The inhibitory effect in Fraxinellone on oxidative stress-induced senescence correlates with AMP-activated protein kinase-dependent autophagy restoration
Abstract
As a natural metabolite of limonoids from Dictamnus dasycarpus, fraxinellone has been reported to be neuroprotective and anti-inflammatory. However, its influence on cellular metabolism remains largely unknown. In the present study, we investigated the effect of fraxinellone on cellular senescence-induced by oxidative stress and the potential mechanism. We found that fraxinellone administration caused growth arrest and certainly repressed the activity of senescence associated β-galactosidase as well as the expression of senescence-associated-genes. Interestingly, this effect of fraxinellone is closely correlated with the restoration of impaired autophagy and the activation of AMPK. Notably, fraxinellone reacts in an AMPK-dependent but mTORC1-independent manner. Together, our study demonstrates for the first time that fraxinellone has the effect on senescence inhibition and AMPK activation, and supports the notion that autophagic mechanism is important for aging prevention. These findings expanded the list of natural compounds and will be potentially utilized for aging decay and/or AMPK activation.
1 INTRODUCTION
Cellular senescence has been proved as a fundamental mechanism of aging and can be triggered by multiple stimuli including oxidative stress (Blagosklonny, 2003; Rubinstein & Kimchi, 2012; Toussaint, Medrano, & Von Zglinicki, 2000; Chen & Amos, 1994; Toussaint et al., 2000). The characters used to feature senescent cells contain cell growth arrest, the upregulated expression of the p53 and p21 genes, the enhanced senescence-associated β-galactosidase staining (SA-β-gal) and the elevated expression of secretory inflammatory cytokines and other factors, known as senescence-associated secretory phenotype (SASP) (Hwang, Yoon, & Kang, 2009; Rodier & Campisi, 2011). Given none of these characters is unique to the senescence state (Collado, Blasco, & Serrano, 2007), concurrent occurrence of the growth arrest together with SA-β-gal staining and SASP has been widely used as reliable markers of cellular senescence.
AMP-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase that plays a key role in regulating cellular energy homeostasis (Xiao et al., 2011). The role of AMPK in preventing ageing/senescence has been suggested in many studies (Apfeld, O'Connor, McDonagh, DiStefano, & Curtis, 2003; Han et al., 2016; Stenesen et al., 2013), and the role of AMPK in aging prevention is generally attributed to its effects on the activation of Sirt1 and FoxO1 (Wang, Liang, & Vanhoutte, 2011; Yun et al., 2014), as well as the suppression of NF-κB and mTORC1 (Salminen, Kaarniranta, & Kauppinen, 2016). Recent studies demonstrate that the anti-senescence effect of AMPK is closely involved in the induction of autophagy, by protecting and restoring autophagy mechanism which is usually harmful to senescent cells (Han et al., 2016; Salminen et al., 2016). It is generally acknowledged that AMPK activates autophagy by suppressing mTORC1 pathway (Mihaylova & Shaw, 2011; Salminen et al., 2016). To investigate the dynamic process of autophagy, monitoring the autophagic flux is usually adopted (Klionsky et al., 2012). Growing evidences indicate that the physiological aging process is associate with the efficiency decline in autophagic degradation, which occurs in autolysosomes stage and largely limits autophagic flux (Levine & Kroemer, 2008; Lipinski et al., 2010; Mijaljica, Prescott, Devenish, 2010).
Fraxinellone is formed by the natural degradation of limonoids isolated from root bark of Dictamnus dasycarpus, the plants widely distribute in North Asia, Europe, and has been used in traditional Chinese medicine to treat rheumatism, jaundice, headache, cough, urticarial, scabies, etc. (Lv et al., 2015; Wang et al., 2013) for centuries. Although a wide range of pharmacological effects of fraxinellone have been reported, including neuroprotection (Yoon, Sung, & Kim, 2008), anti-inflammation (Kim et al., 2009), and vasorelaxation (Yu et al., 1992). However, the effect of fraxinellone on cellular senescence have not been explored. Considering the neuroprotection, anti-inflammation, and vasorelaxation are all beneficial for healthy life span, we speculated that fraxinellone can probably affect the aging process. As the preliminary step to verify this speculation, in the present study, we testified the effects of fraxinellone on the effects of senescence and autophagy in H2O2-treated cellular senescence system and demonstrated its connection with AMPK signaling.
2 MATERIALS AND METHODS
2.1 Reagents
Fraxinellone and berberine were purchased from MUSTBIO technology (Chengdu, China); Compound C was from MCE (Shanghai, China). Hydroxychloroquine (HCQ, Cat#747-36-4), rapamycin (Cat#53123-88-9), and insulin(Cat#11061) were from Sigma–Aldrich (St. Louis, MO). Antibodies against LC3 (Cat#12741, 1:1000), phospho-mTOR (Ser2448) (Cat#5536, 1:1000), phospho-p70S6K (Thr389) (Cat#9206, 1:1000), and p53 (Cat# 2524, 1:1000) were purchased from Cell Signaling Technology (Danvers, MA). Those against phospho-AMPKα (Thr172) (Cat#ab133448, 1:1000), AMPKα (Cat# ab32047, 1:1000), p62 (Cat#3340-1, 1:5000), and LKB1 (Cat#ab63617, 1:1000) were from Abcam (Cambridge, MA). Antibody against β-actin (Cat#20536-1-AP, 1:5000) was from Proteintech (Beijing, China). Alexa Fluor anti-rabbit IgG (H + L) (Cat#1812166, 1:500) was from Thermo Fisher Scientific (San Jose, CA).
2.2 Cell culture and H2O2 treatment
NIH3T3 cells (murine fibroblast line) was purchased from Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences (Shanghai, China), cultured in complete Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS in a humidified atmosphere with 5% CO2 at 37°C. For senescence induction, a modified H2O2 treatment protocol was performed. In brief, cells seeded in 100 mm dishes with 5 × 105 cells/dish density were trypsinized and suspended in phosphate buffer solution (PBS) at 1 × 106 cells/ml density and exposed to 400 μM H2O2 in an Eppendorf tube at 37°C for 45 min. During H2O2 treatment, the tube was turned upside down gently every 5 min. H2O2 treatment was terminated by a 5 min centrifugation at 800 rpm and a washing process. Then the cells were cultured with complete medium. During the adhesion cultivation, the cells accepted different treatments that will be described later in individual figure legends.
2.3 Cell viability assay
H2O2-treated NIH3T3 cells were incubated in complete medium with fraxinellone (0–200 μM) for 3 days. Cell viability was determined by Typan Blue staining and direct cell counting using automated cell counter (Countstar). The results were expressed as mean ± SD of triplicates.
2.4 SA-β-gal staining
Intracellular Senescence-Associated-β-galactosidase (SA-β-Gal) activity was assayed using a SA-β-gal staining kit (Cat#C0602, Beyotime, Beijing, China) according to the manufacturer's instruction, and senescent cells were identified as bluish green stained cells under a phase-contrast microscope. The percentage of SA-β-gal-positive cells in total cells was determined by counting 1,000 cells in eight random fields, for each group. The results were expressed as mean ± SD of triplicates.
2.5 Drug treatments
Fraxinellone was dissolved at a concentration of 1,000 mM in 100% dimethyl sulfoxide (DMSO) as a stock solution, stored at −20°C, and diluted with medium before each experiment. The final DMSO concentration did not exceed 0.1% throughout the study (all the control groups are composed of 0.1% DMSO).
To evaluate the effect of regulator of AMPK activity and autophagy activity on SIPS processing, different chemical reagents were added into the culture medium after H2O2 treatment, with their concentrations indicated as in figure legends. These concentrations were determined by preliminary experiments. The stock solutions of fraxinellone, Compound C, rapamycin, and hydroxychloroquine were dissolved in DMSO, those of metformin, berberine, and insulin, in ddH2O, respectively.
2.6 Transmission electron microscopy
PBS washed cells were collected into 1.5 ml Eppendorf tube using cell scrapers. After centrifuging, 5% Glutaraldehyde was loaded carefully on cell pellets for an overnight fixation at 4°C. Fixed cells were further treated and sliced, and then the images of their ultrastructure were recorded under a transmission electron microscope (H-7650, Japan) in Pathology Department of West China Hospital.
2.7 MRFP-GFP-LC3 expressing cells generation and florescence LC3 puncta assay
NIH3T3 cells were transfected with ptfLC3plasmid (Cat#21074, Addgene, Cambridge, MA), and G418 (Cat#108321-42-2, Sigma–Aldrich) was added for selecting positive cells. As intracellular distribution of LC3 protein was tagged by the fluorescence of RFP and GFP in these cells, images were collected with fluorescent confocal microscope. Quantification of LC3 puncta was performed using Red and Green Puncta Colocalization Macro with ImageJ program, as described (Mizushima, Yoshimori, & Levine, 2010), and the average numbers of LC3 puncta per cell were accounted from the data collected from more than 30 cells. Here, GFP+RFP+puncta are yellow, and GFP-RFP+ puncta are red. Experiments were repeated three times.
2.8 Immunofluorescence
NIH3T3 cells were cultured on glass slides and fixed with cold 4% paraformaldehyde for 10 min, and then permeabilized with 0.5% Triton X-100 in PBS for 10 min on ice. After 30 min of prehybridization in TBST-2% BSA and rinsing twice with PBS, the slides were incubated with p62 antibody in 2% BSA (1:200) for 2 hr at room temperature, rinsed three times with PBS, and incubated with Alexa Fluor anti-rabbit IgG (diluted 1:500 in 2% BSA) for 1 hr at room temperature in the dark. Nuclei were stained with DAPI (Cat#C1002, Beyotime, Beijing, China). Images were taken under confocal fluorescence microscope. The average numbers of p62 puncta per cell were accounted from the data collected from more than 30 cells. Experiments were repeated three times.
2.9 Lentiviral shRNA production
Lentiviral shRNA constructs for Atg5 gene was purchased from GENECHEN (Shanghai, China). The target sequences was: AGAACCATACTATTTGCTT. Lenti-virus was produced by co-transfection of shRNA expression plasmid with plasmids encoding psPAX2 and pMD2.G using Jet PRIME (PolyPlus, Illkirch, France) into 293T cells. Medium was changed 24 hr post-transfection and the medium containing virus was harvested after 72 hr, followed by a centrifugation at 10,000g for 10 min. The supernatant was used to infect NIH3T3 cells in the presence of 5 μg/ml polybrene (Cat#107689, Sigma–Aldrich), or temperately stored at 4°C for less than 3 days. Selection of resistant colonies was initiated 48 hr after using 20 μg/ml puromycin (Cat# A1113803, Life Technologies, Carlsbard, CA).
2.10 DN-AMPKα1 expression cells generation
DN-AMPKα1 expressing cells were generated by transfecting pDN-AMPKα1 plasmid (Jeong et al., 2009) with Jet PRIME. Empty vector-transfected cells were generated in parallel.
2.11 Real-time PCR analysis
Total RNA was isolated from cultured cells using Trizol (Takara, Shiga, Japan) and 1 μg of total RNA was used for reverse transcription by cDNA Synthesis Super Mix (Cat#B24403, Biotool, Shanghai, China). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR Green qPCR Master Mix (Cat#B21203, Biotool, Shanghai, China). PCR reactions were performed in triplicate and the relative amount of cDNA was calculated by the comparative CT method using the 18S ribosomal RNA sequences as control. PCR primer for the p21 gene are 5′-GTGGCCTTGTCGCTGTCTT-3′ (forward) and 5′-GCGCTTGGAGTGATAGAAATCTG-3′ (reverse), for the IL6 gene are 5′-ACTCACCTCTTCAGAACGAATTG-3′ (forward) and 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ (reverse), for IL8 gene are 5′-CTCTTGGCAGCCTTCCTGATTT-3′ (forward) and 5′-CGCAGTGTGGTCCACTCTCAAT-3′ (reverse), for p62 gene are 5′-AGGATGGGGACTTGGTTGC-3′ (forward) and 5′-TCACAGATCACATTGGGGTGC-3′ (reverse) and for 18 s rRNA are 5′-TTGACGGAAGGGCACCACCAG-3′ (forward) and 5′-GCACCACCACCCACGGAATCG-3′ (reverse). Experiments were repeated three times.
2.12 Immunoblotting
Whole cell lysates were collected using ice-cold lysis buffer (50 mM Tris-base, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl, 0.1% SDS, 1% TritonX-100, 1% sodium deoxycholate, 1 mM PMSF, 1 mM DTT, and 1 mM protease inhibitor) and lysis for 30 min following by centrifugation. Protein concentration was determined by BCA method (Cat#CW0014, Cwbio, Beijing, China). And 2.5 × SDS loading buffer were added to the lysates following 10 min of boiling. A total of 30 μg of proteins were loaded on SDS–PAGE gel and separated by electrophoresis, followed by blotting on a PVDF membrane (Cat#IPVH00010, Millipore, Germany). The target proteins were probed by corresponding primary antibodies with optimized conditions and then incubated with the secondary antibody. Immunological signals were surveyed via electrochemical luminescence method, using ECL Plus Western Blotting Reagent Pack (Bio-Rad, Hercules, CA) and Fusion Solo Imaging System (Germany). The band intensities were quantified by FUSION-CAPT analysis Software. Experiments were repeated three times.
2.13 Statistical analysis
Data are expressed as means ± SD from at least three biological replicates. The difference between control and treated samples was examined using the Student's t-test. The difference between multiple groups was examined by one-way ANOVA with Bonferroni post hoc. p < 0.05 is considered to be significant and p < 0.01 was considered highly significant.
3 RESULTS
3.1 Fraxinellone inhibits oxidative stress (H2O2)-induced premature senescence (SIPS)
Using an established cell model for senescence, we first examined the effect of fraxinellone on the viability of NIH3T3 cells (Figures 1a and 1b). As shown in Figure 1b, cell viability did not significantly decrease when incubated with fraxinellone at concentrations up to 200 µM for 72 hr. Then, we evaluated the effect of fraxinellone on senescence by SA-β-gal staining assay and genes expression assay. As shown in Figures 1c and 1d, fraxinellone remarkably reduced the ratio of SA-β-Gal-positive cells, especially when the concentrations range from 50 to 200 µM. Consistently, the expressions of senescence-associated protein of p53 and genes (p21, IL6, and IL8) decreased in the cells with fraxinellone treatment (Figure 1e-h). These results showed that H2O2-induced senescence in NIH3T3 cells was attenuated in the presence of fraxinellone. Given the outcomes of both assays kept unchanged in cells accepting fraxinellone from 50 µM to 200 µM concentrations, we use 50 µM concentration of fraxinellone in following experiments when a single dose is appropriate.

3.2 Fraxinellone inhibits senescence via restoring the H2O2-impaired autophagic flux
As autophagic activity is an emerging concept for ageing/senescence prevention (Rubinsztein, Marino, & Kroemer, 2011), and our previous study revealed that autophagic impairment is a character of H2O2-induced cellular senescence (Han et al., 2016). We next assessed the influence of fraxinellone on autophagic flux. At beginning, we examined the ultrastructure of cells using transmission electron microscopy and found an apparent increase in the number and density of vesicular-like structures, considered as autophagosomes and autolysosomes, distributed in the cytoplasm of senescent cells. In contrary, the number and particular the density of these structures decreased in fraxinellone-treated cells (Figure 2a). Then, we surveyed the status of autophagy flux in cells by three lines of approaches. First, using cells stably expressing tandem mRFP-GFP-LC3, we checked the rate of red-LC3 puncta in cells, which indicates functional autolysosomes and active autophagy flux. We found that the numbers of red LC3 puncta was less than 30% in H2O2-stressed cells while went up to near 50% in fraxinellone treated cells (Figures 2b and 2c). Second, we addressed p62 protein aggregation in cells, as it is a confirmed protein degraded through autolysosomal pathway. In immunoblotting assay, the accumulation of p62 protein in H2O2-treated cells dramatically decreased without a decrease in p62 mRNA level when fraxinellone treatment (Figure 2d–f). In immunofluorescence assay, similar data obtained, as fraxinellone reduced H2O2-evoked p62 protein aggregation (foci-like) in cells (Figures 2g and 2h).
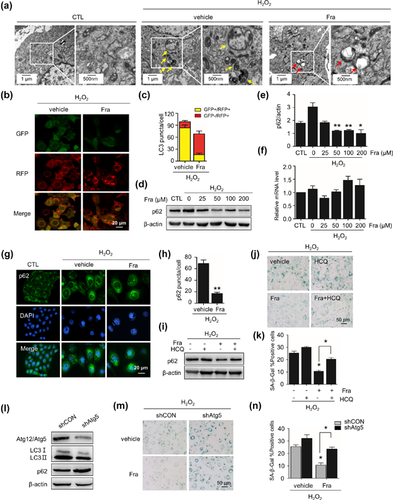
To confirm the alteration of autophagy status caused by fraxinellone, the nature of decrease of p62 protein in fraxinellone-treated cells was further examined through blocking experiments, using a functional inhibitor of lysosomes, hydroxychloroquine (HCQ). As shown in Figure 2i, HCQ treatment did not cause an increase of p62 protein level in H2O2-treated cells, but did it in fraxinellone-treated cells, suggesting that the autolysosomal degradation function largely meliorated in fraxinellone-treated cells. Accordantly, HCQ aggravated H2O2-induced senescence and blunted the protective effect of fraxinellone on senescence (Figures 2j and 2k). The significance of autophagy flux on the effect of fraxinellone was further evidenced by using an Atg5 knockdown cell population with autophagy flux impairment (Figure 2l). As shown in Figure 2m, unlike in normal cells, the effect of fraxinellone on senescence protection decreased in this cell population (Figures 2m and 2n).
Taken together, these findings reveal that fraxinellone can improve the impaired autophagic flux in H2O2-treated cells, which closely associated with the inhibitory effect of fraxinellone on senescence.
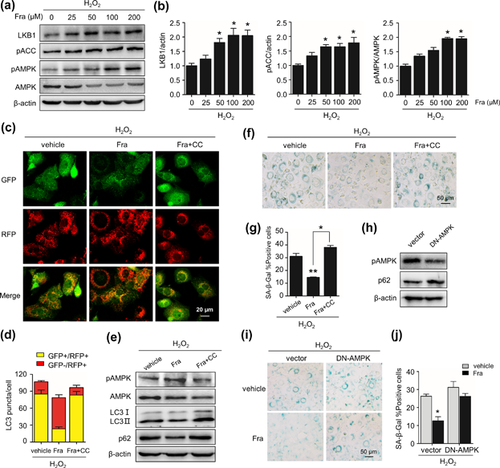
3.3 AMPK mediates fraxinellone-induced autophagy restoration and senescence prevention
Many studies have revealed that the decline in AMPK activity is associated with ageing and senescence (Han et al., 2016; Salminen et al., 2016). Therefore, we examined the association of AMPK activity and the effect of fraxinellone on senescence and autophagy. In H2O2-treated cells, fraxinellone treatment increased the levels of LKB1 protein, a kinase phosphorylating AMPK, and the phosphorylation of both AMPK alpha subunit (Thr172) and ACC (Ser79), a target of AMPK kinase (Figures 3a and 3b). In addition, fraxinellone-prompted phosphorylation of AMPK was blocked by Compound C (CC), a AMPK specific chemical inhibitor (Figure 3e). Importantly, CC blocked the effect of fraxinellone on autophagy restoration, signing as the decrease in red LC3 puncta and the increase in yellow LC3 puncta, accompanied with elevated level of p62 and LC3 proteins in cells treated with fraxinellone and CC combined compared to fraxinellone alone (Figure 3c-e). Simultaneously, the effect of fraxinellone on senescence prevention was blunted by the existence of CC, as indicated by the remarkable increase in SA-β-Gal-positive cells compared with fraxinellone-treated alone (Figures 3d and 3e). Unsurprisingly, overexpression of dominant negative AMPK (DN-AMPK) increased the SA-β-Gal-positive rate in the H2O2-treated cells compared with the cells transfected with the empty vector. Moreover, the anti-senescence effect of fraxinellone was weakened in these cells (Figure 3h–j). These results demonstrate that the effect of fraxinellone on autophagy restoration and senescence prevention is AMPK activation-dependent.
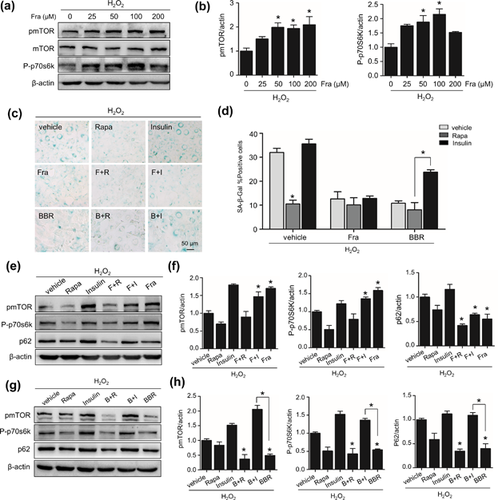
3.4 Fraxinellone ameliorates senescence independent of mTOR
Given mTOR inhibition is a general downstream of AMPK activation and upstream of autophagy activation, we next evaluated whether fraxinellone affects the activity of mTOR pathway. The phosphorylation of mTORC1 and p70S6K, a target protein of mTORC1, was examined. As shown in Figures 4a and 4b, Fraxinellone significantly increased the protein level of pmTOR1 and P-p70S6K in senescent cells with a dose-dependent manner. This result is contrary to that seen when using berberine, an AMPK activator, reduced the phosphorylation of mTORC1 and p70S6K (Figures 4g and 4h). To ensure this unexpected interaction of fraxinellone with mTORC1 activity and clear its significance for the effect of fraxinellone on autophagy and senescence, we tested impacts of mTOR regulators on the function of fraxinellone. As shown in Figures 4g and 4h, mTOR activator insulin restored mTOR and p70S6K phosphorylation which suppressed by berberine, but did not affect the phosporylation of mTOR and p70S6K elevated by fraxinellone (Figures 4e and 4f). These results indicate that fraxinellone is an activator of mTOR rather than an inhibitor likes berberine. Accordant results were obtained by examining the influence of insulin and rapamycin on autophagy flux. The insulin did not affect the relive of p62 accumulation in fraxinellone group but apparently blocked it in berberine group, meanwhile the impact of rapamycin was more distinct in fraxinellone group. Aspect to the senescence inhibition, insulin had no influence on the effect of fraxinellone, while weakened the effect of berberine (Figures 4c and 4d). These results demonstrate that fraxinellone activate mTOR activity. Meanwhile, this mTORC1 activation dose not correlate with fraxinellone-induced autophagy restoration and senescence prevention, in other words, the effect of fraxinellone is mTORC1 activity-independent.
4 DISCUSSION
Though limonoids isolated from Dictamnus dasycarpus possess various bioactivities, the impact of these molecules on aging protection is unknown but interesting. Based on the present study, we demonstrate that fraxinellone can inhibit oxidative stress-induced senescence, and this effect is largely mediated by the activation of AMPK and subsequent autophagy induction. Furthermore, we find that the fraxinellone-evoked autophagy was not dependent on mTORC1 pathway. This study displays the potential of fraxinellone as an anti-aging drug and a mTORC1-independent inducer of autophagy.
Previous information suggested the healthy beneficial pharmacologicaction of fraxinellone (Kim et al., 2009; Wu et al., 2014; Yoon et al., 2008), indicating that fraxinellone can inhibit glutamate-induced neurotoxicity (Yoon et al., 2008), can suppress inflammation induced by LPS through negatively regulating NF-κB (Kim et al., 2009) and can selectively block voltage-dependent Ca2+ channels (Yu et al., 1992). To the best of our knowledge, the present study is the first to report that demonstrates the anti-senescence effect of fraxinellone and elucidates the molecular basis for this effect.
The relationship between aging and autophagy has been established. For example, the expression of the autophagic-related genes is strongly associated with lifespan regulation in flies and worms (Lionaki & Tavernarakis, 2013; Simonsen et al., 2008) and the activation of autophagy evoked by mTOR inhibitor rapamaycin extends lifespan in Drosophila (Kapahi et al., 2004), in addition, our previous study demonstrated that autophagy impairment is positively correlated with senescence development (Tai et al., 2017). For this reason, the restoration of autophagic flux is a reasonable consideration for anti-aging/senescence therapeutics. In this study, several lines of evidence confirmed the effect of fraxinellone on autophagy activation and the causal connection between this effect and the action of fraxinellone on senescence protection, herein being supportive for the theory that autophagy mechanism is necessary for senescence prevention.
Emerging evidences indicate that AMPK plays a key role in the regulation of aging/senescence and autophagy (Apfeld et al., 2003; Ido et al., 2015; Stenesen et al., 2013). Regarding to aging, it is clear that AMPK activator metformin can prevent oxidative stress-induced senescence and AICAR can extend the lifespan of flies and worms (Cabreiro et al., 2013; Ido et al., 2015; Stenesen et al., 2013). As to autophagy, one study showed that under energy stress conditions, LKB1-AMPK pathway facilitates autophagy activation (Liang et al., 2007). Our data presented in the present study is accordant, because fraxinellone not only upregulates LKB1-AMPK pathway in senescent cells but its effects on autophagy restoration and senescence protection can be abolished by AMPK inhibitor. Our observation based on DN-AMPKα overexpression experiments also strengthened the notion that AMPK-dependent autophagy restoration is an important and perspective mechanism for senescence prevention.
One of the unstated findings is the AMPK-dependency but mTOR-independency of the effect of fraxinellone. As reported, AMPK up-regulates autophagy via its role in mTOR inhibition, thereby releases the functions of ULK1 and TFEB, those are key proteins necessary for autophagy initiation and mostly for autolysosomal degradation, respectively (Alers, Löffler, Wesselborg, & Stork, 2012; Roczniak-Ferguson et al., 2012). In addition, many studies have proved the impact of IGF-mTOR pathway in lifespan regulation. For example, the reduction of insulin/IGF-1 signaling promotes longevity in Caenorhabditis elegans (Tullet et al., 2014) and dietary restriction and its inhibitory role in IGF-mTOR signaling extends the lifespan of mice (Mair & Dillin, 2008; Masoro, 2005; Salminen et al., 2016). In a previous study, we revealed that two AMPK activators, metformin and berberine, restore autophagic flux in senescent cells via AMPK-mTORC1 pathway. As shown in the present study, however, mTORC1 seems not involved in autophagy induction in response to fraxinellone treatment, as fraxinellone significantly increased, but not decreased, the phosphorylation levels of mTORC1 and p70S6K in senescent cells. In addition, two mTOR regulators, rapamycin and insulin, have no significant influence on the effect of fraxinellone on senescence prevention and autophagic flux restoration. Herein, we consider that the molecular basis of the effect of fraxinellone probably differs from other AMPK activators, and the relationship between AMPK and mTOR may be more complicated than what we known at this moment.
In summary, using a H2O2-induced premature senescence model, the effect of fraxinellone on senescence repression got explored and an AMPK activation- and autophagy restoration-involved mechanism was demonstrated. Although intensive investigation is needed to elucidate the details for molecular mechanism, our findings suggests that fraxinellone may serve as a new anti-aging drug or novel mTORC1 independent inducer of autophagy.
ACKNOWLEDGMENTS
This work was supported by National Key Research and Development Program (Grant Number 2016YFC1200203) and National Natural Science Foundation of China (Grant Number 81273224). We thank Dr. Yuquan Wei and Canhua Huang for continuous supports, and Dr. Jie Zhang, Ping Lin, and Xiujie Wang for all around convenience.




