TRPC3-mediated Ca2+ signals as a promising strategy to boost therapeutic angiogenesis in failing hearts: The role of autologous endothelial colony forming cells
Abstract
Endothelial progenitor cells (EPCs) are a sub-population of bone marrow-derived mononuclear cells that are released in circulation to restore damaged endothelium during its physiological turnover or rescue blood perfusion after an ischemic insult. Additionally, they may be mobilized from perivascular niches located within larger arteries’ wall in response to hypoxic conditions. For this reason, EPCs have been regarded as an effective tool to promote revascularization and functional recovery of ischemic hearts, but clinical application failed to exploit the full potential of patients-derived cells. Indeed, the frequency and biological activity of EPCs are compromised in aging individuals or in subjects suffering from severe cardiovascular risk factors. Rejuvenating the reparative phenotype of autologous EPCs through a gene transfer approach has, therefore, been put forward as an alternative approach to enhance their therapeutic potential in cardiovascular patients. An increase in intracellular Ca2+ concentration constitutes a pivotal signal for the activation of the so-called endothelial colony forming cells (ECFCs), the only known truly endothelial EPC subset. Studies from our group showed that the Ca2+ toolkit differs between peripheral blood- and umbilical cord blood (UCB)-derived ECFCs. In the present article, we first discuss how VEGF uses repetitive Ca2+ spikes to regulate angiogenesis in ECFCs and outline how VEGF-induced intracellular Ca2+ oscillations differ between the two ECFC subtypes. We then hypothesize about the possibility to rejuvenate the biological activity of autologous ECFCs by transfecting the cell with the Ca2+-permeable channel Transient Receptor Potential Canonical 3, which selectively drives the Ca2+ response to VEGF in UCB-derived ECFCs.
1 INTRODUCTION
Acute myocardial infarction (AMI) results in the irreversible loss of cardiac myocytes, which do not possess the adequate capability to regenerate and restore myocardial mass and ventricular function in the injured heart. As a consequence, the prolonged increase in the workload imposed to remaining myocytes will trigger a maladaptive change in the cardiac structure that ultimately leads to heart failure (HF) (Akhmedov & Marin-Garcia, 2013). Aging is an additional factor in determining the onset of HF. The longer life expectancy has increased the percentage of population, especially in the western countries, persistently exposed to traditional cardiovascular risk factors, such as hypertension, atherosclerosis, and coronary artery disease (Goldwater & Pinney, 2015). The incidence of HF has been reported to increase from <1% in persons aged 20–39 y.o. to >20% in those aged >80 y.o (Jugdutt, 2010). Therefore, HF is a leading cause of mortality and morbidity worldwide by accounting for more than 7 million deaths per year with 500,000 new cases every year and an economic burden of more than $500 billion annually in health care costs (Steg et al., 2012; Young & Schafer, 2015). Unfortunately, HF lacks an effective pharmacological treatment as it is not possible to interrupt or at least reverse disease progression (Koudstaal et al., 2013). The only therapeutic options for end-stage HF remain heart transplantation, which is limited by the paucity of donors, and left ventricular assist devices either as bridge-to-transplant or destination therapy (Koudstaal et al., 2013). An alternative, promising approach to treat advanced HF is represented by cell-based therapy (CBT), which consists in stimulating heart regeneration and halting or reversing myocardial injury by the infusion of autologous stem/progenitor cells with myogenic or angiogenic potential (Passier, van Laake, & Mummery, 2008; Young & Schafer, 2015). A multitude of stem cell platforms have been probed in the last decade for their ability to rescue cardiac structure and function in HF patients, including cardiac progenitor cells (CPCs), adipose-derived stem cells (ADSCs), and bone marrow (BM)-derived mononuclear cells (BM-MNCs) (Moccia, Ruffinatti, & Zuccolo, 2015; Passier et al., 2008; Young & Schafer, 2015). Among the many cellular subsets that comprise the heterogeneous population of BM-MNCs, namely mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and endothelial progenitor cells (EPCs), EPCs are rapidly emerging as one of the most suitable tools to achieve cardiac regeneration and function recovery (Maltais, Perrault, & Ly, 2011; Moccia, Bonetti, et al., 2012; Pfister, Della Verde, Liao, & Kuster, 2014; Young & Schafer, 2015). Many pre-clinical animal studies demonstrated that the direct injection of EPCs into the border zone of infarcted myocardium leads to an increase in capillary density, a significant regeneration of lost myocardium and an improvement of global left ventricular function (Moccia, Bonetti, et al., 2012; Pfister et al., 2014; Young & Schafer, 2015). Nevertheless, the clinical translation of such exciting results produced only modest benefits to the patients and EPCs-based therapy is far from moving from the bench to the bedside. One of the main shortcomings associated to cellular therapeutics is the ability of the infused cells to engraft and proliferate within the harsh microenvironment (i.e., oxidative stress, acidic pH, local ischemia) of the injured heart (Pfister et al., 2014). Understanding the signal transduction pathways that determine EPC activity is indispensable to enhance their regenerative outcome (Moccia, Bonetti, et al., 2012; Moccia, Dragoni, et al., 2012; Moccia, Lodola, et al., 2014). Intracellular Ca2+ signals lie at the center of an intricate network of signalling cascades that finely tune stem cell behavior (Forostyak et al., 2016; Hao, Webb, Miller, & Yue, 2016; Moccia, Ruffinatti, & Zuccolo, 2015; Moccia, Tanzi, & Munaron, 2014; Pinto et al., 2016). Manipulating the Ca2+ response to growth factors and local developmental cues has been shown to redirect stem/progenitor cell fate towards the desired lineage (Kawano et al., 2006; Yang & Li, 2007) and/or to increase the rate of expansion both in vitro (Kong et al., 2008; Ferreira-Martins et al., 2009) and in vivo (Ferreira-Martins et al., 2009). As recently reviewed in (Moccia & Guerra, 2016), recent work has revealed that vascular endothelial growth factor (VEGF) utilizes intracellular Ca2+ oscillations to promote EPC proliferation and in vitro tubulogenesis. Herein, we will speculate about the possibility to re-juvenate EPC phenotype and increase their pro-angiogenic response to VEGF by forcing the autologous cells of the patient, which are harvested from peripheral circulation, to express Transient Receptor Channel Canonical 3 (TRPC3), which drives VEGF-induced Ca2+ oscillations in umbilical cord blood (UCB)-derived cells. We will focus on the so-called endothelial colony forming cells (ECFCs), which represent a truly endothelial progenitor and are endowed with the highest potential to stimulate therapeutic angiogenesis in failing hearts (Medina et al., 2017; Tasev, Koolwijk, & Hinsbergh, 2016).
1.1 Evidence in favor and against EPC application for cellular therapeutics of heart failure
EPCs are mobilized either from the BM or the arterial wall to replace injured/senescent endothelial cells or to reconstruct the local vascular network in ischemic tissues or organs (Leone et al., 2009; Medina et al., 2017; Murasawa & Asahara, 2005). Moreover, EPCs drive the angiogenic switch that promotes cancer growth and metastatization in solid malignancies (Moccia, Zuccolo, Poletto, et al., 2015). Accordingly, hypoxic tissues are featured by a drop in oxygen tension (PO2) that is sensed by the hypoxia-inducible factor 1α (HIF-1α), a transcription factor that controls the expression of a plethora of peptidic growth factors and cytokines, including VEGF, basic fibroblast growth factor (bFGF), placental growth factor (PIGF), angiopoietins, erythropoietin (EPO), and stromal derived factor-1α (SDF-1α) (Moccia, Zuccolo, Poletto, et al., 2015; Semenza, 2007). The consequent release in circulation of this chemokine storm establishes a concentration gradient that is detected by quiescent EPCs, both those residing in the BM niche or in the vasculogenic zone of some arterial vessels (Leone et al., 2009; Watt, Athanassopoulos, Harris, & Tsaknakis, 2010). Once activated, EPCs migrate through the gradient towards the area of higher chemokine concentration, thereby homing to the injury site (Leone et al., 2009). Herein, EPCs sustain vascular regrowth by the paracrine release of further growth factors and cytokines and/or by physically incorporating within neovessels (Leone et al., 2009; Moccia, Tanzi, & Munaron, 2014; Yoder, 2012). We refer the reader to a number of recent reviews which provided an exhaustive description of the molecular mechanisms underlying EPC activation, mobilization, and trafficking to the target tissues (Aicher, Zeiher, & Dimmeler, 2005; de la Puente, Muz, Azab, & Azab, 2013; Leone et al., 2009). Nevertheless, the gasotransmitter nitric oxide (NO) is the key determinant that promotes EPC egression from their residence niche in response to guidance cues (Leone et al., 2009; Murasawa & Asahara, 2005). A number of distinct EPC subsets with different clonogenic and vasculogenic potentials have been described in the literature (Asahara, Kawamoto, & Masuda, 2011; Fadini et al., 2012; Hirschi et al., 2008; Moccia & Poletto, 2015; Watt et al., 2010). Briefly, EPCs may be subdivided in hematopoietic or non-hematopoietic angiogenic cells based on their true belonging to the endothelial lineage (Asahara et al., 2011; Fadini, Losordo, & Dimmeler, 2012; Medina et al., 2017). Hematopoietic EPCs derive from BM-derived HSCs with which they share some surface markers, such as CD133, CD34, Flk-1/KDR, and CXCR4. In addition, although human hematopoietic EPCs express typical endothelial antigens, such as CD144, CD105, lectin UEA-1, and von Willebrand factor (vWF), they present a number of hematopoietic markers, such as CD11b, CD14, CD45, and demonstrate acetylated low-density lipoprotein (AcLDL) intake, which is a function of both endothelial cells and macrophages. Therefore, human hematopoietic EPCs represent a heterogenous population that mainly belongs to the myeloid lineage and shares several features with immune cells, in particular monocyte/macrophages (Asahara et al., 2011; Fadini et al., 2012; Hirschi, Ingram, & Yoder, 2008; Medina et al., 2017). Hematopoietic EPCs include CFU-ECs (Colony forming unit-ECs, CFU-Hill, CFU-End, Early EPCs), which correspond to the first EPC subset originally described by Asahara et al. (1997), and circulating angiogenic cells (CACs), both of which may be released from BM after an ischemic insult (Asahara et al., 2011; Fadini et al., 2012; Hirschi et al., 2008; Moccia & Poletto, 2015). CFU-ECs and CACs cannot differentiate into mature endothelial cells, do not replate into secondary or tertiary colonies and do not assembly into capillary-like structures in Matrigel in the absence of supporting endothelial cells. Yet, they both promote tissue revascularization in vivo by paracrine interaction with local endothelial cells and by the cross-talk with the inflammatory cells recruited to the ischemic microenvironment (Watt et al., 2010). Conversely, non-hematopoietic cells are regarded as truly endothelial progenitors due to their ability to differentiate into spindle-shaped endothelial cells with typical cobblestone morphology, to form capillary-like networks in vitro and patent vessels in vivo, and to maintain a stable phenotype through up to 60 passages with no loss of function. Non-hematopoietic stem cells encompass the endothelial colony forming cells (ECFCs) or late outgrowth EPCs, which lack CD133 and monocytic markers, such as CD11b, CD14, and CD45, while express Flk-1/KDR, and CXCR4, CD144, CD105, lectin UEA-1, vWF, and are positive to AcLDL uptake (Ingram et al., 2004; Yoder, 2012). ECFCs could rescue local blood perfusion either indirectly, through the paracrine liberation of growth factors and cytokines, or directly by serving as building blocks for neovessel formation (Collett et al., 2017; Moccia, Zuccolo, Poletto, et al., 2015; Richardson & Yoder, 2011; Sakimoto et al., 2017; Yoder, 2012). Similar to CFU-ECs and CACs, ECFCs may be selected from peripheral blood (PB) and UCB, although the clonogenic potential of UCB-derived ECFCs (>70 population doublings) is remarkably higher as respect to their peripheral counterparts (20–40 population doublings) (Ingram et al., 2004).
After the seminal discovery of circulating EPCs by Asahara et al. (1997), several pre-clinical studies were conducted to assess the regenerative effect, if any, of BM-derived unselected MNCs and CD34-enriched EPCs in rodent, monkey, and swine models of AMI. These investigations disclosed that cell transplantation enhanced cardiac revascularization, reduced cardiac fibrosis and partially rescued the ventricular function of injured heart (Ben-Shoshan & George, 2007; Sekiguchi, Ii, & Losordo, 2009). Intriguingly, the elimination of MNCs engineered to express a suicide gene under the control of a cardiac or endothelial specific gene promoter after the injection demonstrated that the acquisition of an endothelial, but not cardiac, lineage was indispensable to promote the functional recovery of mouse heart after an ischemic insult (Yoon et al., 2010). Moreover, EPCs were reported to fuse with resident cardiomyocyte, rather than transdifferentiating into cardiac cells, and to preserve myocardial viability in a paracrine manner (Avitabile et al., 2011; Murry et al., 2004; Pfister et al., 2014).
Based on these promising premises, numerous randomized controlled phase I/II trials were launched by either using unselected BM-MNCs or selecting EPCs from the MNC fraction of either BM or PB. In most cases, EPCs were identified as CD34+ cells by using a magnetic cell sorter that did not permit to distinguish between CFU-ECs and CACs, that is, the hematopoietic EPCs, and ECFCs, that is, the truly endothelial EPCs (Moccia, Bonetti, et al., 2012; Psaltis, Spoon, Wong, & Gulati, 2014; Sekiguchi et al., 2009; Young & Schafer, 2015). These trials adopted intracoronary administration as the delivery route of the cells to the ischemic heart. The Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial showed that the intracoronary injection of BM-MNCs after successful percutaneous coronary intervention for treating AMI improved myocardial viability and left ventricular ejection fraction (LVEF) as compared to patients treated with a placebo (Assmus et al., 2002). However, the subsequent Bone Marrow Transfer to Enhance ST-Elevation Infarct Regeneration (BOOST) trial revealed that the improvement in LVEF as relative to placebo-treated patients was significant at 6, but not at 18 months, a feature suggesting that CBT could accelerate the process of heart repair but required an adjuvant treatment to produce an effective benefit (Meyer et al., 2006). Conversely, the injection of BM-MNCs did not cause any significant improvement in ventricular function at 6 months in the smaller Autologous Stem Cell Transplantation in Acute Myocardial Infarction (ASTAMI) trial (Cleland, Freemantle, Coletta, & Clark, 2006). Finally, the Transplantation of Progenitor Cells and Recovery of LV Function in Patients with Chronic Ischemic Heart Disease (TOPCARE-CHD) trial demonstrated that the infusion of approximately 200 million BM-MNCs in patients affected by non-ischemic dilative cardiomyopathy caused a modest improvement in LVEF of ≈4% at 3 months (Assmus et al., 2007). Overall, a number of meta-analysis reviews of CBT trials conducted by administrating un-fractioned BM-MNCs revealed a weak increase in LVEF (no more than 2–4%) and end-systolic volumes and a modest reduction in the infarct size area (Pfister et al., 2014; Psaltis et al., 2014; Young & Schafer, 2015). Moreover, several recent randomized, double-blind, and placebo-controlled clinical studies failed to report any significant benefit in cardiac remodeling and function after BM-MNC infusion (Psaltis et al., 2014; Young & Schafer, 2015). It has, therefore, also been probed the regenerative outcome of CD34-enriched progenitors, which are supposed to exert a major healing effect due to their ability to provide a larger amount of growth factors and cytokines to the injured heart (and, eventually, to directly incorporate into neovessels). However, the multicenter REGENT trial showed that LVEF increased by 3% both in AMI patients treated with unselected BM-MNCs and in those who received CD34+/CXCR4+ selected EPCs during a follow-up of 6 months (Tendera et al., 2009). We refer the reader to a number of recent reviews which provided an excellent discussion of the main pitfalls and hurdles associated to EPC-based therapy of infarcted myocardium and HF (Jujo, Ii, & Losordo, 2008; Moccia et al., 2013; Psaltis et al., 2014; Young & Schafer, 2015).
The discrepancy between the positive results provided by pre-clinical studies and the modest or null benefit imparted by EPCs-based therapy to patients with AMI or HF depends on three main reasons. First, the term EPC includes a heterogeneous population of cells that can be more or less prone to stimulate angiogenesis and post-natal vascularization. The bolus of un-selected BM-MNCs that has been administered in most trials could present variable percentages of CD34+ cells (Quyyumi et al., 2011) and these, in turn, were likely to comprehend different fractions of hematopoietic cells and true endothelial precursors (Psaltis et al., 2014). In this view, the median yield of CD34+/CXCR4+ EPCs that has been obtained by the REGENT trial was 1.90 × 106 versus the 1.78 × 108 BM-MNCs employed in the same study (Tendera et al., 2009). Unfortunately, circulating EPCs are rather scarce in PB, that is, 0.5–2.0 × 104 human EPCs/g body weight, so that the volume of blood volume necessary to achieve a therapeutically relevant amount of EPCs is 12L (Murasawa & Asahara, 2005). Second, EPCs were directly injected into the injured myocardium of ischemic animals and the coronary artery was not reperfused, a manoeuvre that could dilute the cells away from the injured site. Conversely, the cells were intra-coronary delivered by using the stop-flow balloon catheter strategy in human patients, a strategy that, albeit less invasive than local intra-myocardial injection, could reduce the percentage of EPCs effectively homing and engrafting within the target tissue (Moccia et al., 2013). Third, a significant point to support the aim of the present review, while pre-clinical studies have been conducted on young animals, EPCs-based therapy has been mainly devoted to treat aging patients surviving AMI or affected by other forms of HF. This issue deserves particular attention as both the frequency and the biological activity of EPCs is dramatically affected both by aging and by the cardiovascular risk factors that are mainly associated to HF, such as atherosclerosis, smoke, obesity, hypertension, and CAD. Under these conditions, EPCs display a reduced capacity to proliferate, egress from BM, target the ischemic tissue and promote neovascularization (Dimmeler & Leri, 2008; Pesce et al., 2011). Therefore, although a transient increase in the frequency of both hematopoietic proangiogenic cells and ECFCs has been reported to occur immediately after AMI, this response does not produce any benefit to the patients (Massa et al., 2009; Regueiro et al., 2015; Templin, Luscher, & Landmesser, 2011). As discussed in Dimmeler (2010) and Dimmeler and Leri (2008), cardiovascular risk factors could act by altering the BM niche microenvironment, thereby affecting the functional capacity of all its cellular constituents, while aging affects stem/progenitor cells by reducing their telomeric length. A number of treatments have been proposed to improve the therapeutic outcome of autologous EPCs-based therapy. The efficacy of regenerative medicine could be increased by pre-conditioning the cells with a cocktail of small molecules (e.g., statins, peroxisome proliferative activated receptor γ agonists, p38 inhibitors, or endothelial NO synthase enhancers) or by transducing them with pro-survival genes (e.g., Akt, telomerase, or VEGF) with the aim to enhance EPC proliferation, homing, and incorporation within the ischemic heart (Chavakis, Urbich, & Dimmeler, 2008; Dimmeler & Leri, 2008; Murasawa & Asahara, 2005; Seeger, Zeiher, & Dimmeler, 2007). Alternatively, the target tissue (i.e., the ischemic heart) could be pre-activated by the local administration of cytokines, chemokines, and growth factors to expedite EPC recruitment and counteract the harsh conditions of necrotic myocardium (Chavakis et al., 2008; Dimmeler & Leri, 2008; Murasawa & Asahara, 2005; Seeger et al., 2007).
Understanding the signal transduction pathways governing EPC proliferation, survival, homing, and differentiation is, therefore, imperative in order to gain full benefit from their largely underestimated therapeutic potential. In particular, it would be of help to decode the signaling network intracellularly activated by VEGF, which is the most potent EPC activator (Chavakis et al., 2008). At the same time, it is mandatory to find an agreement as regard to the most effective EPC subset and to identify the proper timing and route of cell administration as well as the most efficient dose (Seeger et al., 2007; Young & Schafer, 2015). With these concepts in mind, one should also recall that UCB-derived EPCs have long been known to be more amenable for cellular therapeutics due to their higher frequency as compared to their peripheral counterparts. For instance, UCB contains about 10-fold excess of CD34+ cells as respect to adult PB (Murohara, 2010), while the frequency of UCB- and PB-derived ECFCs amounts to 2.5 cells/ml and 0.05–0.2 cells/ml, respectively (Ingram et al., 2004). Moreover, UCB-derived EPCs have longer telomeres, which augment their regenerative potential by delaying their entrance in senescence, display a faster cell-cycle rate, and possess a greater self-renewal potential as compared to those harvested from PB (Murohara, 2010; Zhang, Yang, & Han, 2006). Several pre-clinical studies confirmed that the local injection of UCB-derived EPCs, sorted either as CD133+ or CD34+ MNCs, promote neovascularization and rescue blood perfusion in mouse models of hindlimb ischemia and AMI (Murohara, 2001, 2010). Unfortunately, transplantation of UCB-derived cells for therapeutic angiogenesis is currently unrealistic as implanted cells would be rejected by the host's immune response (Murohara, 2001, 2010). However, these cells could serve as a template to design the perfect autologous EPC to be used for CBT. The case of ECFCs is particularly intriguing as the proliferative response to VEGF is remarkably higher in UCB-derived cells (up to 100 population doublings) as relative to PB-derived ECFCs (up to 20–40 population doublings) (Ingram et al., 2004). ECFCs are emerging as suitable candidates for cell-based therapy of ischemic diseases by virtue of their ability to paracrinally stimulate local angiogenesis and preserve cardiomyocyte viability and to physically engraft within nascent vasculature (Watt et al., 2010; Yoder, 2012). Accordingly, both PB- and UCB-derived ECFCs may cause de novo vessel formation and functional recovery of blood flow in several rodent models of hindlimb ischemia (Flex et al., 2016; Goto et al., 2016; Kang, Coggins, Xiao, Rosenzweig, & Bischoff, 2013; Patel et al., 2016). A recent study further showed that VEGFR-2 (KDR/Flk-1), the receptor isoform that mediates the pro-angiogenic effects of VEGF on EPCs (Moccia, Dragoni, et al., 2012; Rabbany, Heissig, Hattori, & Rafii, 2003), is also key to ECFCs-dependent revascularization (Joo et al., 2015). A reliable strategy to rejuvenate the reparative phonotype of PB-derived ECFCs (PB-ECFCs) is to genetically intervene on the signalling machinery that perceives and/or translates the mitogenic input of VEGF. In particular, comparing VEGFR-2 signalling in UCB- and PB-derived ECFCs might indicate which component(s) of this pathway is (are) absent or down-regulated in PB-ECFCs, thereby causing their lower sensitivity to VEGF (Ingram et al., 2004). In the following paragraphs, we will survey our recent work aiming at elucidating the differences in the molecular underpinnings of VEGF-evoked pro-angiogenic Ca2+ signals in PB-and UCB-derived ECFCs and how they could be exploited to improve the regenerative outcome of the former.
1.2 The molecular machinery that drives VEGF-evoked Ca2+ signals differs between peripheral blood- and umbilical cord blood-derived ECFCs
It has long been known that an increase in intracellular Ca2+ concentration ([Ca2+]i) plays a key role in the complex and multistepped process of angiogenesis (Moccia, Berra-Romani, & Tanzi, 2012; Moccia, Tanzi, & Munaron, 2014). This notion dates back to the earlier experiments conducted with carboxyamidotriazole, a non-selective inhibitor of a plethora of Ca2+-permeable channels (Dragoni, Turin, et al., 2014; Moccia, Dragoni, et al., 2012; Moccia et al., 2016), that was shown to inhibit angiogenesis both in vitro and in vivo (Kohn, Alessandro, Spoonster, Wersto, & Liotta, 1995; Moccia et al., 2014; Patton, Kassis, Doong, & Kohn, 2003). An elevation in [Ca2+]i represents a critical hub for the intricate network of signaling pathways evoked by extracellular growth factors (Moccia, 2017; Moccia, Berra-Romani, & Tanzi, 2012; Moccia & Poletto, 2015; Moccia, Tanzi, & Munaron, 2014). Upon binding to their specific ligand, tyrosine kinase receptors (TKRs) dimerize and autophosphorylate, a process which leads to the recruitment of phospholipase Cγ (PLCγ), of which two isoforms exist. PLCγ, in turn, cleaves phosphatidylinositol-4,5-bisphosphate (PIP2), a minor phospholipid component of the plasma membrane (PM), into the two second messengers, diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (InsP3) (Berridge, Bootman, & Roderick, 2003). While DAG remains tethered to the inner leaflet of PM, where it may evoke Ca2+ entry by gating some members of the Transient Receptor Channel Canonical (TRPC) family of non-selective cation channels (Moccia, Berra-Romani, & Tanzi, 2012), that is, TRPC3, TRPC6, and TRPC7, InsP3 rapidly diffuses within the cytosol and activates Ca2+ release from the endoplasmic reticulum (ER), the largest intracellular pool of Ca2+, by gating its specific InsP3 receptors (InsP3Rs) (Berridge, Bootman, & Roderick, 2003). Under physiological conditions, InsP3 does so only in the presence of a permissive concentration of Ca2+ in the surrounding microenvironment, so that InsP3 and Ca2+ act as co-agonists of InsP3Rs (Zhang, Fritz, Ibarra, & Uhlén, 2011). InsP3-dependent Ca2+ mobilization may be amplified by the recruitment of neighboring ryanodine receptors (RyRs) through the process of Ca2+-induced Ca2+ release (CICR) (Berridge et al., 2003). More recently, it has been discovered that VEGF may trigger ER-dependent Ca2+ liberation by discharging Ca2+ from closely juxtaposed acidic Ca2+ stores, which release Ca2+ through the nicotinic acid adenine dinucleotide phosphate (NAADP)-gated two pore channels (TPC1 and TPC2) (Favia et al., 2014). The consequent fall in ER Ca2+ concentration ([Ca2+]ER) is detected by the Stromal Interaction Molecule-1 (Stim1), a ER Ca2+ sensor that oligomerizes and translocates into the ER-PM junctions upon store depletion. Once arrived in close apposition, that is, 10–20 nm, to the PM, Stim1 assemblies into spatially restricted clusters or puncta at level of which it binds to and gates at least two Ca2+-permeable channels, such as Orai1 and/or TRPC1 (Ong & Ambudkar, 2015; Prakriya & Lewis, 2015). This mode of Ca2+ inflow has been termed store-operated Ca2+ entry (SOCE) and represents the major route for Ca2+ influx in vascular endothelial cells (Abdullaev et al., 2008; Cioffi et al., 2012; Jho et al., 2005; Li et al., 2011; Sundivakkam et al., 2012; Xu, Cioffi, Alexeyev, Rich, & Stevens, 2015). We will not address here the complex discussion about the molecular details of the physical interaction between Stim1 and Orai1 and/or TRPC1, which has been exhaustively described elsewhere (Cioffi, Barry, & Stevens, 2010; Ong & Ambudkar, 2015; Prakriya & Lewis, 2015). Therefore, growth factors may rely on a vast repertoire of elements from the Ca2+ signaling toolkit to shape the increase in [Ca2+]i that governs endothelial remodeling. It turns out that the waveform of pro-angiogenic Ca2+ signals may change depending on the pathway(s) recruited for Ca2+ delivery. For instance, VEGF has been shown to evoke a biphasic Ca2+ elevation in human umbilical vein endothelial cells (HUVECs), which consists in an initial, rapid Ca2+ release from TPC-2 that is, in turn, propagated to the whole cytoplasm by InsP3R activation (Faehling et al., 2002; Favia et al., 2014). The Ca2+ peak then decays to a plateau phase of intermediate amplitude that is supported by Stim1, Orai1 and eventually TRPC1 (Abdullaev et al., 2008; Li et al., 2011; Sundivakkam et al., 2012). Moreover, VEGF-induced Ca2+ entry in HUVECs may be also sustained by the reverse-mode of the Na+/Ca2+ exchanger (NCX) (Andrikopoulos et al., 2011). Conversely, DAG activates TRPC3 and TRPC6 to mediate the monotonic Ca2+ signal generated by VEGF in HMECs (Cheng, James, Foster, Hancox, & Bates, 2006; Ge et al., 2009; Hamdollah Zadeh, Glass, Magnussen, Hancox, & Bates, 2008). There is evidence that VEGF utilizes the gasotransmitter hydrogen sulphide (H2S) to regulate the interaction between the different components of the Ca2+ toolkit in both HUVECs and HMECs, thereby promoting proliferation and in vitro tubulogenesis (Pupo et al., 2011; Potenza et al., 2014). Unlike VEGF, epidermal growth factor (EGF) was found to trigger asynchronous oscillations in [Ca2+]i in rat cardiac microvascular endothelial cells (CMECs) by promoting a concerted interplay between InsP3-depedent Ca2+ release and SOCE (Moccia et al., 2003). Finally, basic fibroblast growth factor (bFGF) and insulin-like growth factor-I (IGF-I) evoke Ca2+ entry in bovine aortic endothelial cells by engaging TRPC1 through a yet to be elucidated mechanism (Antoniotti, Lovisolo, Pla, & Munaron, 2002; Munaron & Fiorio Pla, 2000). Alternatively, DAG may be converted by DAG lipase into arachidonic acid (AA) and/or AA itself may be released from PM phospholipids upon activation of the cytosolic phospholipase A2 (PLA2) (Antoniotti et al., 2003). Several studies have convincingly shown that AA and its derivatives activate TRP Vanilloid 4 (TRPV4) to promote proliferation and in vitro tubulogenesis in both HMECs and tumor-derived endothelial cells (Dragoni, Guerra, et al., 2015; Pla et al., 2008), although it is not clear whether this signaling axis is actually recruited by any growth factor. The advent of genetic Ca2+ indicators, such as GCaMP6 that can be expressed under an endothelial cell specific promoter, recently permitted to confirm that VEGF-induced Ca2+ oscillations guide the proliferation of stalk cells during sprouting angiogenesis in vivo due to the activation of the Ca2+-dependent transcription factor, nuclear factor of activated T cells (NFAT) (Noren et al., 2016). Conversely, higher concentrations of VEGF, which trigger biphasic Ca2+ signals comprising an initial Ca2+ peak followed by the plateau phase, sustain endothelial cell motility by recruiting the Ca2+-sensitive myosin light chain kinase (MLCK) (Noren et al., 2016). Based on these evidences, therefore, we sought to assess whether intracellular Ca2+ signals were equally important in driving the pro-angiogenic response to VEGF in both PB- and UCB-derived ECFCs. First, we and others found that PB-ECFCs possess many of the components of the Ca2+ toolkit that are recruited by growth factors to stimulate proliferation, in vitro tubulogenesis and sprouting angiogenesis in mature endothelial cells. For instance, PB-ECFCs express all the three known InsP3R isoforms (InsP3R1-3) (Dragoni et al., 2011; Zuccolo, Bottino, et al., 2016), their pattern of expression being InsP3R-3 = InsP3R-2>InsP3R-1, and a functional SOCE, which is mediated by the interplay between Stim1, Orai1, and TRPC1 (Li et al., 2011; Lodola et al., 2012; Sanchez-Hernandez et al., 2010). Moreover, PB-ECFCs express a set of pumps which are devoted to interrupt Ca2+ signals either by extruding Ca2+ across PM, such as Plasma Membrane Ca2+-ATPase 1B and 4B, or by sequestering back Ca2+ into ER lumen, such as Sarco-Endoplasmic Reticulum Ca2+-ATPase 2B (Poletto et al., 2016). Surprisingly, PB-ECFCs lack transcripts encoding for the endothelial NCX isoforms (Poletto et al., 2016), which play a key role in clearing [Ca2+]i in mature endothelium (Berra-Romani et al., 2010; Moccia et al., 2002; Sedova & Blatter, 1999), as well as those encoding for TRPC3, TRPC6, and TRPC7, which are also largely expressed in adult endothelial cells (Moccia, Berra-Romani, & Tanzi, 2012). Likewise, PB-ECFCs do not possess RyRs (Zuccolo, Dragoni, et al., 2016), which are also present in mature endothelial cells (Long, Cook, Wu, & Mitchell, 2007; Zhang et al., 2006). Finally, PB-ECFCs display a remarkable acidic Ca2+ store that can be liberated by NAADP-gated TPC1 channels (Di Nezza et al., 2017; Zuccolo, Dragoni, et al., 2016). In terms of pro-angiogenic Ca2+ signals, TRPV4, albeit functionally expressed, exerts only a weak proliferative action on PB-ECFCs (Dragoni, Guerra, et al., 2015), in marked contrast with its established role in arterial remodeling (Troidl et al., 2010, 2009). At the same way, TRP Melastatin 7 (TRPM7) does not promote ECFC proliferation (Baldoli & Maier, 2012), while TRPV1 stimulates ECFCs to undergo angiogenesis by favoring anandamide intake in a Ca2+-independent manner (Hofmann et al., 2014).
We recently discovered that VEGF triggers asynchronous oscillations in [Ca2+]i in PB-ECFCs by causing a rhythmic discharge of Ca2+ from InsP3Rs which is maintained over time by SOCE activation (Dragoni et al., 2011; Li et al., 2011), as usually observed in non-excitable cells (Berridge, 2007; Putney & Bird, 2008). The dose-response relationship revealed that 10 ng/ml was the most suitable dose to elicit the spiking response, as highlighted by the largest fraction of oscillating cells, the higher amplitude of the first Ca2+ spike, the lower interspike interval (ISI) and the higher number of Ca2+ spikes per unit time (i.e., 1 hr) (Dragoni et al., 2011). While the role played by InsP3Rs has been clearly established, it is still unknown whether NAADP-gated TPC1 contributes to the onset of the Ca2+ response to VEGF, as recently observed in HUVECs (Favia et al., 2014, 2016). It is, however, intriguing that intracellularly delivered NAADP stimulates ECFC proliferation in a TPC-dependent manner (Di Nezza et al., 2017). Parallel work from Prof. Beech's group confirmed that the genetic silencing of Stim1 and Orai1 prevent VEGF-evoked Ca2+ inflow in PB-ECFCs, whereas VEGF promotes Stim1 relocation into sub-membranal puncta (Li et al., 2011). We also showed that VEGF promote PB-ECFC proliferation and in vitro tubulogenesis by promoting the nuclear translocation of another Ca2+-dependent transcription factor, that is, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) (Dragoni et al., 2011). The NF-κB signaling network comprises five monomers (p65/RelA, RelB, cRel, p50, and p52) that assembly into homo- or hetero-dimers that directly bind to DNA to promote gene expression and are modulated by the association with the inhibitory protein IκB through multiple ankyrin repeats (Mitchell, Vargas, & Hoffmann, 2016). NF-κB activation is tightly tuned by a kinase complex formed by the kinases, IKKα and IKKβ, which are in turn assembled by the scaffold protein NEMO/IKKγ (Mitchell et al., 2016). In the absence of extracellular stimulation, NF-κB is prevented from reaching the nucleus by IκB, which masks its nuclear localization sequence. However, a train of repetitive Ca2+ spikes with an ISI of approximately 100–200 sec, which is in the same time range as that described in PB-ECFCs (Dragoni et al., 2011), can recruit Ca2+/Calmodulin-dependent Kinase II (CaMKII) or CaMKIV (Lewis, 2003; Ishiguro et al., 2006; Smedler & Uhlén, 2014), which may hyperphosphorylate IκB at S32 and S36, thereby targeting it for ubiquitination and proteasomal degradation (Mitchell et al., 2016). Consequently, NF-κB is freed from inhibition and may translocate into the nucleus (Lewis, 2003), where it can trigger the transcriptional program that controls VEGF-induced angiogenesis (Grosjean, Kiriakidis, Reilly, Feldmann, & Paleolog, 2006; Kim et al., 2001). In particular, we showed that interfering with the intracellular Ca2+ waves inhibits VEGF-induced IκB phosphorylation (Dragoni et al., 2011) and p65 translocation into the nucleus (manuscript in preparation). Recent work has also shown that SOCE may be selectively coupled to endothelial NO synthase (eNOS) activation in PB-ECFCs (Zuccolo, Bottino, et al., 2016), as also observed in many other cell types (Parekh, 2008). This mechanistic link is, however, not as strong as that described in mature endothelial cells (Berra-Romani et al., 2013; Dedkova & Blatter, 2002). For instance, TRPV4-mediated NO signals are significantly larger as compared to those induced by SOCE in healthy PB-ECFCs (Zuccolo, Dragoni, et al., 2016). The relationship between VEGF, intracellular Ca2+ oscillations and NO production deserves, therefore, future consideration as not only NO induces EPC mobilization from BM (Urbich & Dimmeler, 2004), it also delivers a mitogenic signal to several EPC subsets, including ECFCs (Lu, Wang, & Qian, 2015; Ozuyaman et al., 2005; Zuccolo, Bottino, et al., 2016). Additionally, VEGF caused the phosphorylation of AMP-activated protein kinase (AMPK) to promote ECFC differentiation by recruiting the Ca2+/CaM-dependent protein kinase kinase-β (CaMKKβ) (Li et al., 2008). Of note, VEGF-induced ECFC differentiation also requires the Krüppel-like factor 2 (Klf2) (Song et al., 2013), a zinc finger transcription factor that is rapidly emerging among the key regulators of endothelial biology (Novodvorsky & Chico, 2014). Recent investigations reported about the indispensable role of Ca2+ entry in the endothelial activation of Klf2 (Sathanoori et al., 2015; Wang et al., 2010). Ca2+ entry may indeed induce the Ca2+/CaM-dependent phosphorylation and nuclear export of histone deacetylase 5 (HDAC5), thereby leading to Klf2 activation (Wang et al., 2010). Therefore, there is wide evidence to conclude that intracellular Ca2+ dynamics represents a key node in the multifaceted web of signaling pathways recruited by VEGF in ECFCs.
Relevant to the aim of the present article, a recent study demonstrated that VEGF-induced SOCE is augmented at Day 0 in EPCs (sorted as CD45−, CD34+, KDR+, and CD133+) isolated from PB of AMI patients, while the amplitude of Ca2+ entry returned to that of control cells at Day 7 and 180 (Regueiro et al., 2015). The authors concluded that SOCE up-regulation was responsible for the transient increase in EPC frequency acutely observed in AMI patients, although they could not dissect whether SOCE remodeling was accompanied by a molecular rearrangement of the underlying machinery (Regueiro et al., 2015). To further highlight the tight link between VEGF-induced Ca2+ signals, EPCs and cardiovascular diseases, an investigation conducted on murine CACs showed that SOCE is heavily down-regulated in cells harvested from PB of atherosclerotic mice (Wang et al., 2015). The reduction in SOCE magnitude, which was associated to the down-regulation of Stim1, Orai1, and TRPC1, reduced CAC ability to proliferate and assembly into bidimensional capillary-like structures in Matrigel (Wang et al., 2015). Overall, these findings, although preliminary and requiring further confirmation in ECFCs, strongly indicate that the Ca2+ toolkit is severely altered in PB-derived EPCs in the presence of cardiovascular risk factors prone to induce HF. It turns out that rejuvenating the Ca2+ machinery available to VEGF could represent an alternative strategy to improve the regenerative outcome of EPC-based therapy of ischemic disorders.
Our subsequent investigation revealed that VEGF still evoked asynchronous Ca2+ spikes in UCB-derived (UCB-ECFCs) (Dragoni et al., 2013), yet, a sophisticated statistical analysis demonstrated that the oscillatory activity is significantly stronger as compared to PB-ECFCs (Moccia, Ruffinatti, & Zuccolo, 2015). Accordingly, both the amplitude and the frequency of VEGF-induced Ca2+ oscillations were remarkably increased in UCB-ECFCs (Moccia, Ruffinatti, & Zuccolo, 2015). Surprisingly, these cells lacked InsP3R1 (Dragoni et al., 2013); nevertheless, the combination of both InsP3R2 and InsP3R3 has been shown to generate robust and long lasting intracellular Ca2+ spikes in several cell types (Zhang et al., 2011). Pharmacological manipulation showed that, while the repetitive Ca2+ transients developed downstream of PLCγ activation, Ca2+ inflow was absolutely required to initiate the Ca2+ burst (Dragoni et al., 2013). However, blocking SOCE with the selective drug BTP-2/YM-58483 curtailed, but not prevented the onset of the Ca2+ train. Therefore, a pathway other than SOCE initiated the Ca2+ response to VEGF in UCB-ECFCs (Dragoni et al., 2013). As mentioned above, DAG is synthesized along with InsP3 upon PLCγ activation. Interestingly, our earlier comparison of the Ca2+ toolkit between PB- and UCB-derived ECFCs disclosed that TRPC3 is selectively expressed in the latter, but not in the former (Sanchez-Hernandez et al., 2010). In keeping with this notion, 1-oleyl-2-acetyl-sn-glycerol (OAG), a membrane permeant analogue of DAG, caused a massive entry of Ca2+ in UCB-, but not PB-derived ECFCs. Additionally, the pharmacological and genetic inhibition of TRPC3 prevented both OAG-gated Ca2+ influx and VEGF-induced Ca2+ oscillations and proliferation (Dragoni et al., 2013). These results clearly indicate that the concerted interplay between InsP3-dependent Ca2+ mobilization and SOCE that patterns the Ca2+ response to VEGF in ECFCs is triggered by TRPC3 in UCB-derived cells. In other words, although the waveform of the signal is almost identical between the two cell types, the underlying machinery is more complex in UCB-ECFCs. A cartoon depicting the signalling machinery engaged by VEGF in UCB-ECFCs is illustrated in Figure 1. Although SOCE controls proliferation in both cell types, it is unlikely to underpin their different angiogenic response to VEGF. Accordingly, Stim1, which initiates the assembly of the heteromeric supermolecular complex upon depletion of the ER Ca2+ store, is significantly downregulated in PB-ECFCs, whereas Orai1 is slightly up-regulated and TRPC1 normally expressed (Sanchez-Hernandez et al., 2010). It is currently unclear how TRPC3 initiates the spiking signal. In principle, TRPC3-mediated Ca2+ inflow could load the ER with Ca2+, thereby priming InsP3Rs from the luminal side as extensively illustrated in (Berridge, 2007, 2009). Some hints could be obtained by a recent series of studies that we conducted in primary myelofibrosis-derived ECFCs (Dragoni, Laforenza, et al., 2014; Dragoni, Reforgiato, et al., 2015). Herein, OAG may activate TRPC1 to trigger VEGF-induced Ca2+ oscillations; however, the measurement of [Ca2+]ER revealed that TRPC1 caused an immediate decrease, rather than an increase in ER Ca2+ levels, which reflects InsP3-dependent Ca2+ release (Dragoni, Reforgiato, et al., 2015). Therefore, we speculate that TRPC3 acts by recruiting the PLCγ/InsP3 signalling pathway as clearly demonstrated in DT40 B lymphocytes (Nishida et al., 2003). Herein, TRPC3-mediated Ca2+ inflow is sensed by the C2 domain of cytosolic PLCγ, which is in turn stimulated to translocate towards PM and trigger InsP3-dependent Ca2+ mobilization upon PIP2 hydrolysis (Nishida et al., 2003). Alternatively, TRPC3 could directly induce Ca2+ release by promoting the formation of a ternary complex with InsP3R1 and the receptor for activated C-kinase-1 (RACK1) (Bandyopadhyay et al., 2008; Woodard, López, Jardín, Salido, & Rosado, 2010), but this hypothesis is seemingly ruled out by the fact that InsP3R1 is absent in UCB-ECFCs (Dragoni et al., 2013). The following scenario is, therefore, predicted to occur in these cells. VEGFR-2 activation recruits PLCγ to generate DAG and InsP3. It is conceivable that the amount of InsP3 synthesized is not enough to trigger Ca2+ release, either because of modest PLCγ recruitment to PM or fast InsP3 degradation, so that the further activation of PLCγ by TRPC3 is indispensable to mobilize ER stored Ca2+ and stimulate SOCE (Moccia & Guerra, 2016). This mode of signaling could enhance the oscillatory behavior of [Ca2+]i in UCB-ECFCs as compared to their peripheral counterparts, also because TRPC3-dependent Ca2+ entry could further sensitize InsP3Rs from the cytosolic side through the CICR process (Morgan & Jacob, 1996; Shuttleworth & Thompson, 1996). Interestingly, TRPC3 also controls the development of Ca2+ oscillations during tube formation by EA.hy926 cells plated in Matrigel (Antigny, Girardin, & Frieden, 2012). The question then arises as to whether and how TRPC3 could improve the proliferative capacity of UCB-ECFCs. This issue will be addressed in the next paragraph.
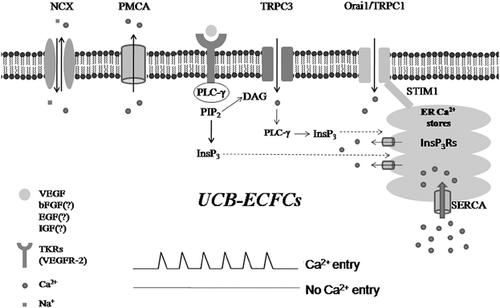
1.3 How could TRPC3 impact on the biological activity of UCB-ECFCs?
Bioelectrical signals, such as those delivered by the opening of an ion channel, finely tune many processes related to tissue regeneration, such as proliferation, migration, cell shape, positional fate, and differentiation (Levin, 2014). Ion channels, and particularly those bearing a significant Ca2+ permeability (Becchetti, 2011), directly feed into epigenetic and transcriptional cascades that shape the gene expression profile orchestrating cellular activity (Levin, 2014). As to Ca2+ signalling, the pattern of intracellular Ca2+ oscillations may dictate the ultimate fate of stem and progenitor cells (Forostyak et al., 2016). For example, the onset of fast Ca2+ spikes during the final commitment period stimulates chicken chondrogenic cell differentiation and cartilage formation (Varga et al., 2011), while the acceleration of spontaneous Ca2+ fluctuations promote human cardiac progenitor cell proliferation (Ferreira-Martins et al., 2009). Similarly, the increase in the frequency of Ca2+ oscillations is associated to an increase in the speed of mouse astrocyte progenitor migration (Striedinger, Meda, & Scemes, 2007).
In principle, TRPC3 could contribute to enhance the proliferative response to VEGF in two ways. First, it could act by simply increasing the frequency of the Ca2+ spikes. As explained above, the complex molecular choreography that sustains VEGF-induced Ca2+ oscillations could shorten the ISI between two consecutive Ca2+ transients either by accelerating InsP3 synthesis during development of the Ca2+ burst or by sensitizing InsP3Rs to ambient InsP3 through an increase in Ca2+ entry. The period of the Ca2+ waves is within the same range as that which has been shown to specifically recruit Ca2+-dependent decoders (Parekh, 2011; Smedler & Uhlén, 2014), such as protein kinase Cγ (PKCγ), Ras, NF-κB, and NFAT, that have all been linked to endothelial cell proliferation (Dragoni et al., 2011; Noren et al., 2016; Wong & Jin, 2005). Accelerating the Ca2+ fluctuations could boost the downstream Ca2+-dependent pathways. For instance, an increase in the average frequency of VEGF-evoked Ca2+ spikes may enhance the nuclear translocation of NFAT, thereby accelerating the rate of stalk cell proliferation during sprouting angiogenesis in vivo (Noren et al., 2016). Likewise, an increase in the frequency of Ca2+ oscillations augments NF-κB activity and, consequently, the expression of genes that play a key role during angiogenesis (Zhu et al., 2008). Finally, decreasing the ISI of the Ca2+ train from 600 s down to 100–200 s significantly enhances the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway (Kupzig, Walker, & Cullen, 2005), which in turn stimulates ECFCs activity and promotes tissue revascularization (Benslimane-Ahmim et al., 2011). Alternatively, TRPC3 could be selectively coupled to one or more downstream signaling pathways, which is a strategy by which Ca2+-permeable channels, such as Orai1, voltage-gated Ca2+ channels, and N-methyl-D-aspartate receptors, boost the strength, fidelity, and reliability of the signal they deliver (Bading, 2013; Moccia, Zuccolo, Soda, et al., 2015; Parekh, 2008; Samanta, Kar, Mirams, & Parekh, 2015). For instance, TRPC3 phosphorylation by PKC results in the assembly of a signaling platform with the Ca2+-sensitive phosphatase calcineurin; as a consequence, the proximal Ca2+ emerging from the channel is directly conveyed to calcineurin, which signals NFAT to initiate the transcriptional program responsible for HL-1 cell growth (Poteser et al., 2011). Moreover, TRPC3-mediated Ca2+ influx is necessary to recruit PKCβ to the PM and to sustain PKCβ-dependent full ERK activation in DT40 B cells through a direct protein:protein interaction (Numaga et al., 2010). In other words, TRPC3 may serve as a platform for signal transduction processes at the PM (Lichtenegger & Groschner, 2014). Furthermore, TRPC3-mediated Ca2+ entry could engage PKCα, thereby promoting IκB-α phosphorylation and the nuclear translocation of NF-κB in mouse airway smooth muscle cells (Song et al., 2016). The same study further showed that TRPC3 stimulates the nuclear relocation of NF-κB by recruiting the calcineurin/IκB-β pathway (Song et al., 2016). Also, TRPC3 may deliver spatially-restricted Ca2+ signals to CaMKII and Rac-1 (Ras-related C3 botulinum toxin substrate1) (Kitajima et al., 2011), a Ca2+-dependent small GTP-binding protein that is key to cellular metabolism by controlling the assembly and activation of NADPH oxidase (Carrizzo et al., 2014). A recent study revealed that VEGF gates TRPC3, thereby causing a massive Na+ influx in HUVEC and causing NCX1 to work into the reverse mode and mediate Ca2+ entry. The TRPC3-induced, NCX-dependent Ca2+ influx may in turn enlist ERK1/2 and enhance in vitro tubulogenesis (Andrikopoulos, Eccles, & Yaqoob, 2017). As anticipate earlier, this mechanism is, however, unlikely to be recruited by TRPC3 in ECFCs, which lack all the endothelial NCX isoforms and do not display detectable Ca2+ signals upon removal of external Na+ (Moccia & Guerra, 2016; Poletto et al., 2016), a procedure which has long been exploited to reverse the Na+ gradient across the PM (Berra-Romani et al., 2010; Moccia et al., 2002). Additionally, TRPC3 could provide an additional source of Ca2+ for eNOS stimulation, as recently shown in porcine coronary artery endothelial cells (PCAECs) (Huang et al., 2011). As anticipated above, besides stimulating EPC egression from BM, NO controls EPC-dependent angiogenesis and vascular remodeling by promoting proliferation and migration and inhibiting apoptosis (Bai et al., 2015; Lu et al., 2015; Ozuyaman et al., 2005; Wu et al., 2016; Zuccolo, Bottino, et al., 2016). The up-regulation of NO synthesis by exogenously expressed TRPC3 could be even more therapeutically relevant in ECFCs. Accordingly, these cells are much more efficient than CFU-ECs at preventing platelet aggregation and thrombus formation, thereby improving the outcome of CBT, due to their ability to release higher amounts of NO (Bou Khzam et al., 2015). The addition of such a powerful source of Ca2+ for eNOS activation would be highly recommendable in ECFCs, as SOCE seems to be only loosely coupled to NO production in healthy PB-ECFCs (Zuccolo, Bottino, et al., 2016). In this perspective, the impact of TRPC3 on ECFCs-based therapy could vary between male and female patients. A recent study demonstrated indeed that TRPC3-dependent Ca2+ influx is tightly linked to NO synthesis in PCAECs isolated from males, while TRPV4 is more suitable to activate the eNOS in females (Wong, Roberts, & Randall, 2015). Finally, TRPC3 may act by transactivating the EGF receptor (EGFR) by recruiting a complex signaling cascade, as recently illustrated in rat cerebral smooth muscle cells. TRPC3-mediated Ca2+ entry engages the Ca2+-sensitive conventional PKC, which in turn favors the association of TRPC3 with the PM metalloproteinase ADAM17 (A Disintegrin and Metalloproteinase 17). This exposes ADAM17 to an extremely localized Ca2+ hotspot that activates ADAM17 to induce heparin-binding EGF (HB-EGF) ectodomain shedding, thereby transactivating EGFR (Wang et al., 2016). This signalling pathway could be potentially relevant also in ECFCs as EGF is a powerful stimulator of angiogenesis (Carmeliet & Jain, 2011; Moccia et al., 2003).
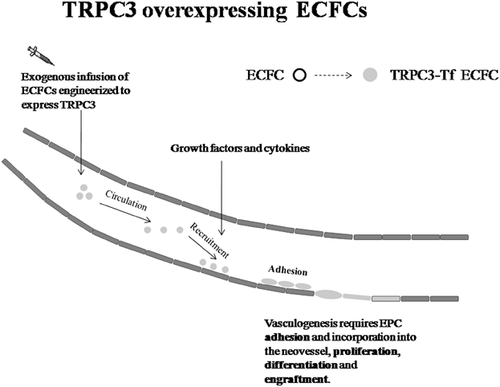
1.4 TRPC3 as a putative target of therapeutic angiogenesis by autologous endothelial colony forming cells
Overall, the observations reported above strongly suggest that the selective expression of TRPC3 could confer UCB-ECFCs with an objective proliferative advantage as compared to PB-derived cells. Therefore, we speculate that TRPC3 could become an exciting candidate to improve the regenerative outcome of CBT by combining the delivery of autologous ECFCs with the most recent gene transfer technologies (Figure 2). The following observations indicate that PB-ECFCs represent an amenable cellular substrate for combining gene therapy with therapeutic angiogenesis (Tasev et al., 2016). First, after their initial discovery, ECFCs were shown to promote tissue revascularization in a multitude of contexts. For instance, earlier work demonstrated that rat ECFCs facilitate fracture repair and new bone formation by inducing angiogenesis at the site of injury (Chandrasekhar et al., 2011). Subsequently, the injection of exogenous ECFCs was shown to promote tissue revascularization in several mouse models of hindlimb ischemia (Flex et al., 2016; Hache et al., 2016; Stalin et al., 2016). Furthermore, ECFC infusion restored local blood perfusion, stimulated angiogenesis and neurogenesis and reduced apoptosis in a mouse model of brain ischemia (Ding et al., 2016). Likewise, the intravitreal administration of ECFCs improved organ function and induced neovessel formation in a mouse model of retinal ischemia (Medina, O'Neill, Humphreys, Gardiner, & Stitt, 2010). Finally, and relevant to the aim of the present review, intramyocardial transplantation of ECFCs enhanced cellular proliferation and secretion of pro-angiogenic cytokines, increased capillary density, reduced myocardial fibrosis and partially rescued cardiac function in several murine models of AMI (Lee et al., 2014; Tasev et al., 2016). Second, ECFCs may be easily engineered to over-express pro-angiogenic signaling proteins (Tasev et al., 2016). Pioneering studies from Asahara's group revealed that the CFU-EC phenotype could be rejuvenated by transfecting the cells with an adenovirus encoding VEGF or hTERT, which dramatically improved the outcome of CBT in a mouse model of hindlimb ischemia (Murasawa & Asahara, 2005). This approach promoted neovascularisation, rescued local blood supply and limited limb necrosis and autoamputation by using a sub-therapeutic dose of cells, that is, 30 times less than that necessary with non-transduced cells (Iwaguro et al., 2002; Murasawa et al., 2002). More recently, ECFCs engineered with an adenovirus expressing either Akt or heme-oxygenase-1 (HO-1) led to enhanced neovascularisation, improved cardiac performance and reduced myocardial fibrosis in a mouse model of AMI (Brunt et al., 2012). At the same way, reconstituting Akt1 activity in ECFCs isolated from South Asian men reversed their inherited reduced reparative capacity in a mouse model of hindlimb ischemia (Cubbon et al., 2014). In this case, autologous ECFCs were transduced with a self-inactivating lentiviral vector (Cubbon et al., 2014). Intriguingly, we also have demonstrated that ECFCs may be reliably transfected with lentiviral vectors encoding for the Ca2+-sensitive probe aequorin (Lim, Bertoli, Sorgato, & Moccia, 2016). Finally, the sub-cutaneous implantation of a vascular network lined by ECFCs engineered to express erythropoietin was able to rescue erythropoiesis in anemic mice (Lin et al., 2011). All these evidences, therefore, suggest that ECFCs constitute an excellent tool for combining gene therapy with cellular therapeutics. Third, previous studies have demonstrated that the Ca2+ signalling machinery may be genetically manipulated to control stem cell fate and behavior. For instance, knocking down Stim1 or Orai1 dampened mouse neural progenitor cell (NPC) proliferation both in vitro and in vivo (Somasundaram et al., 2014). Similarly, down-regulating Stim1 and Orai1 impaired proliferation and migration in human cardiac c-kit+ progenitor cells (Che et al., 2015) and suppressed angiogenic activity in human ECFCs (Li et al., 2011; Lodola et al., 2012). Additionally, genetic silencing of TRP Melastatin 4 (TRPM4) channel prevented adipogenesis in human adipose-derived stem cells (Tran et al., 2014), while aquaporin 4 knockout affected proliferation, motility and neuronal differentiation in mouse NPCs (Kong et al., 2008). Conversely, co-expressing Stim1 and Orai1 or over-expressing InsP3Rs increased the rate of cell growth in Drosophila-derived intestinal stem cells (Deng, Gerencser, & Jasper, 2015). Similarly, up-regulating connexin 43 enhanced extracellular Ca2+ influx and odontoblastic differentiation of human dental pulp stem cells (Li et al., 2015). At the same way, over-expression of InsP3R1 accelerated human myoblast differentiation (Antigny, Konig, Bernheim, & Frieden, 2014), while over-expression of TPC2 prevented mouse embryonic stem cells from entering into the neuronal lineage (Zhang, Lu, & Yue, 2013). In the perspective of therapeutic angiogenesis, no study assessed the function outcome of TRPC3 over-expression on endothelial cells. However, increasing TRPC4 levels in human pulmonary artery endothelial cells (HPAECs) exposed to hypoxic conditions was earlier shown to enhance SOCE and boost proliferation (Fantozzi et al., 2003). Similarly, Mehta's group found that it was possible to increase TRPC6 expression on the PM by manipulating the activity of phosphatase and tensin homologue (PTEN), thereby accelerating angiogenesis (Kini, Chavez, & Mehta, 2010). This result is rather relevant to the aims of the present review as TRPC6 is also gated by DAG and is the closest structural relative of TRPC3 (Moccia, Berra-Romani, & Tanzi, 2012). In this view, VEGF-induced Ca2+ entry and proliferation were enhanced in HMECs transduced with an adenovirus encoding for TRPC6 (Hamdollah Zadeh et al., 2008). Moreover, the ectopic expression of TRPC1 promoted VEGF-dependent angiogenesis in the intersegmental vessels of zebrafish larvae by recruiting the ERK cascade (Yu, Gu, Bu, & Du, 2010). A recent report showed that Ca2+-permeable ion channels may be successfully targeted to favor EPC tropism towards the target issue. In this study, rat spleen-derived EPCs were primed by P2X7 receptor stimulation before being injected into a xenograft model of glioma. This treatment increased the homing of transplanted EPCs to the tumor and accelerated their physical incorporation within neovessels (Fang et al., 2015). Overall, these evidences strongly support the hypothesis that the reparative potential of ECFCs could be enhanced by engineering autologous cells with TRPC3 gene before their injection into the patients.
1.5 Caveats for the use of TRPC3 to improve the therapeutic potential of autologous ECFCs
Although the available evidence suggests that TRPC3 is implicated in the higher proliferative potential of UCB-ECFCs as compared to their peripheral counterparts, and that TRPC3-mediated Ca2+ entry recruits a number of pro-angiogenic Ca2+-sensitive decoders, some caution is warranted before translating these findings into pre-clinical studies. Studies from Vazquez's group demonstrated that the constitutive activity of TRPC3 drives the expression of vascular cell adhesion molecule-1 (VCAM-1) in human coronary artery endothelial cells (HCAECs), thereby favoring monocyte adhesion to the endothelial monolayer (Smedlund & Vazquez, 2008). Subsequently, they showed that pro-inflammatory/pro-atherogenic clues act by increasing TRPC3 and VCAM-1 expression in HAECs (Smedlund, Tano, & Vazquez, 2010). These data were then validated in vivo by using Apoe knockout mice with endothelial specific over-expression of TRPC3 (Smedlund, Birnbaumer, & Vazquez, 2015). In this model, advanced atherosclerotic plaques (16 weeks old) displayed larger size and increased macrophage content as compared to non-transgenic littermates (Smedlund et al., 2015); moreover, endothelial inflammation was noticeable already in the early lesions only in TRPC3-overexpressing mice (Smedlund et al., 2015). Furthermore, constitutive TRPC3-mediated Ca2+ influx triggers the unfolded protein response (UPR) in HCAEs and, in doing so, enhances their susceptibility to ER stress-induced apoptosis (Ampem, Smedlund, & Vazquez, 2016). This finding is consistent with the notion that many pro-atherogenic factors promote cause ER stress and consequently activate the UPR in an attempt to restore ER homeostasis (Tabas, 2010). Therefore, before testing TRPC3-over-expressing ECFCs for their regenerative potential, one should first assess whether: 1) they up-regulate their pro-atherosclerotic/pro-inflammatory signalling pathways and 2) they are more prone to undergo ER stress and UPR. Nevertheless, the pharmacological and genetic inhibition of TRPC3 did not decrease resting Ca2+ levels in UCB-ECFCs (Dragoni et al., 2013), which indicates that the channel is not active in the absence of extracellular stimulation in these cells. Moreover, lentivirus or adenovirus expression could be carefully titrated in order to achieve an amount of channel protein which is sufficient to boost the pro-angiogenic activity of PB-ECFCs without triggering the UPR.
2 CONCLUSION
The advent of cellular therapeutics has been predicted to provide the magic bullet for the treatment of ischemic disorders, such as HF and peripheral artery disease, as well as of other life-threatening pathologies, such as neurodegeneration and retinal degenerative disease. Nevertheless, we are still far from fully exploiting the regenerative potential of autologous stem and progenitor cells for therapeutic purposes. This limit depends on many factors, including, but not limited to, the scarce number of autologous stem and progenitor cells, which requires expensive procedures of ex vivo expansion, the poor understanding of the mechanisms leading to cell differentiation into the desired fate, and the identification of the right dose and of the most proper time for injection. Many strategies, including humoral pre-conditioning and genetic engineering, have been put forward to enhance the ability of autologous EPCs to achieve the structural and functional regeneration of failing hearts. In particular, the rejuvenation of their reparative phenotype could be of significant help to circumvent the main shortcoming related to their therapeutic use, namely their rare number in peripheral blood. This hurdle is even more pronounced in the case of ECFCs, which represent a very exciting tool for CBT of ischemic diseases due to their ability to induce postnatal vascularization by physically engrafting within neovessels. Although no study hitherto assessed whether the pro-angiogenic activity of ECFCs is impaired during ageing or in presence of cardiovascular risk factors, such as atherosclerosis, this is extremely likely to produce an additional shortcoming for their therapeutic application. Our comparative work performed on PB- and UCB-derived ECFCs suggested that TRPC3 gene could be a promising candidate to rejuvenate the bioactivity of ECFCs harvested from PB of aging subjects or of individuals exposed to cardiovascular risk factors. This hypothesis, of course, remains to be supported by a throughout in vitro and in vivo characterization of TRPC3-overexpressing cells, but introducing the Ca2+ toolkit in the list of genes to exploit for achieving the revascularization of ischemic tissues could represent an unexpected advance for CBT.
ACKNOWLEDGMENTS
We are grateful to all our coworkers, who are sharing with us this ECFC trip. This article is dedicated the memory of Prof. Maria Pia Cinelli. We do not have any conflict of interest to declare.




