Completed | NCT03095092 |
| Resveratrol | Tolerability and steady-state pharmacokinetics of BIA 6-512 | Parkinson disease | Completed | NCT03093389 |
| Resveratrol | Pharmacokinetic profile of BIA 6–512 in healthy elderly subjects versus healthy young subjects | Parkinson disease | Completed | NCT03095105 |
| Resveratrol | BDPP treatment for mild cognitive impairment (MCI) and prediabetes or Type 2 diabetes mellitus (T2DM) | Mild cognitive impairment; Alzheimer's disease | Recruiting | NCT02502253 |
| Resveratrol | Tolerability, safety and pharmacokinetics of four single-doses of BIA 6–512 (Trans-resveratrol) and their effect on the Levodopa Pharmacokinetics | Parkinson disease | Completed | NCT03091543 |
| Resveratrol | Effect of BIA 6–512 at steady-state on the Levodopa Pharmacokinetics | Parkinson disease | Completed | NCT03094156 |
| Resveratrol | Effect of BIA 6–512 at steady-state on the Levodopa Pharmacokinetics with a single-dose of Levodopa/Benserazide 200/50 mg or with a single-dose of Levodopa/Benserazide 200/50 mg Plus a Single-dose of Nebicapone 150 mg | Parkinson disease | Completed | NCT03097211 |
| Resveratrol | Short term efficacy and safety of perispinal administration of etanercept in mild to moderate Alzheimer's disease | Alzheimer's disease | Completed | NCT01716637 |
| EGCG | Effects of EGCG (Epigallocatechin Gallate) in Huntington's disease (ETON-study) | Huntington disease | Completed | NCT01357681 |
| EGCG | Sunphenon EGCG (Epigallocatechin-Gallate) in the early stage of Alzheimeŕs Disease | Alzheimer's disease | Completed | NCT00951834 |
| EGCG | Progression rate of MSA under EGCG supplementation as anti-aggregation-approach | Multiple system atrophy | Completed | NCT02008721 |
| Quercetin | Short term efficacy and safety of perispinal administration of etanercept in mild to moderate Alzheimer's disease | Alzheimer's disease | Completed | NCT01716637 |
- The table reports the 21 polyphenol-based clinical trials for neurodegenerative disorders. Completed: The study has ended normally, and participants are no longer being examined or treated. Unknown: A study whose status has not been verified within the past 2 years. Recruiting: The study is currently recruiting participants. Withdrawn: The study stopped early, before enrolling its first participant. Data from www.ClinicalTrials.gov.
4.2 Resveratrol
Resveratrol, the most common member of the stilibene family, is found in wine, red grapes, pomegranates, peanuts, tea, and mulberries (Tellone, Galtieri, Russo, Giardina, & Ficarra, 2015). In vitro and in vivo studies indicate that resveratrol exhibits a wide range of beneficial effects on several human diseases, including ND (Tellone et al., 2015). In an in vitro HD cell model, we showed that dopamine-induced oxidative stress affects the autophagy-lysosomal system and, consequently, cell expressing the mutant polyQ Huntingtin (polyQ-Htt) protein died due to apoptosis (Vidoni et al., 2016). In a more recent study we demonstrated that resveratrol prevents the generation of ROS and restores the level of autophagy related 4A cysteine peptidase (ATG4), a redox-sensitive cysteine-protein involved in the processing of microtubule-associated protein 1A/1B-light chain 3 (LC3), thus protecting the cells from dopamine toxicity (Vidoni, Secomandi, Castiglioni, Melone, & Isidoro, 2017).
Huang, Lu, Wo, Wu, and Yang (2011) evaluated the effect of resveratrol treatment in a rat model of AD. They found that resveratrol is able to protect animals from Aβ-induced neurotoxicity and improve the spatial memory; these protective neuronal effects were associated with a reduction in iNOS levels and lipid peroxidation. An interesting study reported by Karuppagounder et al. (2009) demonstrated, in a mouse model of AD, that resveratrol is able to reduce β-amyloid plaque formation in a brain region-specific manner; the greatest plaque reduction was observed in the hypothalamus, striatum and medial cortex. In 2014 an intriguing study showed that resveratrol may represent a valid therapy for ALS. In a SOD1G93A mouse model of ALS, resveratrol treatment preserved motor neuron function and delayed disease onset. In addition, the authors observed that neuroprotective effect of resveratrol was associated with an increased expression of Sirtuin1 and AMPK, two important mediators involved in normalization of autophagic flux and mitochondrial biogenesis, in mouse ventral spinal cord (Mancuso et al., 2014). The beneficial effect of resveratrol was also demonstrated in a mouse model of HD by Naia and colleagues. The authors showed that resveratrol treatment for 28 days improved learning, motor coordination and expression levels of genes associated with mitochondrial function in HD mice (Naia et al., 2017).
According to data reported by the US National Institute of Health, 12 resveratrol-based clinical trials for ND treatment have been completed or remain ongoing (Table 1) (http://www.clinicaltrial.gov/; searching for: “Neurodegenerative disorders” and “Resveratrol”). The results of the clinical trial entitled “Resveratrol for Alzheimer's Disease” were recently published by Moussa et al. (2017). In this phase 2 randomized, double-blind, placebo-controlled study, 119 subject with mild-moderate AD received oral resveratrol administration (up to 1 g twice daily) for 52 weeks. Compared to the placebo-treated subjects, resveratrol treatment decreased matrix metallopeptidase 9 (MMP9) levels in cerebrospinal fluid, modulated neuroinflammation by increasing human macrophage–derived chemokine (MDC), interleukin-4 (IL-4), fibroblast growth factor 2 (FGF-2) levels and induced adaptive immunity. Taken together these observations suggest that resveratrol may slow down a cognitive decline through a coordinated peripheral and central immune response. These encouraging results are a starting point to further validating the neuroprotective effects of resveratrol (Moussa et al., 2017).
4.3 EGCG
EGCG is the most important phenolic compound in the green tea which is prepared from the leaves of the Camellia sinesis plant. The beneficial effect of green tea in reducing neurodegeneration is widely reported (Jurado-Coronel et al., 2016).
EGCG has been evaluated for its ability to increase intracellular adenosine triphosphate (ATP) levels in human astrocytes and neurons, given mitochondrial dysfunction and energy impairment are key features in many forms of ND. The study demonstrated that EGCG is able to increase ATP production in both cultured cells with different kinetic parameters; in addition, no toxic effects were found (Castellano-Gonzalez et al., 2016). Using both in vitro and in vivo models, Kang et al. (2010) evaluated the role of EGCG in modulating chemically-induced oxidative damage and levodopa (L-DOPA) methylation. L-DOPA, a precursor for the biosynthesis of dopamine, is the most effective drug for PD; it is almost always used in combination with carbidopa, a peripheral dopa decarboxylaae inhibitor, and with COMT, a catecholamine-O-methyltransferase inhibitor. The authors demonstrated that the oral administration of EGCG reduce the accumulation of 3-methyldopa in the plasma and striatum in L-DOPA plus carbidopa treated rats and glutamate-induced oxidative cytotoxicity in mouse hippocampal neuronal cells. The study suggests EGCG may have beneficial effects in PD patients treated with L-DOPA. Avramovich-Tirosh et al. (2007) reported that EGCG in combination with M30, a multi-functional derivative of the non-toxic lipophilic brain-permeable iron chelators VK-28, decreased apoptosis of human SH-SY5Y neuroblastoma cells. Moreover, both compounds reduced the levels of cellular holo-amyloid precursor protein (APP) in neuroblastoma cells. An interesting study conducted by Ding et al showed the protective effects of EGCG in a sevoflurane-induced neurotoxicity neonatal mouse model. The authors demonstrated that EGCG was able to effectively inhibit sevoflurane-induced neurodegeneration of treated mice via the activation of CREB-BDNF/TrkB − PI3 K/Akt signaling pathways (Ding, Ma, Man, & Lv, 2017).
In the US National Institute of Health database, 3 completed EGCG-based clinical trial are reported (Table 1) (http://www.clinicaltrial.gov/; searching for: “Neurodegenerative disorders” and “EGCG”).
The results from EGCG-based studies suggest that the most common green tea polyphenol may have an important role in ND prevention and treatment (Jurado-Coronel et al., 2016).
4.4 Quercetin
Quercetin, a ubiquitous flavonoid, is found in apples, onions, parsley, berries, green tea, citrus fruits, and in some herbal remedies like Ginkgo biloba. Because of its ability to cross the blood brain barrier, it has been shown to prevent and/or modify various behavioral symptoms in brain diseases (Elumalai & Lakshmi, 2016). Xi, Zhang, Luo, Liu, and Yang (2012) reported that quercetin treatment reduces apoptotic rate in H2O2-treated neuroblastoma SH-SY5Y cells. The study suggests a potential protective role of quercetin against oxidative damage. In accordance with these findings, another study showed that the administration of quercetin reduced ROS production, increased mitochondrial manganese-dependent superoxide dismutase (MnSOD) activity and the ratio of B-cell CLL/lymphoma 2 (BCL-2) to BCL2 associated X (BAX) in aluminum-induced oxidative stress rat model (Sharma et al., 2016). Prasad et al. (2013) demonstrated that quercetin supplementation protected rat hippocampal neurons during exposure to hypobaric hypoxia. The authors suggested that the neuroprotective role of quercetin was accomplished through anti-oxidative and anti-apoptotic mechanisms. Recently, Ay and collaborators evaluated the effects of quercetin in a progressive dopaminergic neurodegenerative transgenic mouse model of PD; their data showed reversed behavioral deficits and striatal dopamine depletion due to oral quercetin administration (Ay et al., 2017).
In the US National Institute of Health database only one quercetin-based clinical trial is reported (Table 1) (http://www.clinicaltrial.gov/; searching for: “Neurodegenerative disorders” and “Quercetin”).
5 POLYPHENOLS AND ADULT-ONSET BRAIN TUMORS
Aging leads to an increased risk of adult-onset brain tumors. It is widely demonstrated that increased age is also associated with increased malignancy and low patient survival (Stoll et al., 2013). In addition to age, risk factors such as genetics, exposure to radiations and chemicals, occupational factors, head trauma, cigarette smoking, infections, and diet seem to play a key role in the etiology of brain tumors (Anand et al., 2008).
Glioblastoma multiforme (GBM) is the most common form of age-related primary brain tumor, accounting for more than 60% of all brain tumors in adults. Among primary brain tumors, generally termed gliomas and classified according to their presumed cell of origin, GBM is the most aggressive and has been assigned as grade IV (Stoll et al., 2013). Despite the variety of therapies for GBM, patients over 70 respond poorly compared to younger patients, thus representing a disease with a very poor prognosis for the elderly. To date, there are no definitive treatments for GBM and for this reason researchers are seeking new therapeutic treatments (Cirillo et al., 2014).
Neurofibromatosis type 2 (NF2) is a genetic condition that leads to the development of multiple benign tumors of the central and peripheral nervous system (such as schwannomas, meningiomas, and ependymomas). The majority of NF2 cases are diagnosed in the second or third decade of life (Angelo et al., 2011; Blakeley, 2012). Although the tumors associated with NF2 are benign, NF2 morbidity and mortality is closely related to the location of the tumors as well as to the effects of treatments. To date, no effective therapies other than local treatments are available for this disabling condition; therefore systemic treatment of NF2 patients is still a challenge (Blakeley, 2012).
It was widely reported that a healthy diet based on the consumption of fruit and vegetables is associated with a decreased tumor incidence and mortality (Turrini, Ferruzzi, & Fimognari, 2015).
Currently, researchers are interested in a number of fruits rich in polyphenolic compounds, given their proven chemopreventive and/or chemotherapeutic potential (Turrini et al., 2015). Because of the well known anti-oxidant proprieties, polyphenol supplementation is a valid treatment to counteract high levels of oxidative stress in various types of tumors (Liou & Storz, 2010). In vitro and in vivo studies have demonstrated that polyphenols inhibit MMP-9, cyclooxygenase, hydroperoxidase, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), protein kinase B (AKT) and other cell cycle regulators (Pandey & Rizvi, 2009).
In addition to oxidation prevention, several mechanisms of action have been reported to account for the chemopreventive and therapeutic effect of polyphenols, including the induction of G2/M cell cycle arrest, activation of apoptotic pathways (e.g., p21 and p53), inhibition of invasion and metastasis, reduction in angiogenesis and anti-inflammatory activity (Figure 2) (Pandey & Rizvi, 2009; Rodriguez et al., 2016).
It has been demonstrated that polyphenolic compounds influence the metabolism of pro-carcinogens by modulating the expression of cytochromes P450 (CYPs), key enzymes in cancer formation (Scalbert, Manach, Morand, Remesy, & Jimenez, 2005). Several studies also showed that polyphenols can induce epigenetic changes leading to the prevention of cancer progression (Thakur, Gupta, & Gupta, 2012).
Polyphenolic molecules are involved also in metastasis inhibition via effects on urokinase and matrix metalloproteinases. In addition, they can reduce angiogenesis by modulating vascular endothelial growth factor expression and receptor phosphorylation (Beltz, Bayer, Moss, & Simet, 2006).
Another area of interest regards the use of dietary polyphenols in combination with conventional therapy or cytotoxic factor to treat drug refractory tumor cells (Pandey & Rizvi, 2009).
6 CHEMOTHERAPEUTIC POTENTIAL OF POLYPHENOLS
In recent years there has been growing interest in the chemopreventive and chemotherapeutic benefit of dietary polyphenols. Several studies conducted on cancer cells and animal models have demonstrated the anti-cancer efficacy of several polyphenolic compounds including curcumin, resveratrol, epigallocatechin-3-gallate, and quercetin (Niedzwiecki, Roomi, Kalinovsky, & Rath, 2016). Moreover, numerous studies indicated that the action mechanism of polyphenols can be further enhanced by using them in combination with other similar or different compounds (Niedzwiecki et al., 2016; Pandey & Rizvi, 2009).
6.1 Curcumin
The beneficial effect of curcumin as a treatment for GBM has been investigated by numerous groups.
Several in vitro and in vivo experiments have demonstrated the inhibitory effect of curcumin on GBM proliferation and invasion. An interesting study by Zhuang et al. (2012) showed that curcumin promotes differentiation of glioma-initiating cells by inducing autophagy. Karmakar and collaborators demonstrated that curcumin induces apoptosis in human glioblastoma T98G cells by activating both receptor-mediated and mitochondria-mediated proteolytic pathways (Karmakar, Banik, Patel, & Ray, 2006). Wang et al. (2017) found that curcumin treatment inhibits cell growth, induces apoptosis and suppress migration and invasion by reducing the expression of cancer signaling pathways (i.e., Notch1 and pAKT) and of neuronal precursor cell-expressed developmentally downregulated 4–1 (NEDD4) protein in human glioma cells. Mukherjee et al. demonstrated that the delivery of a glioblastoma-directed adduct of curcumin into the brain of GBM mice caused tumor remission in 50% of the animals (Mukherjee et al., 2016).
Angelo et al. (2011) investigated the effect of curcumin on HEI-193human schwannoma cells and observed a decreased proliferation and increased apoptosis rate following curcumin treatment. The study suggests that patients with NF2 schwannomas may benefit from curcumin administration. Recently, we report the first experience with curcumin supplementation in NF1 patients. We demonstrated that NF1 patients adopting a Mediterranean diet enriched with 1200 mg/day of curcumin exhibited a reduction in the number and volume of cutaneous neurofibromas. Interestingly, in one patient the reduction in volume (28%) of a large cranial plexiform neurofibroma was showed by Magnetic Resonance Imaging. On the contrary, an unenriched Mediterranean diet or Western diet enriched with curcumin exhibited no significant positive effect (Esposito et al., 2017).
To date, one completed curcumin-based clinical trial for GBM treatment is reported in US National Institute of Health database (http://www.clinicaltrial.gov/; searching for: “Glioblastoma multiforme” and “Curcumin”) (Table 2). No curcumin-based clinical trial for NF2 treatment is present (http://www.clinicaltrial.gov/; searching for: “Neurofibromatosis type 2” and “Curcumin”).
| Polyphenol compound | Study title | Condition | Status | Clinical trial ID |
|---|---|---|---|---|
| Curcumin | Curcumin bioavailability in glioblastoma patients | Patient harboring glioblastoma that will undergo surgery | Completed | NCT01712542 |
- The table reports the polyphenol-based clinical trial for glioblastoma multiforme. Completed: The study has ended normally, and participants are no longer being examined or treated. Data from www.ClinicalTrials.gov.
6.2 Resveratrol
Several experimental studies reported the beneficial effect of resveratrol in reducing tumorigenesis and preventing metastasis (Sato et al., 2013; Xiong et al., 2016). Sato et al demonstrated that resveratrol significantly reduced the self-renewal and tumor-initiating capacity of glioma stem cells derived from GBM patients by the activation of p53/p21 pathway (Sato et al., 2013). Xiong et al. (2016) showed that resveratrol significantly inhibits the migration and invasion of glioblastoma cells by means of wound-healing assays. Recently, Cilibrasi et al. (2017) investigated, for the first time, the role of resveratrol on the Wnt signaling pathway in a glioma stem cell line. The authors observed that resveratrol was able to decrease cell proliferation and motility, increase cell mortality and modulate the Wnt signaling pathway and pivotal activators of epithelial-mesenchymal transition process. Interestingly, Yang and colleagues showed that resveratrol was able to reduce tumorigenicity and enhance the sensitivity of GBM-derived tumor initiating cells to radiotherapy through the signal transducer and activator of transcription 3 (STAT3) pathway in an in vitro and in vivo model of GBM (Yang et al., 2012).
To the best of our knowledge there are no studies in literature investigating the role of resveratrol for NF2.
No clinical trials for GBM or for NF2 are present in US National Institute of Health database (http://www.clinicaltrial.gov/; searching for: “Glioblastoma multiforme” and “Resveratrol” and “Neurofibromatosis type 2” and “Resveratrol”, respectively).
6.3 EGCG
The beneficial effect of EGCG in brain tumors has been explored in several studies. A study conducted by Yokoyama, Hirano, Wakimaru, Sarker, and Kuratsu (2001) documented the inhibitory effect of EGCG in three glioma cell lines; the authors also reported that the modulation of epidermal growth factor-1 (EGF-1) may be involved in the effects of EGCG. In 2006 McLaughlin and collaborators evaluated the effect of EGCG in modulating GBM response to ionizing radiation (IR). The study demonstrated that the radioresistance of GBM may be mediated by a mechanism dependent on Survivin (a protein involved in the modulation of apoptosis) in conjunction with Ras homolog gene family, member A (RhoA) and that the combination of EGCG with radiotherapy seems to improve the efficacy of IR treatments (McLaughlin et al., 2006). An interesting study supporting the efficacy of EGCG in combination with cytotoxic factors was conducted by Chen et al. (2011). The researchers demonstrated that EGCG improved the therapeutic effect of temozolomide, a DNA-damaging agent, in a mouse model of glioblastoma (Chen et al., 2011). In 2015 Zhang et al. (2015) studied the molecular mechanisms by which EGCG inhibits the peculiar characteristics of glioma stem cells and its action in synergy with temozolomide. They showed that EGCG treatment inhibited cell viability, neurosphere formation and cell migration and induced apoptosis by downregulating p-Akt and Bcl-2 proteins. They concluded that EGCG administration alone or in combination with temozolomide may be an effective therapy for GBM treatment. Of note is another study conducted by Sui, Wang, Zhu, & Zhang, 2016, in which the authors showed the effect of EGCG in inducing apoptosis and cell-proliferation inhibition by suppressing JAK2/STAT3 signaling pathway in U251 glioblastoma human cells.
To our knowledge there are no studies on the role of EGCG for NF2 treatment. No clinical trials for GBM or NF2 are present in US National Institute of Health database (http://www.clinicaltrial.gov/; searching for: “Glioblastoma multiforme” and “EGCG” and “Neurofibromatosis type 2” and “EGCG”, respectively).
6.4 Quercetin
The beneficial effect of quercetin on glioblastoma cells has been extensively studied. The anti-proliferative and pro-apoptotic effect of rutin, the glycoside form of quercetin, on human GBM cells was demonstrated by Santos et al. (2011) This intriguing study revealed that rutin is able to decrease the viability and proliferation of GL-15 cell lines, leading to decreased levels of phosphorylated extracellular signal-regulated protein kinases 1 and 2 (ERK1/2), two members of the mitogen-activated protein kinase super family involved in cell proliferation and apoptosis modulation. The authors also demonstrated the capacity of rutin to induce apoptosis and astroglial differentiation in GBM cell cultures (Santos et al., 2011). Apoptosis induction in GBM cells was also studied by Jakubowicz-Gil et al. The authors analyzed the effect of quercetin, both applied alone and in combination with temozolomide, on apoptosis and autophagy. Their results showed that quercetin and temozolomide induced apoptosis, whereas no effect on autophagy induction was observed (Jakubowicz-Gil, Langner, Badziul, Wertel, & Rzeski, 2013). An interesting result supporting the effectiveness of quercetin treatment to activate the apoptotic process was reported by Pozsgai et al. (2013). The authors demonstrated that quercetin, alone or in combination with irradiation treatment, induced apoptosis by the cleavage of caspase-3 and poly [ADP-ribose] polymerase 1 (PARP-1) and the suppression of Akt pathway in GBM cultures (Pozsgai et al., 2013). Zhang et al. (2017) reported that rutin treatment increases the cytotoxicity of temozolomide in GBM cells by inhibiting the autophagic process. Interestingly, subcutaneous and orthotopic xenograft studies showed that combined temozolomide/rutin treatment was able to significantly reduce tumor volumes compared to mice receiving temozolomide or rutin alone. Recently, Liu et al. (2017) reported the inhibitory effect of quercetin on cell proliferation, viability, the cell cycle, migration and invasion in U251 glioblastoma human cells. Quercetin treatment also induced apoptosis by modulating the expression levels of apoptotic genes and arresting the cell cycle. In 2015 we reported that the water extract of Ruta graveolens L., known as rue, induced cell death in U87MG, C6 and U138 glioblastoma cultures. Our results also demonstrated that anti-proliferative effect of rue was mediated through ERK1/2 and AKT activation. Moreover, we observed that rutin, the major component of the Ruta graveolens water extract, failed to cause cell death (Gentile et al., 2015).
To our knowledge there are no studies investigating the role of quercetin for NF2 treatment. No clinical trials for GBM or for NF2 are present in US National Institute of Health database (http://www.clinicaltrial.gov/; searching for: “Glioblastoma multiforme” and “Quercetin” and “Neurofibromatosis type 2” and “Quercetin”, respectively).
The results from in vitro and in vivo model of GBM provide evidence that quercetin shows inhibitory effects on cell proliferation, migration, and invasion; in addition, it is able to activate apoptosis. Taken together these evidence suggest a potential clinical application for GBM treatment.
7 POLYPHENOL BIOAVAILABILITY: A LIMITATION FOR THEIR CLINICAL POTENTIAL
Despite the numerous in vitro and in vivo studies demonstrating the beneficial effects of diet polyphenols for brain disease treatment, their low oral bioavailability and inefficient delivery system are the major issues limiting their application in the functional food and medical fields. In addition to low bioavailability, the diversity in structure and molecular weight of polyphenolic compounds may account for the ambiguous results deriving from pre-clinical and clinical studies (D'Archivio et al., 2010; Lewandowska et al., 2013). Bioavailability is commonly defined as “the fraction of an ingested compound that reaches the systemic circulation and specific sites where it can exert its biological action” (D'Archivio et al., 2007, 2010). The bioavailability of polyphenols is largely dependent upon modifications occurring at the levels of the first-pass metabolism, the degree of permeation in the intestinal tract and their solubility and cell-membrane permeability (Lewandowska et al., 2013; Spagnuolo et al., 2016). According to their solubility and cell-membrane permeability polyphenols are classified in three main groups (i) low solubility and low cell-membrane permeability compounds (e.g., curcumin); (ii) low solubility and high cell-membrane permeability (e.g. resveratrol); and (iii) high solubility and poor cell-membrane permeability (e.g., EGCG) (Hu, Liu, Zhang, & Zeng, 2017). The capacity of polyphenols and their metabolites to cross the blood-brain barrier (BBB), a selective barrier that is permeable to nutrients and actively limits the passage of many substances, also have to be taken also into account. The permeation rate through the BBB of polyphenols is largely dependent on their lipophilicity rate (Vauzour, 2012). Taken together these studies show that only a fraction of polyphenols ingested is able to reach the bloodstream and target tissues. The current challenge for the scientific community is to design an efficient polyphenol delivery system able to enhance their solubility and cell permeation.
In recent year, polymeric nanoparticle-based delivery systems able to encapsulate and preserve polyphenolic compounds have been developed. Among these, food-grade macromolecules seem to be the best delivery system because they are safety, biodegradable, biocompatible, and also bio-functional (Hu et al., 2017). Although several in vitro and in vivo studies have demonstrated the capacity of nanoparticle-delivery systems to improve the polyphenol bioavailability, more efforts are needed to improve their application in medical practice.
8 CONCLUSIONS
Current literature data suggest that polyphenolic compounds may be useful for brain aging-related disorders, such as NDs and adult-onset brain tumors. Although the mechanisms for beneficial polyphenol effects have yet to be completely investigated, it is clear that they involve a decrease in oxidative stress and inflammatory signaling, as well as increased activity in protective signaling pathway. Pre-clinical and clinical evidence shows that polyphenolic compounds are safe and able to interact in synergy with commonly used therapeutic treatment. Because of the low bioavailability of polyphenols, new delivery system have to be explored to reach the therapeutic levels in blood stream and in target organs, such as the brain. A promising solution lies in the use of polymeric nanoparticle-based delivery systems able to encapsulate and preserve polyphenolic compounds, thus enhancing their stability, solubility and cell membrane permeation. However, additional clinical trials are necessary to confirm the efficacy of polyphenol administration for brain-related disorders.
ACKNOWLEDGMENTS
This work was in part supported by the Pennsylvania Department of Health for Sbarro Health Research Organization (SHRO) and The Ken&Ann Douglas, Charitable Foundation Trust. Dr. Tiziana Squillaro has been awarded a fellowship entitled “Nanodispositivi per il rilascio controllato di molecole bioattive a povera farmacocinetica per il trattamento di malattie neurodegenerative” granted by Regione Campania “RIS 3–POR FSE CAMPANIA 2014/2020 Asse III, Obiettivo specifico 14”. We are grateful to Elizabeth McGarry for her critical reading of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.




