Apelin/APJ system: A novel potential therapy target for kidney disease
Abstract
Apelin is an endogenous ligand of seven-transmembrane G protein-coupled receptor APJ. Apelin and APJ are distributed in various tissues, including the heart, lung, kidney, and even in tumor tissues. Studies show that apelin mRNA is highly expressed in the inner stripe of kidney outer medulla, which plays an important role in process of water and sodium balance. Additionally, more studies also indicate that apelin/APJ system exerts a broad range of activities in kidney. Therefore, we review the role of apelin/APJ system in kidney diseases such as renal fibrosis, renal ischemia/reperfusion injury, diabetic nephropathy, polycystic kidney disease, and hemodialysis (HD). Apelin/APJ system can improve renal interstitial fibrosis by reducing the deposition of extracellular matrix. Apelin/APJ system significantly reduces renal ischemia/reperfusion injury by inhibiting renal cell death. Apelin/APJ system involves the progression of diabetic nephropathy (DN). Apelin/APJ system also predicts the process of polycystic kidney disease. Besides, apelin/APJ system prevents some dialysis complications in HD patients. And apelin/APJ system alleviates chronic kidney disease (CKD) by inhibiting vascular calcification (VC). Overall, apelin/APJ system plays diversified roles in kidney disease and may be a potential target for the treatment of kidney disease.
1 INTRODUCTION
Angiotensin 1 receptor related protein APJ, a seven-transmembrane G protein-coupled receptor, is first found by O'Dowd in 1993 (O'Dowd et al., 1993). The amino acid sequence of APJ shares 31% homology with angiotensin II type −1 receptor (AT-1), and even 54% in the hydrophobic transmembrane region (O'Dowd et al., 1993). APJ is also known as the orphan nuclear receptor until the discovery of apelin. Apelin, an endogenous ligand of APJ, is isolated from bovine stomach tissue in 1998 (Liu et al., 2010; Tatemoto et al., 1998). Recent studies suggest that Elabela is a novel endogenous ligand for APJ (Xie, Lv, & Chen, 2014). The human apelin gene (APLN) is located in chromosome Xq25-26, which contains 3 exons and 2 introns (Lee et al., 2000). APLN encodes a 77 amino acids apelin precursor (pre-apelin). Pre-apelin has 76–95% homology in the rat and mouse. Pre-apelin contains an N terminal and a C terminal with potential proteolytic cleavage sites. The C terminal contains a sequence of biological activity and a region specific which can bind to the APJ, while the amino acid sequences between tryptophan 55 and phenylalanine 77 is completely conserved among different species (Medhurst et al., 2003). Pre-apelin can be divided into different apelin peptide including apelin-12, apelin-13, apelin-17, and apelin-36. Among these, apelin-13 (42–77) and apelin-36 (65–77) are the most widely studied. Some studies show that apelin-13 is one of the most important subtype in human plasma (Zhen, Higgs, & Gutierrez, 2013).
Apelin and APJ are widely expressed in many tissues, such as heart, kidney, and blood vessels (Li et al., 2013; Liu, Chen, & Chen, 2016; Lv, Li, & Chen, 2013; Xie et al., 2015; Yu et al., 2014). A large amount of evidence indicates that apelin/APJ system plays an important role cardiovascular system (Barnes, Japp, & Newby, 2010; Galanth, Hus-Citharel, Li, & Llorens-Cortes, 2012; Tycinska, Lisowska, Musial, & Sobkowicz, 2012; Yu et al., 2014). In the cardiovascular system, apelin/APJ system can reduce blood pressure, promote the formation of new blood vessels, reduce arterial tension, and the heart preload (Yu et al., 2014). In addition, apelin/APJ system can regulate gastrointestinal function and insulin sensitivity, promote cell proliferation and migration, induce angiogenesis, and regulate immune function (Adam et al., 2016; Castan-Laurell et al., 2011; Lv et al., 2016; Tiani et al., 2009; Wang et al., 2004; Zhou, Cao, & Chen, 2016). Various researches show that apelin may play a role in body fluid homeostasis (Najafipour, Soltani Hekmat, Nekooian, & Esmaeili-Mahani, 2012). Collectively, these suggest that apelin/APJ system may be involved in a wide range of physiological and pathological processes.
Recently, more and more studies show that apelin/APJ system plays an important role in kidney disease. Han, Wang, Diao, and Liu (2016) find that apelin/APJ system may exert potential therapeutic value for the treatment of vascular calcification (VC) in chronic kidney disease (CKD). Guo et al. (2015) find that apelin/APJ system induces podocyte dysfunction in diabetic nephropathy (DN) through endoplasmic reticulum (ER) stress induced by decreased proteasome activities in podocytes. In this paper, the role of apelin/APJ system in renal disease is reviewed.
2 EXPRESSION OF APELIN/APJ SYSTEM IN KIDNEY
Apelin/APJ system is expressed in kidney. And as APJ mRNA expression is found in the rat kidney, the role of apelin/APJ system gets much attention in kidney (Hosoya et al., 2000; O'Carroll, Selby, Palkovits, & Lolait, 2000). Studies show that apelin/APJ is expressed in kidney. APJ mRNA is expressed in all nephron segments in the rat kidney(Hus-Citharel et al., 2008; Sagiroglu et al., 2012). Additionally, apelin immunoreactivity is detected in the human collecting tubules (De et al., 2002). Moreover, apelin is mainly expressed in renal vascular endothelial cell (Kleinz & Davenport, 2004). The level expression of apelin mRNA is the highest in the inner medulla of kidney outer medulla (Hus-Citharel et al., 2008). And apelin is intimate correlated with water and sodium balance in the inner stripe of kidney outer medulla (Khan et al., 2010).
The expression of apelin/APJ system may play an important role in kidney. The mRNA of apelin is expressed in all renal elements, which may regulate the function of the renal tubule. Studies also find that apelin is distributed in the urinary catheter, suggesting that apelin may be a pro drainage peptide in this site (Najafipour et al., 2012). Additionally, apelin/APJ system regulates renal hemodynamics and diuresis in the pre- and post- glomerular microvasculature. More importantly, the expression of apelin/APJ system potentially occur some changes in kidney disease. Zhang et al. find that the number of glomeruli with apelin positive staining is increased in type 2 diabetic mice (KK mice) compared to that in control mice (Zhang, Wang, Wang, Yin, & Zeng, 2013; Guo et al., 2015). Higher levels of apelin are detected in patients with type 2 diabetes and in the kidneys of type 2 diabetic mice (Zhang et al., 2013). Autosomal dominant polycystic kidney disease (ADPKD) patients are characterized by low apelin levels (Lacquaniti et al., 2013). Nishida et al. (2012) find that both apelin and APJ receptor mRNAs are upregulated at day 7 after unilateral ureteral obstruction (UUO). These results indicate that apelin/APJ system is linked to kidney disease, which is likely to predict the progression of certain kidney diseases.
3 THE ROLE OF APELIN/APJ SYSTEM IN KIDNEY DISEASES
3.1 Apelin/APJ system attenuates renal fibrosis
Renal fibrosis is a major hallmark in the occurrence and progression of CKD (Mack & Yanagita, 2015; Roberts, Cowan, Alexander, Robson, & Dwyer, 2014). Extracellular matrix (ECM) protein is a typical marker of renal fibrosis, which increased the degree of renal damage in the glomerular and renal interstitial abnormalities (Sun, Qu, Caruana, & Li, 2016). The endless healing process causes renal fibrosis when the normal wound healing response fails. Studies indicate that the level of apelin is elevated when mice are subject to UUO surgery and then treat with subsequent losartan treatment. Furthermore, losartan alleviates UUO-induced renal fibrosis via the apelin/APJ/Akt/eNOS pathway (Huang, Chen, Lu, & Li, 2016a). And apelin/APJ system can activate the endothelial nitric oxide synthase (eNOS) pathway (Ashley, Chun, & Quertermous, 2006; Huang et al., 2016a). Apelin/APJ system, interacting with losartan exerts a synergistic effect in attenuating renal fibrosis (Nishida et al., 2012). In vitro, apelin inhibits epithelial–mesenchymal transition (EMT) induced by the TGF-β (transforming growth factor-β) in HK-2 cells. In the UUO model, apelin significantly reduces the expression of TGF-β1 and its receptor, as well as interstitial matrix components. Apelin also ameliorates renal interstitial fibrosis by suppressing tubular EMT in a smad protein-dependent mechanism (Wang et al., 2014). All of these findings show that apelin/APJ system can reduce the deposition of ECM and improve renal interstitial fibrosis. Therefore, apelin/APJ system has potential renoprotective effects, which is an effective agent for retarding CKD progression.
3.2 Apelin/APJ system alleviates renal ischemia/reperfusion injury
Renal ischemia/reperfusion (I/R) injury is the leading cause of acute kidney injury (AKI) during surgery, arterial occlusion, shock, and organ transplantation (Yamamoto et al., 2011). Renal I/R can cause tubular epithelial cell damage, including renal tubular obstruction and decreased NaCl resorption. Chen et al. (2015) show that apelin-13 can significantly reduce renal tubular lesions and renal cell death. Apelin-13 inhibits up-regulation of TGF-β1, the level of H3K4me2 and Kmt2d (the histone methyltransferase of H3K4me2) induced by I/R or H/R (hypoxia/reperfusion) injury in glomerular mesangial and tubular cells (Figure 1) (Chen et al., 2015). All of the above results suggest that apelin-13 alleviates acute renal injury. Sagiroglu et al. (2012) also find the intraperitoneal injection of apelin increases the glomerular filtration rate, and decreases the degree of kidney damage in the renal I/R rats. TGF-β1, a major member of the TGF family, is closely associated with the fibrosis of renal disease and inflammation (Choi, Ding, & Kim, 2012). Bircan, Cakir, Kirbag, and Gul, (2016) detail the possible protective effect of apelin-13 on the kidney I/R injury. Results show that apelin-13 treatment can increase serum blood urea nitrogen (BUN), creatinine (CRE), Cl, and tumor necrosis factor alpha (TNF-α) levels, and decrease Na, total protein, albumin, total antioxidant status (TAS) levels. Therefore, the inhibition of TGF-β1 may be related to the protective effect of apelin-13 on renal injury. Besides, apelin-13 increases the antioxidant enzyme activity, prevents the lipid oxidation and improves the renal functions in renal I/R.
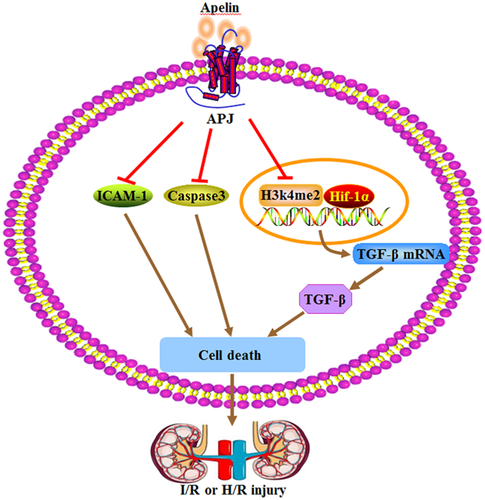
3.3 Apelin/APJ system induces or prevents DN
DN is one of the main complications in diabetes, and is also the most common etiology of the end-stage renal disease (Maezawa, Takemoto, & Yokote, 2015). The early clinical manifestations of DN include glomerular hypertrophy and thickening of glomerular basement membrane (Cooper, 2001). With the progress of the disease, high glomerular filtration can lead to proteinuria, eventually developing end-stage renal disease (Susztak & Bottinger, 2006). Studies show that apelin/APJ system plays an important role in the DN. Compared with healthy subject, patients with type 2 diabetes appears to higher serum levels of apelin. And urinary albumin and serum apelin levels are positively correlated. Additionally, apelin increases trace protein in the urine and the ratio of CRE in KK mice (Zhang et al., 2013). Apelin increases the migration, proliferation, and tropism of glomerular endothelial cell in dose dependent. The increasing of apelin promotes the permeability of glomerular endothelial cells, and increases vascular endothelial growth factor and the expression of adenosine diphosphate (Zhang et al., 2013). Some studies also suggest that apelin/APJ system can induce the produce of endothelial cells by an autocrine or paracrine manner (Cox, D'Agostino, Miller, Heimark, & Krieg, 2006). Therefore, the apelin can promote angiogenesis of the glomerulus, forming abnormal blood vessels by adjusting the vascular endothelial growth factor in type 2 diabetes mellitus. Meanwhile, the permeability of glomerular endothelial cells is enhanced through adjusting the vascular endothelial growth factor. And the expression of adenosine diphosphate enhances the permeability of glomerular endothelial cells.
Apelin also plays an important role in regulating DN blood vessel function. The decrease of APJ protein and increase of contractile responding to Ang II and Ang IV are exhibited in renal arteries from db/db mice. But, after apelin supplement, the abnormal renal vascular tone in responses to Ang II and acetylcholine in diabetic mice is reversed. These beneficial effects of apelin are thought to result mainly from activation of eNOS phosphorylation and enhancement of NO generation (Zhong et al., 2007).
Studies show the exogenous apelin can slow the progress of DN. Day, Cavaglieri, and Feliers, (2013) study the mice with type 1 diabetes received daily subcutaneous injections of apelin for 2 or 14 weeks. The study finds that apelin does not affect the diabetic rat's blood glucose, body weight, and blood pressure, but inhibit the whole kidney and glomerular hypertrophy, as well the renal inflammation. And apelin can decrease the expression of monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule 1 (VCAM1), the activation of NF-κB and the infiltration of monocyte (Figure 2) (Day et al., 2013). The above results show that apelin can protect DN. Apelin may be used as a new tool for the treatment of DN. In vivo studies also show that apelin has an effect on the treatment of DN. Silva et al. (2013) find the correlation between different apelin levels and cardiovascular mortality, hospitalization rates, renal function, and cardiovascular risk factors in patients with type 2 diabetes. The study finds that the patients with higher baseline apelin level group have a higher survival in 83 months, apelin level is an independent predictor of death and hospitalization. Apelin levels are negatively correlated with cardiovascular risk factors, but are positively correlated with the levels of estimated glomerular filtration rate (eGFR) (Silva et al., 2013). These results suggest that apelin may be as predictive factors for cardiovascular death and hospitalization, and even can play a role in treatment. What is the mechanism that apelin affects the progression of DN? Chen et al. (2014) find that apelin may inhibit the progress of DN by adjusting the histone acetylation. In spontaneous DN mice model, the tail vein injection of apelin-13 can reduce the glomerular filtration rate, proteinuria, glomerular hypertrophy, and glomerular mesangial expansion, lower histone acetylation and kidney inflammation. Application of apelin-13 in glomerular mesangial cells can not only suppress the height of histone acetylation induced by hyperglycemia and inflammatory factor, but also raise histone deacetylase1 (Chen et al., 2014).
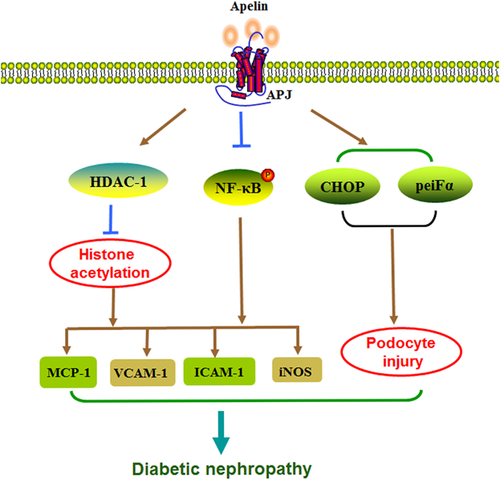
However, some studies believe that apelin may also promote the progress of DN. Podocyte damage is associated with the disease progression of DN. Guo et al. (2015) find that apelin can induces podocyte function disorder through the inhibition of the proteasome activity, in turn, promote the progress of DN disease. Apelin can increases proteinuria in kk-Ay mice and reduces podocyte proteins expression. It is consistent with the increase of glomerular APJ levels in patients with DN and mice. The increased expression of APJ in high glucose cultivate podocyte and injection of apelin-13 in kk-Ay mice celiac causes the foot process protein level decreased and permeability increased. All of these dysfunction is associated with the decrease of 26s proteasome activation and the crease of many ubiquitin proteins in kk-Ay mice and podocytes. Because the apelin can also increase C/EBP homologous protein (CHOP) and the levels of peiFα (phosphorylates eukaryotic translation initiation factor α), so these effects may be associated with ER stress (ERS) (Guo et al., 2015).
Why does this contradiction arise? First of all, the focus of the research is different. The main focus is on ERS and oxidative stress (OS). The interaction mechanism between ERS and OS is not clear. The role of apelin/APJ system in this regard is worthy of further study and attention. Second, DN is a complex process. The role of apelin is associated to many factors of the occurrence and development of DN, such as insulin resistance, obesity, and glycolipid metabolism disorders. Therefore, the role of apelin/APJ system in DN disease progression is controversial, which still needs further research.(Table 1)
| Kidney disease | Experiment model | Treatment | Indicators | Effect | References |
|---|---|---|---|---|---|
| Renal fibrosis | Nondiabetic, clinically stable haemodialyzed patients with or without CAD | Investigated plasma apelin levels | Apelin may be associated with cardiac function | Renal fibrosis | Malyszko, Malyszko, Kozminski, and Mysliwiec (2006) |
| In UUO mice | Angiotensin II type 1receptor | Increase apelin, p-Akt, eNOS | Nishida et al. (2012) | ||
| Renal ischemia/reperfusion injury | Male rats with renal I/R injury | Apelin-13 treatment | Reverse against acute renal injury via suppressing TGF-β | Chen et al. (2015) | |
| The I/R rats | Intraperitoneal injection of apelin | BUN, creatinine, GFR, the degree of pathological damage | Glomerular filtration rate is higher, the lower the degree of kidney damage | Sagiroglu et al. (2012) | |
| Diabetic nephropathy | Type 2 diabetes | Albuminuria and serum apelin | Apelin higher, urinary albumin was positively correlated with serum apelin upregulated VEGFR2 and Tie2 | Zhang et al. (2013) | |
| Diabetic db/db mice | Apelin receptor APJ Ang II and Ang IV | Diminished expression of APJ protein Enhanced contractile responses to Ang II and Ang IV | Zhong et al. (2007) | ||
| Kidney disease in mice with established type 1 diabetes | Injections of apelin, for 2 or 14 weeks | MCP1, VCAM1, NF-κB | Whole kidney and glomerular hypertrophy, inhibit MCP1, VCAM1 expression, NF-κB activation and monocyte infiltration | Day et al. (2013) | |
| ADPKD | ADPKD patients | Apelin level Copeptin level | Apelin levels were lower, higher copeptin levels apelin and copeptin showed a very good diagnostic profile in identifying ADPKD progression. | Lacquaniti et al. (2013) | |
| Apelin/TGF-β1 | Apelin levels were lower TGF-β1 levels were higher | Kocer et al. (2016) | |||
| Hemodialysis (HD) | Children with CKD stage 5 on haemodialysis and peritoneal dialysis. | Adiponectin, apelin, chemerin, omentin, resistin and vaspin | Apelin, omentin, and resistin was higher vaspin, adiponectin, and chemerin was significantly lower | Szczepanska et al. (2015) | |
| HD patients | Apelin/PTH | PTH > 300 pg/ml had significantly higher plasma apelin levels | Mafra et al. (2012) | ||
| Apelin-36 levels | LDL/TNF-α, IL-6, leptin, and CRP | No difference with apelin-36 levels | Leal et al. (2012) | ||
| Apelin-12 levels | Apelin-12 levels were significantly higher. TNF-α, CRP, and LDL (−) levels were higher | ||||
| Creation of a-v fistula might contribute | Plasma Apelin, resistin, visfatin, and vWF | Malyszko et al. (2011) | |||
| PD patients | Investigated apelin-36 levels | Left atrium diameter; diastolic BP, EF, total cholesterol, LDL-cholesterol, HDL-cholesterol, parathyroid hormone, and ALP levels | Negatively with age and left atrium diameter; positively with diastolic BP, EF, total cholesterol, LDL-cholesterol, HDL-cholesterol, parathyroid hormone, and ALP levels. | Karadag et al. (2014) | |
| Patients chronically treated with hemodialysis | Plasmatic osmolality, AVP, apelin, BP, hsCRP, and β2-microglobulin | Apelin rises plasmatic osmolality, AVP, BP, decrease apelin is inversely related to hsCRP | Cernaro et al. (2012) |
- 13-week-old male diabetic C57BL/KsJ (db/db).PTH, parathyroid hormone; EF, ejection fraction; ALP, alkaline phosphatase; CRP, C-Reactive protein; AVP, arginine-vasopressin; BP, mean blood pressure; hsCRP, high-sensitivity C-reactive protein; vWF, von Willebrand factor.
3.4 Apelin/APJ system predicts on the progress of polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life-threatening genetic disease, promoting the progression of end-stage renal disease and cardiovascular disease (Perrone, Ruthazer, & Terrin, 2001). Polycystic kidney disease is characterized by thickening of the tubular basement membrane, renal interstitial fibrosis, and the formation of renal cysts, resulting in structural changes in the kidneys, and ultimately the formation of end stage renal disease (Grantham, 1996). Some scholars evaluate the correlation between apelin and TGF-β1 and ADPKD. They find that the level of apelin is lower, and the level of TGF-β1 is higher in ADPKD patients. At the same time, apelin is negatively correlated with TGF-β1, and is positively correlated with eGFR (Kocer, Karakukcu, Ozturk, Eroglu, & Kocyigit, 2016). Lacquaniti et al. (2013) also show that apelin level is lower, and the copeptin is higher in 52 patients with ADPKD compared with healthy subjects. Analysis of receiver operating characteristic curve (ROC) find that apelin and copeptin shows good receiver operating characteristic curve, in the process of identifying ADPKD disease (Lacquaniti et al., 2013). Cox proportional hazards regression model also show that apelin can predict on the progress of kidney disease. Compared to the eGFR, apelin may appear to a better role in predicting ADPKD disease process, and provides more information.
3.5 Apelin/APJ system promotes dialysis complications in HD
HD is one of the effective alternative treatment approaches for patients with end-stage renal disease. The concentration of apelin increase in the CKD5 stage patients treated with blood and peritoneal dialysis (Szczepanska et al., 2015). In peritoneal dialysis patients, apelin-36 level has is a negatively correlated with age and left ventricular diameter. The diastolic blood pressure, ejection fraction, total cholesterol, LDL cholesterol, HDL cholesterol, parathyroid hormone, and alkaline phosphatase (ALP) levels are positively correlated with apelin-36 level. The linear analysis results show that the diastolic blood pressure, LDL cholesterol, ALP, and ejection fraction are the independent determinants of apelin-36 (Karadag et al., 2014). Thus, apelin/APJ system plays an important role in capacity adjustment, cardiovascular function, lipid metabolism, and bone mineral disorders in patients with peritoneal dialysis. Mafra et al. (2012) also report that apelin levels and PTH levels are positively correlated in HD patients. Additionally, the level of apelin level is significantly increased in the patients with the PTH > 300 pg/ml compared to the PTH < 300 pg/ml (Mafra et al., 2012), And apelin can protect bone dialysis patients. Leal et al.(2012) find that the apelin-36 level has no difference in HD, but the apelin-12 level is much higher in HD patients. The level of TNF-α, c-reactive protein, and LDL (−) are far higher than that of control group in dialysis patients (Leal et al., 2012). However, apelin-12 and apelin-36 are not associated with inflammation and OS markers. Thus, plasma apelin level seems to be not associated with cardiovascular risk in HD patients. Interestingly, the apelin level is increased in the process of dialysis treatment, but decreased in the plasma osmotic pressure, arginine vasopressin (AVP), and mean blood pressure. Thus, apelin is a negatively correlated with high-sensitivity c-reactive proteins (Cernaro et al., 2012), while AVP/apelin can balance the change of dialysis plasma osmotic pressure in the process of dialysis. Therefore, AVP/apelin can be used as markers for arterial low or high pressure. In the stability of dialysis patients, apelin is associated with echocardiographic parameters, diabetes, coronary artery disease, and chronic heart failure, cardiac function classification, and blood lipid, high-sensitivity c-reactive protein, vascular pseudo willebrand factor, residual kidney function. Multivariate analysis shows that apelin level is significantly associated with ejection fraction, diabetes, arteriovenous fistula position (Malyszko, Kozminski, Malyszko, & Mysliwiec, 2011). Therefore, apelin may be associated with the pathology physiology of cardiovascular disease and chronic heart failure. El-Shehaby, El-Khatib, Battah, and Roshdy, (2010) also find that apelin level in dialysis patients is a positive correlation with left ventricular end systolic diameter, left ventricular end-diastolic diameter, interventricular septum, the right ventricle, the left ventricle, and the aorta, but is negatively correlated with high-sensitivity c-reactive proteins and IL-6 (El-Shehaby et al., 2010).
3.6 The other role of apelin/APJ system in kidney disease
VC is associated with increased risk of cardiovascular events, which are the most common cause of death in patients with CKD (Giachelli, 2004). It is reported that apelin/APJ system can effectively restrain the osteoblast differentiation in the calcification of vascular smooth muscle cells (Yuan et al., 2015). Shan et al. (2011) indicate that apelin inhibits the VC in CKD condition. Additionally, apelin-13 can inhibit calcium deposition by inhibiting osteoblast transforming gene BMP-2, OPG, and Cbfa1 induction in human arterial smooth muscle cells (HASMCs) (Han et al., 2016). These effects are mediated by sodium phosphate coordinated transport protein expression and phosphate intake. In phosphate-induced mineralization of human aortic smooth muscle cells (HASMCs) and in adenine-induced CKD rats with aortic calcification, apelin ameliorates VC by suppressing the osteoblastic differentiation of VSMCs and the downregulation of Pit-1 (Han et al., 2016). These results suggest apelin may have potential therapeutic value for treatment of VC in CKD. Malyszko, Malyszko, Pawlak, Wolczynski, and Mysliwiec, (2008) study the association between apelin and coronary artery disease (CAD) in kidney allograft recipients. These results show that apelin is significantly reduced in kidney allograft recipients with CAD. And apelin level can be predicted by the presence of CAD, endothelial damage, or inflammation. Apelin may be associated with inflammation and its clinical consequences (Malyszko et al., 2008).
4 THE POTENTIAL THERAPEUTIC TARGET OF APELIN/APJ IN KIDNEY DISEASES
The above-mentioned research results demonstrate that the apelin/APJ system plays an important role in the process of occurrence and development of kidney diseases such as renal fibrosis, renal I/R injury, DN. Therefore, the apelin/APJ system may become one of the potential drug targets for the treatment of kidney diseases (Cao, Li, & Chen, 2015).
Studies report some small molecule agonists and antagonists targeting APJ. The natural peptide agonists of APJ include apelin-13 (Maguire, Kleinz, Pitkin, & Davenport, 2009), Pyr-apelin-13 (Maguire et al., 2009), apelin-13 Q1A (Fan et al., 2003), apelin-13 H7A (Fan et al., 2003), apelin-13 P12A (Fan et al., 2003), apelin-12 C1 (Hamada et al., 2008), and so on (Huang, Chen, Lu, & Li, 2016b). Apelin-13 treatment markedly reduces the injury-induced tubular lesions, renal cell apoptosis, and normalized the injury induced renal dysfunction (Chen et al., 2015). Pyr-apelin-13 exerts a protective effect on the diabetic kidney (Day et al., 2013). Apelin-13prevents the lipid oxidation and improves the renal functions when I/R increases the antioxidant enzyme activity in a dose dependent manner (Bircan et al., 2016).
In addition, the natural antagonist isoforms of APJ include apelin-13 F13A (Lee et al., 2005), apelin-13 (13[D-Phe]) (Lee et al., 2005), apelin-13 (F13[D-Ala]) (Lee et al., 2005), apelin-13 C1 (Macaluso, Pitkin, Maguire, Davenport, & Glen, 2011), apelin-13 C2 (Macaluso et al., 2011), and so on. F13A can abrogate the process where microalbuminuria and podocyte foot process effacement are aggravated via treating with apelin. F13A restores the increase of the polyubiquitinated proteins in kk-Ay mice caused by apelin (Guo et al., 2015).
Therefore, drugs targeting for apelin may apply to the treatment of kidney diseases. Studies show that puerarin down regulates apelin and exerts a protective effect on renal hypertension (Jin et al., 2009). Huang, Bai, Chen, Wang, and Ding, (2013) also find that puerarin decreases the expression of apelin in ischemic and non-ischemic kidneys. These results suggest that puerarin regulates blood pressure and protects target organs which may be in part because of apelin/APJ pathway. In conclusion, every drug targeting for APJ and apelin become the reliable tools to explore the mechanism of kidney diseases.
5 CONCLUSIONS AND PERSPECTIVES
In conclusion, apelin/APJ system plays a variety of biological function in kidney disease. Apelin/APJ system is a critical mediator of renal fibrosis, renal I/R injury, DN, polycystic kidney disease, HD, and CKD. Apelin/APJ system plays a dual role in some kidney diseases such as CKD. As a new member of the renin-angiotensin system (RAS), apelin/APJ system has key functions in dilate blood vessels, lower blood pressure, increase myocardial contraction force, and so on. However, apelin/APJ system may promote some kidney diseases progress including renal I/R injury, HD, ADPKD. Therefore, the role of apelin/APJ system in kidney disease is complicated.
More studies are required to explore new underlying mechanisms and roles of apelin/APJ system in kidney disease. Further exploration the role of apelin/APJ system in kidney disease will facilitate the discovery of potential targets for diseases. And the findings of new and effective drugs target for apelin/APJ system will alleviate the sufferings of patients or cure these kidney diseases.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (grant numbers: 81470434,81503074), Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study (Hunan Provincial Education Department document (Approval number: 2014–405)).




