Multifaceted role of IL-21 in rheumatoid arthritis: Current understanding and future perspectives
Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune inflammatory disorder designated with hyperplastic synovium, bone destruction and cartilage degradation. Current therapies involve targeting major cytokines and inflammatory mediators involved in RA to alleviate the pain and provide a temporary relief. Interleukin 21 (IL-21), a recently identified cytokine is known to possess a versatile role in modulating the cells of the RA synovium. Over the past decade, the pleiotropic role of IL-21 in RA pathogenesis has been implicated in several aspects. T helper 17 (Th17) and follicular T helper cells (Tfh), being the key immunomodulators of the RA synovium secrete high amounts of IL-21 during disease progression. Several studies have provided experimental evidences elucidating the multifaceted role of IL-21 in RA disease progression. IL-21 has the potential to activate T cells, B cells, monocytes/macrophages and synovial fibroblasts in RA pathogenesis through activation of JAK-STAT, MAPK and PI3K/Akt signaling pathways. Till date, therapies targeting Th17 cells and its inflammatory cytokines have been under investigation and are subjected to various clinical trials. This review showcases the role of IL-21 in RA pathogenesis and recent reports implicating its function in various immune cells, major signaling pathways, and in promoting osteoclastogenesis.
1 INTRODUCTION
Rheumatoid arthritis (RA) is a systemic, inflammatory autoimmune disorder with numerous manifestations caused due to intricate chain of events (Trouw, Pickering, & Blom, 2017). Cells of the leukocyte lineage such as: Monocytes-macrophages, neutrophils, mastocytes, and subsets of T & B cells majorly contribute to the pathogenesis of RA by secreting various cytokines and chemokines (Shikhagaie, Germar, Bal, Ros, & Spits, 2017). Tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and interleukin 17 (IL-17) are the key mediators of RA pathogenesis (McInnes & Schett, 2017). Inhibiting the effect of these cytokines has been the target of interest, mainly aimed at alleviating the inflammation in the synovium (Burmester & Pope, 2017). Monoclonal antibodies (mAbs) against TNFα (Infliximab, etanercept, and adalimumab) and IL-1β (anakinra and canakinumab) have been used in recent years in suppressing the inflammation at the joint space of RA patients (Cohen & Kay, 2017; Szondy & Pallai, 2017). Howbeit, due to several side effects after prolonged usage and ineffectiveness in curing the disease, the quest for effective and a safer therapy for RA are still an unsolved mystery.
Interleukin 21 (IL-21), a dual role cytokine discovered during the year 2000 shares similar homology with IL-2 family of cytokines (IL-2, IL-4, and IL-15) (Leonard & Spolski, 2005). IL-21 interacts with gamma chain (γc) of IL-21 receptor (IL-21R) expressed in various immune cells of the leukocyte lineage. IL-21 is predominantly secreted by T helper 17 (Th17) follicular T helper (Tfh) and natural killer T (NKT) cells (Varricchi et al., 2016). In normal physiology, IL-21 helps in promoting NK cells activation, CD4+ T cell effector function and B cell proliferation to plasma cells for antibody production (Davis et al., 2007; Spolski & Leonard, 2008). IL-21/IL-21R have been shown to mediate various autoimmune disorders such as Sjögren's syndrome, systemic lupus erythematosus (SLE), multiple sclerosis and type I diabetes (Ferreira et al., 2015; Ghalamfarsa et al., 2016; Huang et al., 2016; Kwok et al., 2015). In recent years, IL-21 has been found to be a key player in RA pathogenesis and progression (Kwok et al., 2012; Xiang, Jin, et al., 2016; Xing, Yang, et al., 2016). In RA pathogenesis, IL-21 receptor (IL-21R) is highly expressed on CD4+ T cell subsets, macrophages, dendritic cells and synovial fibroblasts (Lubberts, 2010). These immune cell subtypes recognizes the IL-21 in the microenvironment to carry out several intricate chains of events (Andersson et al., 2015). IL-21 has been implicated to be an important target in RA therapy and several studies have been put forth to substantiate its role through activation of signaling pathways and in promoting inflammatory condition (Jang et al., 2009; Young et al., 2007). Various signaling pathways, including MAPK, PI3K/Akt and JAK-STAT have recently been identified to be initiated and controlled through IL-21/IL-21R interaction in RA disease models (Kim, Kim, Kim, Cho, & Lee, 2015; Xing, Zhang, et al., 2016). Recent reports have also identified that IL-21 promotes bone erosion by contributing to osteoclasts differentiation either in a RANKL dependant/independent manner (Kim et al., 2015; Kwok et al., 2012; Xing, Zhang, et al., 2016). All these reports collectively suggest that IL-21 plays a major role in RA progression and makes it a key cytokine for targeted therapy. In this review, we address the implications and recent reports of IL-21 in mediating the pathogenesis of RA with relation to immune cells, signaling pathways, and in promoting osteoclastogenesis.
1.1 Action of IL-21 on immune cells in RA pathogenesis
IL-21 plays a central role in the activation and proliferation of various immune cells including Th17 cells, Tfh, B cells, FLS and macrophages involved in RA pathogenesis. In conjunction with other inflammatory cytokines revolving around the RA synovium, IL-21 also plays a pleiotropic role in mediating several inflammatory processes. Recent reports substantiating its effect on various immune cells involved in RA pathogenesis and progression will be discussed in the subsequent sections.
1.2 T helper 17 (Th17) cells
T helper 17 (Th17) cells are a subset of T lymphocytes, which play a central role in RA pathogenesis through the secretion of various inflammatory cytokines (TNFα, IL-1β, and IL-6), effector cytokines (IL-17, IL-21, IL-22, and IL-26), and chemokines (CCL20 and GM-CSF) (Lubberts, 2010; Zhou et al., 2007). Various studies have reported the role of these effector molecules, but less focus has been showcased on the newly identified cytokines (IL-21, IL-22, and IL-26) (Tian & Zajac, 2016). Parrish-Novak et al. (2000) initially reported the role of IL-21 in T cell proliferation and NKT cells maturation. Th17 cells secrete large amounts of IL-21 when triggered synergistically by transforming growth factor beta (TGF-β) and IL-6 secreted by various immune cells infiltrated in the synovium (Korn et al., 2007; Nurieva et al., 2007; Parrish-Novak, Foster, Holly, & Clegg, 2002). IL-21 acts as a co-stimulatory molecule through promoting CD4+ T cell activation, survival, uncontrolled proliferation, and pro-inflammatory cytokines secretion in RA disease condition (Li, Shen, Kong, & Liu, 2006). IL-21 amplifies the inflammatory proliferation of Th17 cells in the synovium with further stimulation from IL-23, which is majorly secreted from RA-FLS cells (Korn, Bettelli, & Oukka, 2009). Rasmussen et al. (2010) provided evidences for the involvement of IL-21 in increasing the number of CD4+ T cells in the synovium of RA patients, supporting the pathogenic importance of this cytokine. The current understanding thus substantiates the role of IL-21 in various immune cell processes. However, their role in RA pathogenesis is poorly understood and still requires much more understanding through molecular mechanistic studies.
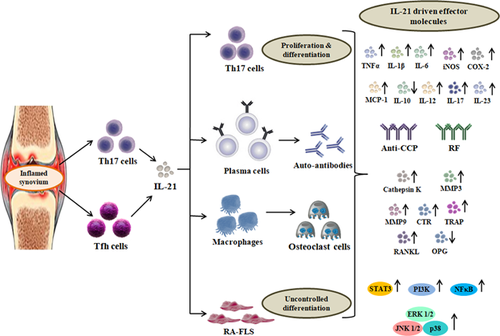
So far studies revealing the effect of IL-21 on Th17 cell function have been seen in regard with several aspects. For instance, Niu et al. (2010) reported that IL-21 potentially promoted Th17 cells differentiation and proliferation through suppression of Foxp3 expression and upregulation of RAR related receptor (RORC). Furthermore, they proved that Th17 cells present in the synovial fluid of RA patients produced high amounts of IL-21 and IL-17, providing evidence for the co-stimulatory nature of IL-21 (Niu et al., 2010). Another study carried out by Marijnissen et al. (2014) explored that IL-21R deficiency in adjuvant induced arthritis (AIA) and Streptococcus cell wall (SCW) arthritis showed reduced inflammation and joint destruction accompanied by decreased IL-17+ IFN-γ+ T cells in IL-21−/−mice. This study elucidates the role of IL-21 in Th17 cell activity in RA pathogenesis and mark an essential note on how it mediates the disease condition (Marijnissen et al., 2014). A step further, recently a study conducted by Roeleveld et al. (2017) depicted the role of IL-21 in promoting Th17 cell differentiation and established a cumulative effect of IL-6 and IL-21 mediated Th17 cell proliferation in collagen induced arthritis (CIA) model of RA. Furthermore, they proved that blockade of IL-6/IL-21 (anti-IL-6R and sIL-21RFc) synergistically reduced the disease development, Th17 subset differentiation and proliferation (Roeleveld et al., 2017). Conclusively, these reports provide new insights with the self proclaimed co-stimulatory effects of IL-21 cytokine on Th17 cells in RA pathogenesis (Figure 1).
1.3 Follicular T helper cells (Tfh) and B cells
Follicular T helper cells (Tfh) are the major T cell lineage which promotes B cells maturation and proliferation to produce antibodies for regulating humoral immune response (Qi, 2016; Walters & Vinuesa, 2016). During a disease condition, Tfh cells produce large amounts of IL-21 for B cell differentiation to mediate pathogenic clearance after which it is subdued (Read, Powell, & Oestreich, 2016). Bryant et al. (2007) reported that huge quantities of immunoglobulin (Ig) subtypes (IgG, IgM, and IgA) were mediated through the presence of IL-21 as a precursor for Tfh cell activation. On the contrary, during an autoimmune condition such as RA, there is uncontrolled differentiation of Tfh cells, resulting in B cell activation and production of auto-antibodies such as anti-CCP (Carbone et al., 2013). Several studies provide evidence for the role of IL-21 producing Tfh cells in RA pathogenesis at the molecular level.
The levels of IL-21 in the serum of RA patients are a direct indicator of B cell activation (Gottenberg et al., 2012). To elucidate this phenomenon, Liu et al. (2012) provided evidences that RA patients expressed high levels of IL-21 in the serum which correlated with DAS28 scoring and anti-CCP antibody with increased frequency of Tfh cells. Exogenous treatment of IL-21 on in vitro cultures of Tfh cells from RA patients supported B cell activation/proliferation and secretion of auto-antibodies via IL-21/IL-21R pathway (Liu et al., 2012). A study conducted by Ma et al. (2012) on Tfh cells from RA patients and healthy individuals established the role of IL-21 via promoting the expression of B cell lymphoma 6 (Bcl-6) transcription factor expression. Furthermore, it was found that the frequency of Tfh cells was increased with elevated levels of IL-21 and anti-CCP antibody, thus elucidating the role of IL-21 in promoting B cell proliferation (Ma et al., 2012). Wang et al. (2013) measured the population of B cells, Tfh, and IL-21 level in RA patients. Tfh specific markers such as: programmed cell death protein 1 (PD-1) and inducible T-cell costimulator (ICOS) were at higher levels in RA patients compared to that of healthy controls, making it an important prognostic marker for detecting RA (Wang et al., 2013). Another report provided by Block and Huang, (2013) depicted that IL-21 deficient T cells did not promote the auto-antibody production and were dependant on normal Tfh proliferation independent of IL-21. Subsequently, they proved that IL-21 action is required on B cells rather than Tfh cells for promoting autoimmune arthritis condition (Block & Huang, 2013). Interestingly, another study proved that IL-21 has the potential to enhance Tfh and B cell population; subsequently reducing the follicular regulatory T (Tfr) cells in a BDX2 mice model of arthritis. This study proves that IL-21 secretion is a key factor in balancing the Tfh and Tfr cell subsets (Ding et al., 2014). Furthermore, Meguro et al. (2015) proposed that Bcl-3 promoted IL-21 producing Tfh cells in RA patients and the levels decreased after silencing the Bcl-3 expression. Apparently, another report predicted that IL-21R expressing B cells promoted elevated levels of IgM and IgG auto-antibodies in collagen induced model of arthritis (CIA). A knockout model of IL-21R repressed the disease model of CIA with reduction in number of IL-21R+ B cells (Sakuraba et al., 2016). On the contrary, Ota et al. (2016) proved that the activation of B cells via BCR and CD40 resulted in RANKL production and was suppressed after treatment with IL-21. Recently it was reported that Tfh cells promoted B cell proliferation in RA through SLAMF5 interaction and altered the expression levels of Bcl-6 and PR domain containing zinc finger protein 1 (BLIMP1) in an IL-21 dependant manner (Rao et al., 2017). Conclusively, all these reports elucidate the potential of IL-21 producing Tfh cells on proliferation and maturation of B cells for autoantibody production in RA disease condition (Figure 1).
1.4 Monocytes/macrophages and osteoclastogenesis
Monocytes/macrophages being the sentinels of the immune system perform several functions such as antigen processing/presentation and phagocytic clearance of pathogens entering into the host system. During a disease condition such as RA, macrophages in the joint space secrete large amounts of inflammatory cytokines, chemokines, growth factors, and co-stimulatory molecules leading to the infiltration of other immune cells (Cuda, Pope, & Perlman, 2016; Roberts, Dickinson, & Taams, 2015). Macrophages express a wide variety of receptors, including IL-21R for interacting with the released cytokines in the microenvironment to mediate various inflammatory processes during RA pathogenesis (McInnes, Buckley, & Isaacs, 2016).
Jungel et al. (2004) initially elucidated that synovial macrophages isolated from RA patients expressed large amounts of IL-21R. Furthermore, T cells isolated from synovial fluid and peripheral blood of RA patients stimulated with IL-21 showed elevated levels of Th1 cytokines. Subsequently, another report proved that the levels of inflammatory cytokine production were alleviated when IL-21R was blocked with IL-21R/Fc fusion protein (Andersson, Feldmann, & Brennan, 2008). These initial reports showed that macrophages possess the potential to recognize the IL-21 revolving around the synovium to mediate various inflammatory responses during RA pathogenesis.
Monocytes/macrophages in the presence of monocyte colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa B ligand (RANKL) results in uncontrolled osteoclast differentiation leading to bone erosion during RA disease progression (Sims & Romas, 2015; Wicks & Roberts, 2016). RANKL is predominantly secreted by RA-FLS under the stimulation of various pro-inflammatory cytokines secreted from the inflammatory Th17 cells (Lavocat, Osta, & Miossec, 2016; Tsushima, Okazaki, Ishihara, Ushijima, & Iwamoto, 2015). Studies have implicated that Th17 cells predominantly secrete these cytokines under inflammatory micro-environment provoked through various immune cells (Kim & Moudgil, 2017). Inflammatory Th17 cells predominantly secrete IL-21 as discussed previously, have the costimulatory potential by itself and promote the production of inflammatory cytokines responsible to mediate osteoclastogenesis.
IL-21 by itself possesses the property to promote osteoclastogenesis in the presence or absence of RANKL. Kwok et al. (2012) demonstrated that IL-21 synergistically with minimal levels of RANKL and M-CSF promoted osteoclast differentiation of CIA bone marrow macrophages when co-cultured with FLS from RA patients. Another study carried out by Kim et al. (2015) using PBMCs to study osteoclast differentiation showed that IL-21 mediated osteoclastogenesis with an elevated RANKL expression in RA-FLS. Recently, Xing, Zhang, et al. (2016) demonstrated that IL-21 induced osteoclastogenesis in RAW 264.7 macrophage cell line independent of RANKL and predicted that it was mediated through activation of PI3K/Akt signaling pathway. These reports thus prove the pro-inflammatory potential of IL-21 in macrophages and in promoting RANKL dependant/independent, uncontrolled differentiation of monocytes/macrophages to osteoclasts resulting in bone erosion (Figure 1).
1.5 Fibroblast-like synoviocytes (FLS)
Fibroblast-like synoviocytes (FLS) also called as type B synoviocytes are a unique population of cells present in the lining of the synovial region (Lefevre, Meier, Neumann, & Muller-Ladner, 2015). The normal functioning of the FLS cells is to assure the structure and integrity of the joints through regulation of synovial fluid and ECM of the joint region (Bustamante, Garcia-Carbonell, Whisenant, & Guma, 2017). However, in the case of RA, FLS cells display pleomorphic changes and undergo tumor like proliferation, giving it an aggressive phenotype leading to inflammation, joint destruction, and pannus formation (Turner & Filer, 2015). Under inflammatory micro-environment, RA-FLS majorly secrete inflammatory cytokines (TNFα, IL-1β, and IL-6), inflammatory mediators (COX-2 and iNOS) and effector molecules (RANKL, GM-CSF, and IL-23) (Asif Amin, Fox, & Ruth, 2017; Bustamante et al., 2017). Various cytokines synergistically promote the proliferation and survival of RA-FLS cells in the synovium (Rockel & Kapoor, 2016). However, the role of IL-21 in mediating FLS proliferation and survival in RA pathogenesis is in its preliminary phases.
In an early report provided by Jungel et al. (2004) showed that RA-FLS proliferation was upregulated in the presence of IL-21, independent of major pro-inflammatory cytokines namely: TNFα and IL-1β which negatively impacted on bone destruction and cartilage degradation. Kwok et al. (2012) elucidated that IL-21 induced the production of RANKL in FLS cells isolated from RA patients and promoted osteoclastogenesis. A recent study showcased the role of IL-21 in promoting the production of TNFα and IL-6 in RA-FLS mediated through MAPK, STAT3, and PI3K/Akt signaling cascade pathways (Xing, Yang, et al., 2016). Similarly, another study carried out by Xing, Jin, et al. (2016) elucidated the pro-inflammatory action of IL-21 on RA-FLS by promoting the secretion of various MMPs (2, 3, 9, and 13) and activation of signaling pathways including: STAT3, ERK 1/2, and PI3K/Akt. Furthermore, a recent report provided by Lebre et al. (2017) substantiated the pro-inflammatory effect of IL-21 through upregulation of MMP-1 and MMP-3. This study further showed that blocking IL-21 producing CD4+ T cells coupled with anti-TNFα therapy suppressed the proliferation of RA-FLS cells (Lebre et al., 2017). Conclusively, all these reports suggest that IL-21 is important for RA-FLS proliferation to mediate the chain of events resulting in joint destruction and cartilage degradation (Figure 1).
1.6 IL-21 dependant signaling pathways in RA
In RA pathogenesis, IL-21 is recognized by the γ chain of IL-21R expressed on the surfaces of Th17 cells, Tfh, FLS and macrophages, which activates several signaling pathways involved in cell survival, proliferation and in mediating inflammation. Extensive studies carried out in the recent years have provided evidences for the involvement of IL-21 in promoting several signaling cascade mechanisms which will be discussed in the upcoming sections.
1.7 JAK-STAT pathway
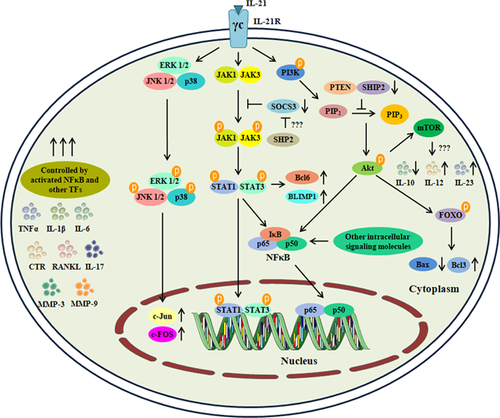
Signal transducer and activator of transcription 3 (STAT-3) is a transcription factor designated to play a pivotal role in various cellular processes such as: initiating immune response, cell survival, and proliferation (Hillmer, Zhang, Li, & Watowich, 2016; Vogel, Milner, & Cooper, 2015). In normal physiological condition, STAT-3 is dormant in the cytosol. During RA pathogenesis, pro-inflammatory cytokines such as: TNFα, IL-6, IL-17, and IL-23 activate STAT-3 through the phosphorylation of Janus kinases (JAKs). After activation, STAT-3 forms dimers and translocates into the nucleus leading to upregulation of inflammatory cytokines and mediators (Cacalano, 2016; Weyand, Zeisbrich, & Goronzy, 2017). STAT-3 plays an essential role in Th17 cells differentiation and proliferation. Recent evidences have proved that in RA disease condition, Th17 cells survival is driven through STAT-3 activation in an IL-21/IL-21R dependant manner (Ryu et al., 2015). IL-21 is designated to have the potential to activate Janus kinase 1 (JAK1) and signal transducer and activator of transcription 3 (STAT-3) (Spolski & Leonard, 2014; Yuan & Wang, 2016). Preliminary reports provided by Wei, Laurence, Elias, and O'Shea, (2007) elucidated that IL-21 promotes the proliferation of Th17 cells and induces the production of IL-17 in a STAT-3 dependant manner.
Diehl et al. (2008) initially predicted that activation of STAT signaling was mediated through IL-21 dependant manner in B cells which expressed elevated levels of Bcl-6 and BLIMP1. Similarly, IL-21 treated B cells proliferated in a STAT-3 dependant manner thereby promoting elevated humoral immune response (Diehl, Schmidlin, Nagasawa, Blom, & Spits, 2012).
IL-21 also promotes the secretion of pro-inflammatory cytokines such as IL-1β in dendritic cells through STAT-3 dependant mechanism (Wan et al., 2015). In par with these reports, a recent study carried out by Ryu et al. (2015) proved that blockade of IL-21R with rhIL-21R-Fc effectively suppressed the STAT-3 dependant inflammatory process in Th17 cells thereby suppressing the B cells mediated antibody production in CIA model of arthritis. They further provided a wider role of IL-21 in promoting Tfh cells development with enhanced B cell proliferation via the activation of STAT-3 pathway (Ryu et al., 2015). Furthermore, Meguro et al. (2015) proved that IL-21 producing Tfh cells proliferated via Bcl-3 over expression through STAT-3 activation. Silencing Bcl-3 suppressed the action of IL-21 mediated cellular proliferation of Tfh cells in RA patients through the inhibition of STAT-3 signaling (Meguro et al., 2015). When B cells were co-stimulated with IL-21, CD-40 and BCR, phosphorylation of STAT-1 and STAT-3 takes place via activation of Bruton's tyrosine kinase (Btr). The level of phospho-Btr thus directly correlates with the levels of RF-positive cells (Wang et al., 2015). Similarly, Xing, Yang, et al. (2016) provided evidences that IL-21 induced proliferation of RA-FLS and secretion of TNFα and IL-6 through the activation of STAT-3 signaling pathway. Subsequently, another report elucidated a novel mechanism through which IL-21 was able to activate STAT-3 and increased MMP-3/9 levels (Xing, Jin, et al., 2016). This study utilized the STAT-3 inhibitor STA-21, which preferentially inhibited STAT-3 activation and thereby blocking IL-21 action owing to the conclusion that it serves as a therapeutic target for disease attenuation (Xing, Jin, et al., 2016). Lin et al. (2016) further unravelled the downstream signaling of IL-21 mediated STAT-3 activation through therapeutic targeting using SM934 (artemisinin analogue). These findings substantiated that the derivative was able to suppress IL-21 producing Tfh and Th17 cells thereby suppressing their proliferation through STAT-3 signaling and antibody dependant immune feedback (Lin et al., 2016). Overall from these reports, it is evident that IL-21 possesses the potential to activate STAT-3 signaling in immune cells involved in RA pathogenesis (Figure 2).
1.8 MAPK signaling pathway
Mitogen-activated protein kinases (MAPKs) are a family of protein kinases (specific for serine, threonine, and tyrosine residues) that play a crucial role in cell division, cellular survival/proliferation and apoptosis (Khorasanizadeh, Eskian, Gelfand, & Rezaei, 2017; Segales, Perdiguero, & Munoz-Canoves, 2016). Cellular stress and mitogenic stimuli serves as the major activators of MAPK signaling pathway (Ruiz, Coderre, Allen, & Des Rosiers, 2017). The activation of MAPK is regulated through JNK, ERK and p38 protein kinases. MAPKs play a crucial role in controlling the gene expression of pro-inflammatory molecules during disease progression (Broome & Datta, 2016; Gadina, Gazaniga, Vian, & Furumoto, 2017). In a systemic disease like RA, MAPKs are potentially driven to mediate the cellular proliferation and regulation of various inflammatory cytokines revolving around the synovial lining. MAPKs are regulated through multiple inflammatory cytokines and mediators circulating in the synovium (Corre, Paris, & Huot, 2017; Malemud, 2017). One such mechanism which is recently been known to promote the MAPK pathway activation is through the IL-21/IL-21R dependant interaction in the cells.
An early report provided evidences that IL-2 family of cytokines, which includes IL-21, activated MAPK signaling pathway, and was negatively regulated through dual specificity phosphatase 5 (DUSP5) (Kovanen et al., 2003). The role of IL-2 family of cytokines established a relationship between T cells survival and maintaining the cell survival/proliferation through MAPK signaling (Benczik & Gaffen, 2004). These reports were further proved by Zeng et al. (2007), which utilized various members of the IL-2 cytokines and their effect on isolated spleenocytes and Ba/F3 cells. This study proved that IL-21 was able to activate MAPK signaling pathway and mediate cellular proliferation/survival (Zeng et al., 2007).
To substantiate its role in RA pathogenesis, very few reports have been put forth to elucidate the mechanism behind IL-21 mediated MAPK activation. For instance, Xing, Yang, et al. (2016) mechanistically proved that IL-21 was able to activate ERK 1/2, which are essential for p38MAPK regulation. Similarly, Xing, Jin, et al. (2016) predicted the same outcome in RA-FLS cells from patients and proved that p38MAPK expression was found upregulated in comparison with osteoarthritic (OA) control cells. In par with these outcomes, IL-21 has recently been identified to activate MAPK signaling in fibroblasts and human derived peripheral blood monocytes (Vallieres & Girard, 2017; Wang et al., 2017). These evidences collectively provide insights into IL-21 mediated MAPK signaling pathway in immune cells of the RA synovium (Figure 2).
1.9 PI3K/Akt signaling pathway
Phosphoinositide-3 kinase (PI3K) and protein kinase B (Akt/PKB) signaling is involved in several cellular driven processes including cell survival and uncontrolled growth of cells (O'Donnell, Massi, Teng, & Mandala, 2017; Tang, Wang, Hemmings, Ruegg, & Xue, 2017). In RA pathogenesis, PI3K activation results in similar environment as witnessed in a cancer condition, promoting the survival of various immune cells such as: monocytes/macrophages, dendritic cells, and FLS in the joint space resulting in bone erosion and cartilage degradation (Malemud, 2015, 2017). PI3K mediates the activation of Akt in the cytosol through phosphorylation and controls the activation of major elements involved in cell survival and proliferation such as: Nuclear factor kappa B (NFκB), c-AMP response element-binding protein (CREB), mechanistic target of rapamycin (mTOR), and phosphotidylinositols (PtdIns) (Fruman et al., 2017). Subsequently, suppressing the action of natural inhibitors such as: p27, forhead proteins (FOXOs), SH-2 domain containing inositol 5-phosphatase 2 (SHIP2), and phosphatase and tensin homolog (PTEN) (Cheung & McInnes, 2017; Lien, Dibble, & Toker, 2017). Several pro-inflammatory cytokines from T cells (majorly from Th17 cells) drives the activation of PI3K-Akt signaling. IL-21, which is majorly secreted by Th17 cells in the RA synovium, has been recently demonstrated to possess the potential to activate PI3K/Akt signaling in immune cells involved in RA pathogenesis (Xing, Jin, et al., 2016; Yang, et al., 2016).
Initially Zeng et al. (2007) provided evidences to prove that IL-21 modulates the functional aspects of T cells, B cells, Tfh and in promoting the survival of macrophages in a PI3K dependant manner. IL-21 has been attributed to promote CD4+ T cells proliferation to promote CD86+ expression in B cells essential for production of auto-antibodies (Attridge et al., 2014). These parameters provided initial evidences to prove that IL-21 was involved in activation of PI3K/Akt for cellular survival and proliferation. Its implication in RA pathogenesis has also been recently reported in various immune cells that undergo uncontrolled growth and differentiation. For instance, a study carried out by Xing, Yang, et al. (2016) provided evidences that IL-21 mediated the proliferation of RA-FLS resulting in overproduction of TNFα and IL-6 which was suppressed after IL-21RFcmAb treatment. Similarly another study carried out by Xing, Jin, et al. (2016) provided evidences that PI3K/Akt signaling attributed the proliferation of RA-FLS and treatment with LY294002 (PI3K specific inhibitor) suppressed the IL-21/IL-21R mediated activation. Furthermore, recently Xing, Jin, et al. (2016) provided the evidences for involvement of PI3K/Akt pathway in cellular proliferation and promoting osteoclastogenesis in an IL-21 dependant manner independent of RANKL in RAW 264.7 macrophages. Recently, a study demonstrated that IL-21 enhanced the proliferation and phagocytic activity of monocytes/macrophages in a PI3K/Akt dependant manner which further showcases the potential of this cytokine (Vallieres & Girard, 2017). These reports thus prove that PI3K/Akt plays a pivotal role in cellular survival of monocytes/macrophage and RA-FLS cells in an IL-21 dependant manner (Figure 2).
1.10 Therapeutic targeting of IL-21 and its effector molecules
In recent years, various therapies have targeted the inflammatory mediators and cytokines to alleviate the pain and reduce the disease aggression. Current treatment strategies mainly target the more pronounced pro-inflammatory cytokines namely: TNFα and IL-1β, but less attention has been given towards the newly identified pro-inflammatory cytokines of Th17 cells origin. The aggressive behaviour of various immune cells in the synovial region is mainly due to the combined effect of these cytokines. For therapeutically targeting these molecules, one must keep in mind its action and its potential in the disease condition. In this regard, IL-21 controls the action of major immune cells that mediate the RA pathogenesis and progression through activation of other cytokines, transcription factors, and inflammatory molecules. So far, several studies under clinical trials has been undergoing to block the action of IL-21/IL-21R driven molecules, which in the near future will serve as a much better approach for curing the RA disease condition (Table 1).
| Therapeutic target | Compound (trade name) | Nature of compound | Clinical trial status |
|---|---|---|---|
| IL-17 | ABBV-257 ABT-122 AMG-827 Bimekizumab CNTO-6785 LY2439821 Secukinimab | IL-17 & TNFα bispecific Ab IL-17 & TNFα bispecific Ab IL-17RA specific mAb IL-17A and IL-17F bispecific mAb IL-17A specific mAb IL-17A specific mAb IL-17A specific mAb | NCT02531178 (Phase I— completed) NCT01853033 (Phase I—completed) NCT02433340 (Phase II—completed) NCT00771030 (Phase II—completed) NCT02430909 (Phase II—recruiting) NCT01909427 (Phase II—completed) NCT02349295 (Phase III—ongoing) Phase II—Cancelled |
| IL-21/IL-21R | NNC0114-0006 Tocilzumab NNC114-0005 | IL-21 specific mAb IL-21 specific mAb IL-21 specific mAb | NCT01647451 (Phase II—completed) NCT02569736 (Phase I—ongoing) NCT01647451 (Phase II—completed) |
| MMP-3 | Tanomastat | MMP-3 specific inhibitor | Phase III—cancelled |
| MMP-9 | GS-7545 | MMP-9 specific mAb | NCT02176876 (Phase I—completed) |
| RANKL | Denosumab | RANKL specific mAb | NCT0 2418273 (Phase II—ongoing) NCT0 0095498 (Phase II—completed) NCT0 1770106 (Phase III—completed) NCT0 1973569 (Phase IV—completed) |
| MAPK | ARRY-371797 BMS-582949 PH-797804 SB-681323 VX702 | Small molecule p38 inhibitor | NCT0 0303563 (Phase II—completed) NCT0 0089921 (Phase II—completed) NCT0 0205478 (Phase II—completed) NCT0 0383188 (Phase II—completed) NCT0 0605735 (Phase II—completed) |
| JAK-STAT | Baricitinib Tofacitinib Decernotinib | Small molecule JAK 1/3 inhibitor Small molecule JAK 1/3 inhibitor Small molecule JAK 1 inhibitor | NCT02066389 (Phase II—completed) Approved NCT01754935 (Phase II—completed) |
| PI3K-Akt | CDZ173 | PI3Kδ specific inhibitor | NCT02775916 (Phase II—ongoing) |
- Notes: MMP-3: matrix metalloproteinase 3; MMP-9: matrix metalloproteinase 9; IL-17: Interleukin 17; IL-21 receptor: Interleukin 21R; JAK-STAT: janus kinase − signal transducer and activator of transcription; MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3 kinase; RANKL: receptor activator of nuclear factor kappa B ligand. Information from the clinicaltrial.gov website (http://clinicaltrial.gov/), accessed June 2017.
2 CONCLUSIONS
IL-21, a cytokine which is majorly produced by lymphocytes (Th17, Tfh, and NK cells) initiates a chain of reaction through interaction with major cells of the synovium (macrophages, dendritic cells, and synovial fibroblasts) expressing IL-21R (Schematically represented in Figure 1). Overall, IL-21 possess the potential to activate various signaling pathways including STAT-3, PI3K/Akt, MAPK and several other downstream elements resulting in aberrant cellular proliferation and inflammatory processes in RA pathogenesis (Schematically represented in Figure 2). The pleiotropic role of IL-21 in RA pathogenesis and disease progression makes it a major target of interest for therapeutic treatment. Our current understanding thus provides a clear picture about the relevance of IL-21 in RA pathogenesis and thus makes it an important mediator for therapeutic targeting. Several inhibitors targeted against IL-21 mediated effector molecules are under clinical trials that are undertaken to reduce the inflammatory processes in RA pathogenesis. Howbeit, therapeutic targets mediated towards IL-21/IL-21R signaling in RA pathogenesis needs to be explored in a mechanistic approach to block essential mediators required for RA disease progression.
CONFLICTS OF INTEREST
The authors report no conflicts of interest. The authors alone are solely responsible for the content and preparation of the manuscript.




