CRISPR editing in biological and biomedical investigation
Abstract
Recently, clustered regularly interspaced short palindromic repeats (CRISPR) based genomic editing technologies have armed researchers with powerful new tools to biological and biomedical investigations. To further improve and expand its functionality, natural, and engineered CRISPR associated nine proteins (Cas9s) have been investigated, various CRISPR delivery strategies have been tested and optimized, and multiple schemes have been developed to ensure precise mammalian genome editing. Benefiting from those in-depth understanding and further development of CRISPR, versatile CRISPR-based platforms for genome editing have been rapidly developed to advance investigations in biology and biomedicine. In biological research area, CRISPR has been widely adopted in both fundamental and applied research fields, such as accurate base editing, transcriptional regulation, and genome-wide screening. In biomedical research area, CRISPR has also shown its extensive applicability in the establishment of animal models for genetic disorders especially those large animals and non-human primates models, and gene therapy to combat virus infectious diseases, to correct monogenic disorders in vivo or in pluripotent cells. In this prospect article, after highlighting recent developments of CRISPR systems, we outline different applications and current limitations of CRISPR use in biological and biomedical investigation. Finally, we provide a perspective for future development and potential risks of this multifunctional technology.
1 INTRODUCTION
Few technological innovations may truly transform the landscape of genetic manipulations in biological and biomedical research, whereas the recently developed innovative genome editing tool- CRISPR/Cas9 has led to such a paradigm shift. CRISPR was conceived after a yogurt company identified it as an unexpected defense mechanism in bacteria to fight off viruses in 2007 (Travis, 2015). Further insight arrived in 2012, Cas protein was firstly demonstrated to cleave specific DNA sequences guided by short synthetic guide RNAs in vitro (Gasiunas, Barrangou, Horvath, & Siksnys, 2012). In vivo demonstrations followed in 2013, with the labs of Church, Doudna and Zhang quickly exhibiting the power of CRISPR-mediated genome modification in mammalian cells (Cong et al., 2013; Jinek et al., 2013; Mali, Yang, et al., 2013). In the short period since then, CRISPR/Cas revolution has ushered in an era of its own, by introducing a new scientific verb and giving rise to the now commonplace phrase: “CRISPR it!” (Dow, 2015). Owing to its capacity of scalable, affordable, and easy to engineer, CRISPR/Cas technologies fuel biological and biomedical investigations through efficient and versatile genetic modifications listed but not limited as deletion, knock-in, RNA regulation and chromatin modification of the targeted gene loci in various cell types and living organisms (Cong et al., 2013). All those promising applications made CRISPR/Cas systems to be selected by Science as “2015 Breakthrough of the Year” (Travis, 2015).
Classical gene editing efforts most relied on the endogenous cellular homologous recombination (HR) pathway, that the insertion of exogenous DNA sequence must be mediated by long homologous sequences flanking the target genomic DNA site (Smithies, Gregg, Boggs, Koralewski, & Kucherlapati, 1985). Spontaneous HR via transfecting exogenous donor DNA sequence is very inefficiently, at rates of 1 in every 103–109 cells, depending on the cell type and cell state (Thomas & Capecchi, 1986). Despite the low efficiency of editing by spontaneous HR, this approach could also induce undesired integration events, in which the exogenous DNA sequence is inserted into the genome at random sites more frequently than at the desired locus (Lin, Sperle, & Sternberg, 1985). So far, the most successful use of spontaneous HR was shown in mouse embryonic stem cells (ESCs), allowing researchers to generate geneticaly modificated mice with a desired genotype efficiently (Capecchi, 1989). However, this ESCs plus HR system is still not available for genetic modifications in large animals because no well characterized ESCs have yet been isolated. Based on that the introduction of a double stranded break (DSBs) within genome would stimulate cellular DNA repairing (Rouet, Smih, & Jasin, 1994; Rudin, Sugarman, & Haber, 1989), the programmable genome engineering nucleases are employed to introduce DSBs, which provide a key advance to overcome the above obstacles in large animals and non-human primates genome editing. After DSB, two different pathways mediate cellular DNA repairing, including non-homologous end joining (NHEJ), which might lead insertion or deletion of a small number of nucleotides (Indels) at the break, and homology-directed repair (HDR), which might result in specific base-pair changes via introducing a donor template at the break (Prinz, Amon, & Klein, 1997; Symington & Gautier, 2011).
Former programmable genome engineering nucleases, including zinc-finger nucleases (ZFNs) and transcription-activator-like effector nucleases (TALENs), have been developed to trigger precise genome editing in mammalian by several orders of magnitude to enhance the overall efficiency of genetic modifications (Boch et al., 2009; Porteus, 2006; Rouet et al., 1994). ZFNs are the first synthetically engineered genome editing agents which combine the binding module zinc-finger protein (ZFP) with the restriction enzyme domain FokI (an endogenous restrictive endonuclease from Flavobacterium okeanokoites) (Kim, Cha, & Chandrasegaran, 1996). For genome editing, a pair of ZFPs need to bind regions flanking the target locus to form a FokI dimer, which is necessary to induce DSBs (Cathomen & Keith Joung, 2008). Similar to ZFNs, TALENs are comprised of a non-specific FokI nuclease domain and a customizable DNA-binding domain (TALE), again introducing DSBs through a FokI dimer (Boch et al., 2009). The design of ZFNs is complicated by their extensive protein-DNA contacts and the cloning of TALEN genes is impeded by their highly repetitive nature (Komor, Badran, & Liu, 2017). In addition, each new target locus requires the design, sequence synthesis, and validation of a new ZFNs or TALENs (Komor et al., 2017; Miller et al., 2011; Urnov, Rebar, Holmes, Zhang, & Gregory, 2010). Despite these challenges, ZFNs or TALENs have been successfully used to generate genetically modified large animals (Whyte et al., 2011; Yang et al., 2011; Yao, Huang, & Zhao, 2016), and in 2012, the scientific society rated TALENs as one of the top ten scientific Breakthrough, 2012. Regardless of the initial successes, significant technical barriers to genome editing via ZFNs and TALENs limit their range of future applications.
Along with continuous improvement, CRISPR based genome editing technologies have overcome those technical barriers and leapfrogged ZFNs and TALENs, been broadly used and will continue to transform biological and biomedical research. In this review, we summarize the recent progress of CRISPR/Cas mediated manipulation of mammalian genomes and their applications in basic life science, biotechnology, and medicine.
2 IMPROVED UNDERSTANDING OF CRISPR/Cas SYSTEMS
The progresses of CRISPR/Cas technology in the recent 3 years was stunning to watch. As detailed structures and biological functionalities being gradually revealed, a more comprehensive insight of the endogenous CRISPR/Cas systems has formed and inspired us to exploit their gene editing capacity to the full.
2.1 Brief introduction of CRISPR/Cas systems
CRISPR/Cas systems have evolved as a component of acquired immune system in prokaryotes (Barrangou et al., 2007). This immune defense system functions in three phases successively, including adaptation, expression, and interference. During adaptation, short pieces of foreign DNA are captured and integrated as “spacer” elements into the CRISPR loci. Then, the assembled CRISPR locus (“spacers” separated by repeat regions) is transcribed to yield a pre-crRNA, which is processed to generate crRNA. The crRNA could identify and combine to the target sequence using simple Watson–Crick base pairing and guide Cas effector proteins to disrupt the target sequences (Barrangou et al., 2007; Horvath & Barrangou, 2010; Wiedenheft, Sternberg, & Doudna, 2012).
CRISPR/Cas systems have been classified into three major types (I, II, and III), and 12 subtypes (Cas1 to Cas12), given their genetic content, protein structural and functional differences (Makarova, Aravind, Wolf, & Koonin, 2011; Makarova, Wolf, & Koonin, 2013). The core defining features of those CRISPR/Cas types and subtypes are the Cas genes and the protein they encode, which are highly genetically and functionally diverse (Makarova, Aravind, et al., 2011; Makarova, Aravind, Grishin, Rogozin, & Koonin, 2002; Makarova et al., 2013; Makarova, Grishin, Shabalina, Wolf, & Koonin, 2006; Makarova, Haft, et al., 2011). Genetically, Cas1 and Cas2 universally occur across all types and subtypes, whereas Cas3, Cas9, and Cas10 have been defined as the signature genes for Types I, II, and III, respectively (Barrangou & Marraffini, 2014).
The most well studied endogenous system, the Streptococcus pyogene (Sp) Class 2 type II system, has only one effector protein-Cas9, which includes two functional units, Cas9 HNH nuclease domain can cleave the complementary strand to the crRNA-guide sequence, whereas Cas9 RuvC-like domain can cleave the noncomplementary strand. The dual-tracrRNA: crRNA, when engineered as a single RNA chimera, can bind to the target genomic locus adjacent to a protospacer motif (PAM)-NGG and generate site specific DSBs, highlighting the potential to exploit the system for RNA-programmable genome editing (Figure 1a) (Cong et al., 2013; Gasiunas et al., 2012; Jinek et al., 2012). In living cells, DSBs activate and recruit the NHEJ or HR enzymatic machinery to repair DNA injuries, thus provide opportunities to introduce desired changes to the sequence (Symington & Gautier, 2011). The simple and effective introduction of DSBs makes CRISPR/Cas9 a very powerful tool, which offers an unprecedented range of targets in a large variety of functional domains within various genomic sites.
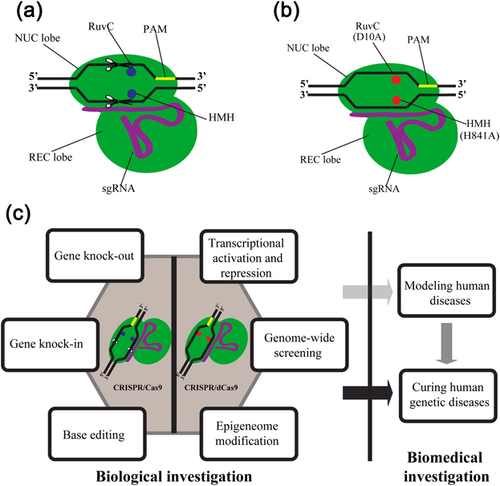
2.2 Natural and engineered CRISPR enzymes
There are several natural CRISPR nucleases, which have now been widely investigated and used for mammalian genome editing. Each CRISPR nuclease has unique characteristics-size, PAM requirement, and location of the introduced DSB within the protospacer-that have been fully reviewed in a recent paper by Komor et al. (2017). The most commonly used CRISPR associated protein is, SpCas9, the 1,368-residue Cas9 protein, which occurs on average every 8–12 bp in the human genome and targets the simple PAM of NGG (Haft, Selengut, Mongodin, & Nelson, 2005). The Staphylococcus aureus (Sa) Cas9 analog (SaCas9) offers a smaller size (1,053 residues) that facilitates some of the applications like gene therapy, but requires a more-complex PAM of NNGRRT (Ran et al., 2015). Cpf1, a smaller and less complex Cas9 orthologue, has further simplified delivery and multiplex gene editing. It was derived from a type V CRISPR system, and the preassembled, recombinant Cpf1 ribonucleoproteins can recognize PAM sequences (5′-TTTN-3′) located 5′ of the target site, with few off-target effects (Zetsche et al., 2015). Besides, Cas12a (presents in type V-A systems) contains a RuvC-like domain and a NUC domain, and does not require tracrRNA for recognition (Makarova, Zhang, & Koonin, 2017). Another effector protein Cas13a (C2c2) is an RNA-guided ribonuclease with two HEPN domains, and has been found to possess RNA-guided single-stranded RNA degradation activity (RNase) (Abudayyeh et al., 2016). Although, these natural CRISPR nucleases have offer multifarious choices to meet different kinds of biological studies, the requirements of more versatile genome editing still exist.
After the natural CRISPR systems were discovered, researchers continued to modify the structures of the core Cas proteins to broad their functionality and overcome inherited limitations. By mutating three to four bases, the variants of SpCas9 can target different PAMs, like NGA, NGAG, and NGCG, with high efficiencies and specificities (Kleinstiver, Prew, Tsai, Topkar, et al., 2015). An engineered variant of SaCas9 was used to relax the PAM requirement from NNGRRT to NNNRRT (Kleinstiver, Prew, Tsai, Nguyen, et al., 2015), and the engineered variant of FnCas9 was used to relax the NGG to YG (Hirano et al., 2016). These critical advances expand the number of target loci amenable to RNA-guided genome editing. In order to reduce off-target cleavage and enhance on-target specificity, a nickase mutant of Cas9 (Cas9n) was designed (Ran, Hsu, Wright, et al., 2013). By introducing an aspartate-to-alanine (D10A) mutation in the RuvC domain, Cas9n only produces single-strand breaks (SSBs) instead of DSBs. And a pair of nickases enables production of crew cut DSBs, in which paired sgRNAs matching is required to complete DNA cleavage at the targeted site, thus significantly minimizing off-target effects by 50–1,000 folds (Ran, Hsu, Lin, et al., 2013; Ran, Hsu, Wright, et al., 2013). Additionally, by fusing of dCas9 (catalytically inactive Cas9) and FokI nuclease, fCas9 can improve targeting specificity by over 140-fold (Guilinger, Thompson, & Liu, 2014). In order to increase efficiency of targeted gene insertions, Cas9 was fused to a peptide derived from human Geminin (Gem), and the Cas9-Gem produced 36% indel-free clones (Howden et al., 2016). Aside from gene perturbations, researchers have also attempted to manipulate gene expression at the transcriptional level using the easy and precise gRNA targeting system, these applications had been reviewed in detail in this review (part 3.2).
2.3 Delivery systems of CRISPR/Cas agents for genome editing in vivo
A key technological barrier for CRISPR-based gene editing in vivo is an efficient and safe delivery system. Since the CRISPR system components are all macromolecules and can not spontaneously enter cells, several systems have been developed to deliver CRISPR system proteins or their encoding genes into cultured mammalian cells and living organisms. These delivery systems can be classified as non-virus and virus. Non-virus delivery systems include electroporation or nucleofection, lipid-based transfection, cationic peptides, and microinjection (Cockrell & Kafri, 2007; Komor et al., 2017; Luo & Saltzman, 2000; Maasho, Marusina, Reynolds, Coligan, & Borrego, 2004; Moreno & Mali, 2017; Wang et al., 2016; Yin, Kanasty, et al., 2014; Zeitelhofer et al., 2007). Virus delivery systems rely on natural viral vectors, such as retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses (AAVs). Because viruses have naturally evolved to transduce mammalian cells efficiently, these vectors have been the preferred format for gene delivery over the past few decades (Moreno & Mali, 2017). The former used non-viruses systems or viral vectors have offer an opportunity to realize the robust gene editing facility of CRISPR, as a matter of course, transmembrane delivery agents developing is considered as a most important companion system to CRISPR based technologies, and attract more attention to optimize it (Komor et al., 2017; Moreno & Mali, 2017).
2.4 Strategies to improve cellular HDR to NHEJ
Although the efficiency of CRISPR/Cas mediated gene deletion has been relatively high, site directed mutagenesis has room for further improvement. The major drawback in using CRISPR/Cas systems for accurate genomic editing is the problematic introduction of stochastic insertions and deletions of nucleotides by NHEJ, as unwanted byproducts, at the target locus (Shibata & Jeggo, 2014; Symington & Gautier, 2011). In most cases, precise HDR genome editing is preferred over NHEJ. Several efforts have been made to favor HDR over NHEJ, including engineer artificial CRISPR/Cas based systems or use some chemicals to inhibit NHEJ DNA repair. Commonly, DNA nicks result in HDR rather than NHEJ, this make gRNA-guided Cas9n with a RuvC or HNH mutation has the mean to produce a nick instead of a DSB in the targeted region (Cong et al., 2013; Gasiunas et al., 2012; Mali, Aach, et al., 2013; Ran, Hsu, Lin, et al., 2013), the mixture of nick productions and HDR has effectively modified mammalian genomes in the proposed area (Cong et al., 2013; Dianov & Hubscher, 2013). But other report revealed that this single-nickase strategy results in decreased editing efficiencies mediated both by NHEJ and HDR compared to that of wild-type Cas9, which might comprise its application (Komor et al., 2017).
In order to improve the product selectivity of genome editing to favor precise allele replacement, small molecules have been used to inhibit or improve certain endogenous DNA repair components to support the error-free HDR outcomes over NHEJ. Small molecules inhibiting DNA ligase IV or DNA-dependent protein kinase (DNA-PKcs) (both involved in NHEJ), have been successfully employed to enhance HDR in cell lines and mice (Srivastava et al., 2012; Maruyama et al., 2015; Robert, Barbeau, Ethier, Dostie, & Pelletier, 2015; Vartak & Raghavan, 2015). Additionally, administration of RS-1, a small molecule activator of Rad51 (a protein involved in HDR) with Cas9, a sgRNA, and a donor DNA template, can prefer HDR-mediated genome editing by two to five folds at different loci in cells (Song et al., 2016). Based on the fact that HDR rate is dependent on many variables, especially the cell cycle, target cells are arrested in G-phase by blocking M-phase before delivering Cas9 ribonucleotide protein complexes (RNP) (Lin, Staahl, Alla, & Doudna, 2014). Although HDR mediated DNA repair can be enhanced by using those exogenous molecules, such treatments are typically very perturbative to cells, which might limit its applications (Komor et al., 2017; Song et al., 2016).
Besides, for the sake of facilitating site-specific transgene integration during the G1 phase of the cell cycle, when DNA repair predominates by NHEJ, the SpCas9 nuclease is fused to a peptide derived from the human Geminin protein (SpCas9-Gem). Although the frequency of HR was similar for SpCas9-Gem and SpCas9, a marked reduction in the capacity for SpCas9-Gem to induce NHEJ-mediated indels is observed at the target locus (Howden et al., 2016). Further, based on CRISPR/Cas9 technology, a homology-independent targeted integration (HITI) strategy is devised, which allows robust DNA knock-in in both dividing and non-dividing cells in vitro and, more importantly, in vivo (e.g., in neurons of postnatal mammals) (Suzuki et al., 2016). A site specific DSB generated both in the genome and the donor plasmid using the CRISPR/Cas9 system can be efficiently used to target ∼5 kb plasmids into mammalian genomes via HITI (Bachu, Bergareche, & Chasin, 2015). Inspired by this, we generated site-specific Uncoupling protein 1 (UCP1) knock-in pigs efficiently and reconstituted UCP1 expression in porcine white adipose tissue via such HITI strategy (unpublished data). Recently, microhomology-mediated end joining (MMEJ)- and homology-mediated end joining (HMEJ)-based strategies are reported to have the capable of integrating long exogenous DNA fragments into the genome at relatively high frequencies (Figure 2a) (Nakade et al., 2014; Yao et al., 2017), which suggested that these HDR dependent and independent strategies are most sensible to introduce site-specific transgene integration without the cell cycle dependence and is free from the exogenous molecule cytotoxicity.
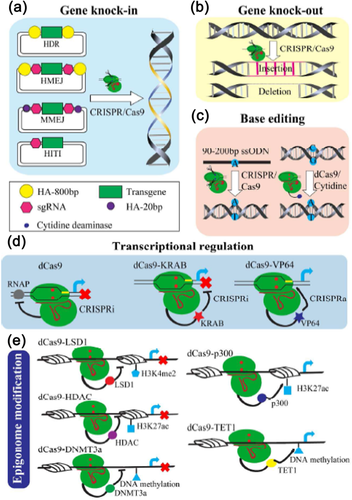
3 BIOLOGICAL APPLICATIONS OF CRISPR/Cas SYSTEMS
Benefiting from the above in-depth understanding and further development of CRISPR, versatile CRISPR-based platforms for genome editing have been rapidly developed to advance investigations in biology and biomedicine (Figure 1c). In biological research area, CRISPR has been widely adopted in both fundamental and applied research fields, such as genomic editing especially “base editing,” transcriptional regulation such as epigenetic modifications and high-flex genome-wide screening. In biomedical research area, CRISPR has also shown its extensive applicability in the establishment of animal models for genetic disorders especially those large and non-human primates models and the revolution of gene therapy for human diseases with a genetic component.
3.1 Permanent genome editing via CRISPR
CRISPR based systems are efficient to create various genetic editing formats like gene knock-out (Figure 2b), knock-in (Figure 2a) as well as single nuclease mutation (Figure 2c) (Cong et al., 2013). Noteworthy, single nuclease mutation represents the most suitable way to mimic single nuclease polymorphisms (SNPs) and to explore their function in mammalian development, physiology, and human diseases.
SNPs are highly abundant, stable, and distributed throughout the mammalian genome. Ten million SNPs have been estimated to exist in the human genome, nearly one SNP for very 290 base-pairs (Nakken, Alseth, & Rognes, 2007; Wu & Jiang, 2013). These variations are associated with diversity in the population, individuality, susceptibility to diseases, individual response to medicine, productive traits of livestock, and so on (Ai et al., 2015; Chanock, 2001; Martin, Boomsma, & Machin, 1997; Pirmohamed & Park, 2001; Shastry, 2002). In human familial inheritance diseases, SNPs are usually identified as crucial genetic changes (Manolio, 2010). CRISPR/Cas9 mediated genetic modification provides an efficient tools for introducing such delicate changes into the genome by DNA repair through the HDR pathway (Cong et al., 2013). Donor templates, which are essential to HDR mediated single nucleotide mutation, can be mainly supplied with chemically synthesized single-stranded DNA oligonucleotides (ssODN) of 90–200 nt in length containing the cleaved target and the introduced mutation (Figure 2c) (Lin, Staahl, et al., 2014; Yang et al., 2013). Current ssODN-mediated HDR still suffer from low efficiency (<5%), causes by many factors, including cell state and cell type, and is competitive with NHEJ (Yang et al., 2013). Thereby, a more robust agent is in urgent to be developed to introduce point mutation within the genome. A novel strategy to introduce point mutations in an RNA-programmed manner that does not rely on DSBs and HDR has been developed (Komor, Kim, Packer, Zuris, & Liu, 2016). Through conjugation of dCas9 with an enzymatic or chemical catalyst that mediates the direct conversion of one base to another, RNA-programmed DNA base editing provides a solution for inducing SNPs into the mammalian genome (Komor et al., 2016). In details, under the direction of a sgRNA, the engineered dCas9-cytosine deaminase fusion protein leads C to U conversion on single-stranded DNA, within a small window (3–5 nt) of the protospacer (Harris, Petersen-Mahrt, & Neuberger, 2002; Jiang et al., 2016; Komor et al., 2016). So far, such DSBs and HDR independent base editing technology has been successfully used in rice, tomato, human cells, and mice zygote to induce single base editing within the genome (Kim, Ryu, et al., 2017; Komor et al., 2016; Shimatani et al., 2017; Zong et al., 2017). Noteworthy, base editing efficiencies (15–75%) are much higher than the efficiency of HDR-mediated point mutation (<5%) (Komor et al., 2016; Ran, Hsu, Wright, et al., 2013). In addition, DNA indels is minimized because base editing avoids making DSBs (Nishida et al., 2016). This was confirm by the results of Digenome-seq, which is sensitive enough to capture off-target sites, that the off-target frequency of dCas9-cytosine deaminase is no more than 0.1% (Kim, Lim, et al., 2017). In order to increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions, five C to T (or G to A) base editors that use natural and engineered Cas9 variants with different PAM specificities are built to expand the number of sites (Kim, Komor, et al., 2017). Additionally, mutated cytidine deaminase domains are engineered to narrow the width of the editing window from −5 nt to as little as 1–2 nt (Kim, Komor, et al., 2017).
In summary, the newly developed “Base editing” expands the scope and efficiency of genome editing of point mutation without the need for a foreign DNA donor or DSBs cleavage, would accelerate SNPs associated genome modification in a wide array of mammalians and food plants.
3.2 Transcriptional regulation (CRISPRi and CRISPRa) through CRISPR
The complexity and dynamic transcriptional regulation of a number of genes and their pathways play a key role in a wide range of cellular activities such as genome replication, repair, cell division, and inheritance. Regulating a certain gene's expression is critical to decipher its function, and can not only be achieved by completely disrupted via permanent genome editing, but also can be obtained by partially reduced via temporary RNA regulation (Figure 2d), such as CRISPR mediated interference (CRISPRi) or activation (CRISPRa).
3.2.1 CRISPRi
The capacity that the CRISPR/Cas9 system can be designed to identify and bind a specific region of the genome, inspired several groups to develop transcriptional repressors and activator based on it. To adapt CRISPR for transcriptional regulation, two mutations are made in the RuvC (D10A) and HNH (H840A) active sites of the Cas9 endonuclease to inactive it's catalytic function without affecting its binding ability, and the mutated Cas9 is customarily called “dead” Cas9 or dCas9 (Figure 1b) (Bikard et al., 2013; Gilbert et al., 2013). dCas9 itself alone is able to act as a transcriptional repressor, simply exploiting its high affinity for targeting DNA and ability to sterically hinder the transcriptional activity of RNA polymerase (RNAP) (Qi et al., 2013). Furthermore, a dCas9 can fuse to a repressor domain such as KRAB, to trigger heterochromatin mediated gene silencing at the transcription start site (TSS), this approach is termed CRISPRi and leads to transcriptional reduction of the target gene (Gilbert et al., 2013; Qi et al., 2013).
3.2.2 CRISPRa
Besides CRISPRi system, several dCas9-based approaches have been successfully used to activate transcription of genes from their endogenous genomic locus, bypassing the need to clone and express the often times-large ORFs in mammalian cells (Chen et al., 2013; Farzadfard, Perli, & Lu, 2013; Maeder et al., 2013; Mali, Aach, et al., 2013; Perez-Pinera et al., 2013). For example, the transcriptional activation domain VP64, which consists of four tandem copies of the Herpes Simplex Viral Protein 16 (VP16), can be fused directly to the C terminus of dCas9 and used to increase the expression of a wide variety of genes (Maeder et al., 2013; Mali, Aach, et al., 2013; Perez-Pinera et al., 2013). Similar to CRISPRi, the target site is crucial for CRISPRa to obtain maximal activation. Typically the region upstream of the TSS (−400 to −50 nt) is considered to be the optimal target site for CRISPRa sgRNAs (Gilbert et al., 2014; Konermann et al., 2015). However, this is mutually exclusive with the CRISPRi target space downstream from the TSS (0 to +500 nt). Most importantly, it is essential to know the location of the TSS to effectively activate expression and secondly, activation of multiple transcripts from one and the same TSS cannot be controlled individually.
3.2.3 Epigenome editing through CRISPRi or CRISPRa
The realization that phenotypic inheritance is not merely a consequence of genetic processes, but also implicates responses to environmental stimuli is one of the great discoveries in 20th century (Noble, 2015). The conventional view that elucidating the mechanisms for translating genes into proteins can account for a panoply of diseases has proven incomplete. Landmark studies point to epigenetics as a missing piece of the puzzle. Today, epigenetics is a dynamic and prolific field broadly aimed at studying fundamental processes related to mitotic and meiotic stable and heritable changes-in gene expression or cellular phenotype-that occur without alteration of DNA sequences (Goldberg, Allis, & Bernstein, 2007). However, former technological limitations have hindered the studying of specific roles for histone post-translational modifications, DNA modifications, and non-coding RNAs in regulation of the epigenome and chromatin structure. This feature highlights CRISPR systems, including CRISPR/Cas9, as novel tools for targeted epigenome editing (Liu, Wu, et al., 2016). Epigenome editing, the precise placement of epigenetic marks with programmable DNA binding domain (DBD) fusions, is a valuable tool for biological investigation by enabling control over gene regulation without changing DNA sequence (Figure 2e) (Keung, Joung, Khalil, & Collins, 2015). Mechanisms of epigenome editing include regulating transcription, altering post-translational histone modifications, modifying DNA methylation, and modulating regulatory element interactions. Now several epigenome editing effectors have been fused with synthetic DBDs to modify target loci (Thakore, Black, Hilton, & Gersbach, 2016).
A CRISPR/Cas9-DNA methyltransferase 3A (DNMT3A) fusion was used to induce DNA methylation at specific loci in the genome. DNA methylation was induced at up to 50% of alleles for targeted CpG dinucleotides. DNA methylation levels peaked within 50 bp of the short guide RNA (sgRNA) binding site and between pairs of sgRNAs (Anton & Bultmann, 2017; Liu, Wu, et al., 2016; McDonald et al., 2016; Okada, Kanamori, Someya, Nakatsukasa, & Yoshimura, 2017; Vojta et al., 2016). Researchers also demonstrated that fusion of Tet1 with a dCas9 can enable targeted DNA methylation editing. Targeting of the dCas9-Tet1 to unmethylated promoter sequences caused activation of an endogenous reporter and upregulate transcription of the target genes, these fusions can induce over 90% demethylation of CpG islands within a 200-bp range of the target site (Anton & Bultmann, 2017; Liu, Wu, et al., 2016; Morita et al., 2016; Okada et al., 2017; Xu et al., 2016).
Additionally, researchers have developed a histone-modifying CRISPR-based tool in which the catalytic domain of human acetyltransferase p300 was fused to the C terminus of dCas9. This fusion catalyzes histone H3 lysine 27 (H3K27) acetylation at loci up to thousands of base pairs from the sgRNA-specified locus and results in transcriptional activation of genes (Hilton et al., 2015; Okada et al., 2017). The impressive histone-modifying CRISPR-based tool in which histone deacetylase (HDAC) was fused to dCas9 has been built and it's range of activity, specificity and expression of target gene in two different cell types have been assessed. The findings demonstrate that the chromatin environment is an important element to consider when utilizing this synthetic HDAC (Kwon, Zhao, Lamonica, & Zhou, 2017). Alternatively, the histone demethylase LSD1 has been fused to dCas9, allowing for demethylation of dimethylated histone H3 lysine 4 (H3K4me2) at sites >350 bp from the sgRNA binding site (Kearns et al., 2015).
Notably, CRISPR based transcriptional regulations have set a precedent for epigenetic modifications, and will transformed the life sciences through forming complementary with permanent genomic editing.
3.3 Genome-wide screening mediated by CRISPR
The simplicity of programming the CRISPR/Cas9 to modify specific genomic loci suggests a new way to interrogate gene function on a genome-wide scale (Shalem et al., 2014). Genome-wide, targeted loss-of-function screening through CRISPR/Cas9 in human and mouse cells provide an alternative screening system to RNA interference (RNAi) and have been used to reveal new mechanisms in diverse biological models (Koike-Yusa, Li, Tan, Velasco-Herrera Mdel, & Yusa, 2014; Shalem et al., 2014; Wang, Wei, Sabatini, & Lander, 2014; Zhou et al., 2014). Indeed, CRISPR/Cas9 system has greatly expanded the toolbox for mammalian genetics, enabling the rapid generation of isogenic cell lines and mice with modified alleles (Wang, Wei, et al., 2014).
Researchers deliver sgRNA libraries and Cas9 into cells then screen the treated cells for a phenotype of interest (Sanjana, Shalem, & Zhang, 2014; Shalem, Sanjana, & Zhang, 2015) and identified genes involved in cancer progression (Chen, Sanjana, et al., 2015; Shalem et al., 2014; Shi et al., 2015; Jaiswal et al., 2017), drug resistance (Sanjana et al., 2014; Wang, Wei, et al., 2014), the immune response (Parnas et al., 2015), vulnerability to bacterial toxins (Liu, Gallay, et al., 2017; Zhou et al., 2014), and epigenomic regulatory element screening (CERES) in the native genomic context (Klann et al., 2017).
Together, these studies demonstrate that Cas9 has enhanced the ability to screen the modifications in whole genome scale in a single experiment. These types of studies provide researchers with powerful tools to dissect complex cellular signaling pathways, determine gene function, identify targets for therapeutic intervention, and predict drug side effects.
4 CRISPR-BASED GENOME EDITING IN BIOMEDICAL INVESTIGATION
Thousands of human diseases are known to have a genetic component, although the penetrance of such effect and the contribution of environment influence are highly variable. Genetically modified animal models have played a critical role in understanding genetic disease pathophysiology and in developing novel therapeutic agents and treatments. No doubt the establishment of tailored animal models have been benefited from the recently developed genome editing platforms. Further, CRISPR/Cas9 mediated genome editing showed promising applications in gene therapy which can prevent or treat genetic diseases directly via genetic intervention by targeting selected genomic locus.
4.1 Creation of genetically modified pigs and non-human primates for modeling human diseases via CRISPR/Cas9
Compared with the widely used mammalian models like rodents, pigs share better similarities in anatomical, physiological, and genomic characteristics with human, which make it highly suitable for resembling human diseases. The emergence of nuclease-mediated genome editing technologies have revolutionized the creation of genetically modified (GM) pig models with highly complex pathophysiologies and comorbidities. In the previous review, we summarized the progress of recently developed genome editing technologies tailored disease models that have been generated in various disciplines (Yao et al., 2016). Notably, pigs are thought as the most suitable animals to supply organs to humans (Xenotransplantation), considering the high degree of similarity between humans and pigs.
However, before pig organs can be transplanted into humans, several hurdles must be overcome, including hyperacute rejection, post-hyperacute rejection, cell-mediated rejection, nonvascular rejection, coagulation dysregulation, inflammation, and porcine endogenous retroviruses (Cooper, Ekser, Ramsoondar, Phelps, & Ayares, 2016). To repress hyperacute rejection in pig-to-human transplantation, porcine alpha1, 3-galactosyltransferase (GGTA1) gene was deleted through HR (Lai et al., 2002), ZFNs (Bao et al., 2014), TALENs (Kang et al., 2016), and CRISPR/Cas9 (Petersen et al., 2016) in succession. Transplantation of hearts from GGTA1 knock-out (KO) pigs into baboons extended the survival process of pig hearts in baboons to 179 days, which was associated with the prevention of hyperacute rejection (Kuwaki et al., 2005). Then, in order to repress severe acute humoral xenograft rejection, which is critical to prolong the survival of GGTA1 KO pig's kidney in baboons (Chen et al., 2005), cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH)/GGTA1 double KO pigs were created using ZFNs, TALENs, or CRISPR/Cas9 (Fischer et al., 2016; Miyagawa et al., 2015), and the xenoantigenicity was further reduced compared with that of the only GGTA1 KO pigs (Lutz et al., 2013). On the other hand, to eliminate the risk of in vitro transmission of PERVs to human cells, sixty-two copies of porcine endogenous retroviruses (PERVs) were simultaneously disrupted by the CRISPR/Cas9 system in PK15 cells, which is admired as another robust application of CRISPR technology in modifying the pig genome for xenotransplantation (Yang et al., 2015).
Beyond the strategy of genetically modifying pigs to resemble humans at the genome level, it is reported that a functional pancreas can be regenerated via complementing pancreatogenesis-disabled embryos with allogenic normal blastomeres, indicating the promise of generating functional organs from xenogenic pluripotent stem cells (PSCs) in pigs, including human induced pluripotent stem cells (iPSCs) or ES cells (Matsunari et al., 2013). Former evidence showed that interspecific chimeras can be generated through injecting mouse or rat PSCs into rat or mouse blastocysts, respectively, and thus confirming that PSCs can contribute to xenogenic development between mouse and rat (Kobayashi et al., 2010). Recently, it is reported that human iPSCs can robustly engraft in both pig and cattle pre-implantation blastocysts, and is able to generate differentiated progenies in post-implantation pig embryos (Wu et al., 2017). These studies suggested that the production of functional human organs in pigs can be achieved through combining CRISPR/Cas9 and human iPSCs, regenerating human organs in the organogenesis—disabled porcine embryos induced by genomic editing, which and is predicted to overcome the difficulties of using pig organs for xenotransplantation more efficiently.
Non-human primates are genetically and phenotypically close to humans, particularly in regards to anatomy, physiology, cognition, and gene sequences (Zhang et al., 2014). Therefore, they are optimal animal models for genetic modifications in an attempt to understand human evolution and human biology, especially in neurobiology. Be aware of this, lentivirus-based transgenic cynomolgus monkeys (Macaca fascicularis) expressing human MeCP2 in the brain were created and exhibited autism-like behaviors and show germline transmission of the transgene, indicate the feasibility and reliability of using genetically engineered non-human primates to study brain disorders (Liu, Li, et al., 2016). With the development of gene-editing technologies, TALEN-edited MECP2 mutant cynomolgus monkeys were created to model a neuro developmental disorder, Rett syndrome (RTT), which is caused by loss-of-function mutations in the human MECP2 gene (Liu et al., 2014), through a battery of behavioral analyses, a series of physiological, behavioral, and structural abnormalities resembling clinical manifestations of RTT were observed (Chen, Yu, et al., 2017). Be worth raising, CRISPR/Cas9 has opened a more impressive era to genome manipulation in non-human primates, by co-injection of Cas9 mRNA and sgRNAs into one-cell-stage embryos, simultaneous disruption of two target genes (Ppar-γ and Rag1) in one step was enabled, and no off-target mutagenesis was detected by comprehensive analysis (Niu et al., 2014). Morever, CRISPR/Cas9 delivered complete gene knockout at high efficiencies (100% on Arntl and 91% on Prrt2) in monkey embryos (Zuo et al., 2017), disrupt the monkey dystrophin gene to model human Duchenne muscular dystrophy (DMD), a recessive X-linked form of muscular dystrophy at a rate up to 87% (Chen, Zheng, et al., 2015), obtained the first live p53 biallelic mutant monkey, and achieved homology directed repair (HDR)-driven gene editing with nucleotide-level precision in monkey embryos (Wan et al., 2015), these all demonstrated the usefulness of CRISPR/Cas9 in rapidly establishing gene-edited monkey models (Zuo et al., 2017).
In summary, genetic conventional models like mice has become commonplace, but it has made breakthroughs in the construction of large animal models such as pigs and non-human primate benefiting from the development of Cas9.
4.2 Gene therapy mediated by CRISPR/Cas technology
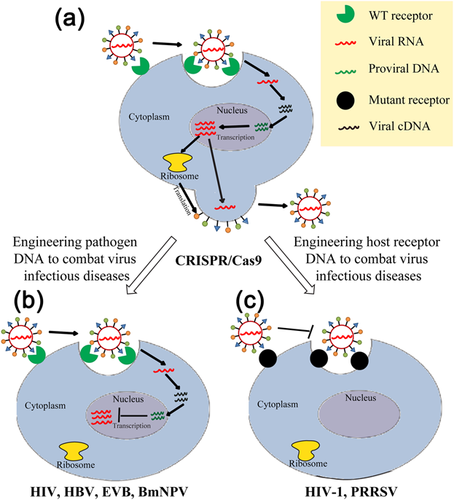
Gene therapy, to prevent or to treat diseases via genetic intervention by targeting selected genomic locus, can be achieved through forward gene therapy and reversed gene therapy. Typically, normalizing an abnormal gene or restoring the function a dysfunctional gene directly, is called forward gene therapy (Pan et al., 2013). Meanwhile, abolishing the function of a gene to balance a complicated regulatory system or depleting cellular molecules essential for a disease mechanism as in pathogen infections, is called reversed gene therapy (He et al., 2011). Gene therapy also can be used to engineer pathogen DNA to combat virus infectious diseases, edit host receptor DNA to combat virus infectious diseases and correct monogenic disorders.
4.2.1 Engineering pathogen DNA to combat virus infectious diseases
Own to its high targeting efficiency, CRISPR/Cas9 have been used to engineer pathogen genome such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), Epstein-Barr virus (EVB), and silkworm Bombyx mori nucleopolyhedrovirus (BmNPV) for therapeutic purposes (Figure 3b).
HIV-1
The first report to experimentally explore the gene therapy potentials of CRISPR/Cas9 system is against HIV infection in human cells (Ebina, Misawa, Kanemura, & Koyanagi, 2013). As is known, the HIV provirus is flanked by identical viral long terminal repeat (LTR) sequences, thereby, targeting the LTR mediated by CRISPR could cleave the virus at both ends. Ebina et al. (2013) firstly showed that HIV-1 LTR sequence can be mutated via CRISPR/Cas9 in vitro, and resulted in removal of the integrated proviral DNA from the part of the host cells and a significant drop in virus expression. Another independent study harnessed CRISPR/Cas9 to mutant HIV-1 LTR U3 region and gained similar results (Hu et al., 2014). Additionally, targeting specific viral reading frames by CRISPR/Cas9 system can also affect viral protein function (Liao et al., 2015). These results suggested that novel technologies aimed at disrupting HIV-1 provirus may be capable of eradicating viral genomes from infected individuals.
HBV
Persistence of hepatitis B virus (HBV) covalently closed circular DNA (cccDNA) under current antiviral therapy is a major barrier to eradication of chronic hepatitis B (CHB). With the cccDNA-specific gRNAs, the CRISPR/Cas9 system significantly reduced the production of HBV core and surface proteins in human cell lines and mice models (Lin, Yang, et al., 2014; Seeger & Sohn, 2014; Ramanan et al., 2015). These data suggest that the CRISPR/Cas9 system could disrupt the HBV-expressing templates both in vitro and in vivo, indicating its potential in eradicating persistent HBV infection.
EBV
Latent viral infection is a major obstacle for effective antiviral treatment and presents a continuous risk to the host. The dormant viral genome during latent infection provides few therapeutic targets other than itself for antiviral drug development (Wang & Quake, 2014). Epstein–Barr virus (EBV) establishes a lifelong persistent infection in 95 % of all adults. Although it causes no pathological manifestation in healthy carriers, EBV infection is aetiologically associated with different types of lymphoid and epithelial malignancies, such as Burkitt's lymphoma, Hodgkin's disease, nasopharyngeal carcinoma, and gastric cancer, in a small subset of individuals (Raab-Traub, 2012). With the EBV genome specific gRNAs, the CRISPR/Cas9 mediated effectively editing of the EBV genome in several human cell lines, and proliferation arrest and apoptosis in EBV-infected cells (Ramanan et al., 2015; Wang & Quake, 2014). Taken together, these work provided that CRISPR based gene editing is efficient to resist latent viral infection.
BmNPV
BmNPV is a major viral pathogen that causes substantial economic losses to mass silkworm rearing in silkworm-raising countries such as China (Jiang & Xia, 2014; Khurad et al., 2004). A novel antiviral strategy by combining transposon-based transgenesis and the CRISPR/Cas9 system is performed for the direct cleavage of BmNPV genome DNA to promote virus clearance in silkworms (Chen, Hou, et al., 2017). This approach will not only contribute to modern sericulture but also shed light on future antiviral therapy.
Additionally, the development of the RNA-targeting CRISPR/Cas9 system has expanded the scope of genetic engineering of pathogens to target RNA viruses, which have no DNA stages in their life cycles. With the engineered RNA-targeting guide RNA, the Francisella novicida Cas9 (FnCas9) can inhibit a human +ssRNA virus, hepatitis C virus, within eukaryotic cells (Price, Sampson, Ratner, Grakoui, & Weiss, 2015). This work reveals a versatile and portable RNA-targeting system that can effectively function in eukaryotic cells and be programmed as an antiviral defense.
4.2.2 Engineering host receptor DNA to combat virus infectious diseases
On the contrary, engineering the genome of host receptor of integrated virus by CRISPR/Cas9 provides another strategy for combating those virus infectious diseases and set a precedent in the fighting with human HIV and porcine reproductive and respiratory syndrome virus (PRRSV) (Figure 3c).
HIV-1
It is suggested that CD4 is required for HIV-1 entry into target cells, and either CCR5 or CXCR4 plays as a co-receptor. However, the targeting of CD4 or CXCR4, is not advisable since these receptors has several essential roles for normal cellular metabolisms (Nagasawa et al., 1996). It is suggested that the antagonist of CCR5 has been shown to be effective in inhibiting HIV-1 entry into cells and is well tolerated (Woollard & Kanmogne, 2015). Additionally, individuals harboring a rare homozygous 32-bp deletion in the CCR5 gene (CCR5Δ32) are reported to be naturally resistant to R5-tropic HIV-1 infection and with otherwise healthy (Samson et al., 1996; Biti et al., 1997). It is reported that combine TALENs or CRISPR/Cas9 together with a piggyBac transposon donor sequence can reproduce the naturally occurring CCR5Δ32 deletion in induced pluripotent stem cells (iPSCs) seamlessly. These modified iPSCs were differentiated into monocytes/macrophages and demonstrated their resistance to HIV-1 challenge (Ye et al., 2014). It showed that a single round transduction of lentiviral vectors expressing Cas9 and CCR5 sgRNAs into HIV-1 susceptible human CD4+ cells yields high frequencies of CCR5 gene disruption. CCR5 gene-disrupted cells are not only resistant to R5-tropic HIV-1, including transmitted/founder (T/F) HIV-1 isolates, but also have selective advantage over CCR5 gene-undisrupted cells during R5-tropic HIV-1 infection (Wang, Ye, et al., 2014). The primary CD4(+) T-lymphocytes are efficiently transduced, CCR5 expression is disrupted, via constructing chimeric Ad5F35 adenoviruses carrying CRISPR/Cas9 components, and the positively transduced cells are conferred with HIV-1 resistance (Vartak & Raghavan, 2015). Thus, silencing of CCR5 via Cas9 and CCR5-specific sgRNAs could be a viable alternative strategy for engineering resistance against HIV-1.
PRRSV
Porcine reproductive and respiratory syndrome (PRRS) is the most economically important disease of swine in North America, Europe, and Asia, costing producers in North America more than $600 million annually (Whitworth et al., 2016). It has been suggested that CD163 is the receptor for entry of porcine reproductive and respiratory syndrome virus (PRRSV) into host cells (Van Breedam et al., 2010). The CRISPR/Cas9 is used to generate pigs lacking functional CD163, and these animals are resistant to the PRRSV isolate NVSL 97–7895, a well-characterized, relatively virulent viral isolate that is commonly used in experimental PRRSV infection trials (Whitworth et al., 2016). The scavenger receptor cysteine-rich domain 5 (SRCR5) region of CD163 was shown to be an interaction site for the PRRSV in vitro. To explore the function of domain 5 in PRRSV infection, genetically modified pigs with a normal SRCR domain 5 or the SRCR domain 5 replaced with a synthesized exon encoding a homolog of the hCD163L1 SRCR domain 8 (domain swap) were generated by CRISPR/Cas9 technology (Burkard et al., 2017; Wells et al., 2017). Infection studies with different PRRSVs revealed that CD163 is likely to be the receptor for all PRRS viruses and that domain 5 knockout pigs are resistant to these viruses (Wells et al., 2017). Refining the modification of CD163 provides the opportunity to breed pigs that are resistant to PRRS while retaining the important biological functions associated with CD163 (Burkard et al., 2017). This strategy might hint us the solutions of making human resistant to virus infection.
4.2.3 Correcting monogenic disorders in vivo
The combination of Cas9, guide RNA, and repair template DNA can induce precise gene editing, generate the correction of genetic diseases in adult mammals.
Muscular dystrophy
Duchenne muscular dystrophy (DMD) is a fatal disease of skeletal muscle and heart, occurring in 1 out of 5000 male births (Fairclough, Wood, & Davies, 2013); patients typically die from cardiopulmonary failure with an average life expectancy of 25 years (VandenDriessche & Chuah, 2016). This disease is attributed to mutations in the gene encoding dystrophin, a vital protein linking the cytoskeleton of the myofibers and cardiomyocytes to the extracellular matrix (Campbell & Kahl, 1989; Ervasti, Ohlendieck, Kahl, Gaver, & Campbell, 1990). DMD has always been considered a prime target for gene therapy, because its genetic etiology is well understood and conventional treatments are not effective in halting disease progression (Koch, 2016).
Three independent groups demonstrated the feasibility of removing DMD mutations from the genomes of mdx mice model via the CRISPR/Cas9 gene-editing system. The frequently used mdx mouse model has a nonsense mutation (or a stop codons) in exon 23 of the Dmd gene, which disrupts the transcription of Dmd mRNA and the expression of dystrophin (Fairclough et al., 2013; Long et al., 2016; Negishi et al., 2014; Wu et al., 2012). In common, normal Dmd mRNA is rescued through destroying exon 23 to correct the nonsense mutation or to delete the faulty stop codons by CRISPR/Cas9. Additionally, the adeno-associated virus-9 (AAV-9)- or AAV-8 delivery system are used to transport CRISPR/Cas9 component into mdx mice cells, which enhanced the expression of truncated but functional dystrophin and improved muscle function of mdx mice (Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016). Taken together, the AAV-CRISPR/Cas9 gene-editing system seems effective for modifying such gain-of-function mutations in the Dmd gene via local or systemic administration and has the potential to improve pathological manifestations of patients suffering from DMD (Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016).
Liver disease
The liver disease is a particularly suitable model for gene repair-based therapy because the repaired hepatocytes will expand and repopulate the liver (Aponte et al., 2001). In hereditary tyrosinemia type I (HTI) patients, mutation of fumarylacetoacetate hydrolase (FAH), the enzyme involved in the last step of catalyzing the tyrosine catabolic pathway, leads to accumulation of toxic metabolites and severe liver damage (Paulk et al., 2010). The common-used Fah−/− mouse model is caused by a G to A point mutation in the last nucleotide of exon 8, which can be termed as loss-of-function mutation (Paulk et al., 2010).
Delivery of components of the CRISPR/Cas9 system resulted in initial expression of the wild-type Fah protein in ∼1/250 liver cells, and the expansion of Fah-positive hepatocytes rescued the body weight loss phenotype (Yin, Xue, et al., 2014). The efficiency of correction was >6% of hepatocytes after a single application, suggesting potential utility of Cas9-based therapeutic genome editing for a range of diseases (Yin et al., 2016). Hepatocytes from tyrosinaemia type I are converted into the benign tyrosinaemia type III by deleting hydroxyphenylpyruvate dioxigenase (Hpd) in vivo using CRISPR/Cas9 system, and the edited hepatocytes (Fah−/−/Hpd−/−) display a growth advantage over non-edited hepatocytes (Fah−/−/Hpd+/+), and in some mice, almost completely replace them within 8 weeks (Pankowicz et al., 2016).
Huntington's disease
Huntington's disease (HD) is a neurodegenerative disorder caused by CAG repeat expansion (>36 repeats) within the first exon of the huntingtin (HTT) gene, which encodes a polyglutamine (poly Q) tract in the N-terminal region of HTT and leads to a wide range of cellular dysfunctions (Bates et al., 2015). Suppressing the expression of mutant HTT (mHTT) has been explored as a therapeutic strategy to treat Huntington's disease, and current RNA interference-based approaches including siRNA and antisense oligonucleotides for lowering mHTT expression have been efficacious in mouse models, but rely on SNPs that are specific to the mutant allele (Carroll et al., 2011; Drouet et al., 2014). Given the truth that loss of Htt in mice can lead to embryonic lethality, specifically mitigate mHTT expression from the mutant allele is critical for Huntington's disease therapy. Thereby, SNPs that either cause or destroy PAM motifs critical for CRISPR-selective editing of one allele versus the other in cells from HD patients and in a transgenic HD model harboring the human allele are distinguished and used to mediate alleles specific genome editing in heterozygosity with the mutant allele in vitro and in vivo (Monteys, Ebanks, Keiser, & Davidson, 2017). But it remains unknown whether depletion of HTT in the adult brain, regardless of its allele, could be a safe therapy. Yang et al. (2017) report that permanent suppression of endogenous mHTT expression in the striatum of mHTT-expressing mice (HD140Q-knockin mice) using CRISPR/Cas9-mediated inactivation effectively depleted HTT aggregates and attenuated early neuropathology. Furthermore, the reduction of mHTT expression in striatal neuronal cells in adult HD140Q-knockin mice did not affect viability, but alleviated motor deficits (Yang et al., 2017). This study showed that non-allele-specific CRISPR/Cas9-mediated gene editing could be used to efficiently and permanently eliminate toxicity in the adult brain, also opened up a new avenue for treating other neurodegenerative diseases caused by the gain-of-function mechanism (Yang et al., 2017).
All in all, these studies indicate that CRISPR/Cas9-mediated genome editing is possible to cure genetic disorders in adult animals under certain conditions, including such gain-of-function mutation related diseases including DMD and Huntington's disease (Long et al., 2016; Nelson et al., 2016; Tabebordbar et al., 2016; Yang et al., 2017), and those liver genetic diseases (Pankowicz et al., 2016; Yin et al., 2016; Yin, Xue, et al., 2014). However, much more efforts should be done to stimulate accurate genomic editing without any off-target byproducts, thereby correct those loss-of-function mutations in human beings.
4.2.4 Repairing genetic disorders in pluripotent cells
The advent of human-induced pluripotent stem cell (hiPSC) and targeted genome editing technologies have provided a unique opportunity to establish cellular models of disease, and to repair those genetic disorders in hiPSC from individual patients (Bassett, 2017). Specific mutations have been introduced into iPSCs and iPSC-derived cells using Cas9 and HDR-mediated genome editing for the study of genetic diseases (Hou et al., 2013; Park, Dwyer, Congiusta, Whelan, & Theodoropoulos, 2015; Rong, Zhu, Xu, & Fu, 2014; Yang et al., 2013). This has enabled analysis of isogenic cell pairs that differ in a single genetic change, which allows a thorough assessment of the molecular and cellular phenotypes that result from this abnormality. Importantly, this establishes the true causative lesion, which is often impossible to ascertain from human genetic studies alone. These isogenic cell lines can be used not only to understand the cellular consequences of disease mutations, but also to perform high throughput genetic and pharmacological screens to both understand the underlying pathological mechanisms and to develop novel therapeutic agents to prevent or treat such diseases. In the future, optimizing and developing such genetic manipulation technologies may facilitate the provision of cellular or molecular gene therapies, to intervene and ultimately cure many debilitating genetic disorders (Bassett, 2017).
5 CONCLUSION
The discovery and characterization of CRISPR systems have transformed the studying in life sciences. The development of new CRISPR associated engineer technologies such as investigating new natural CRISPR enzymes, expanding the targeting scope of Cas9 and improving the DNA specificity of CRISPR-based agents have driven and accelerated this transformation. During the preparing of this manuscript, one group describes a CRISPR/Cas9-based approach for inserting a poly(A) transcriptional terminator into both alleles of a targeted gene to silence protein-coding and non-protein-coding genes, which often play key roles in gene regulation but are difficult to silence via insertion or deletion of short DNA fragments (Liu, Han, et al., 2017). The other group develops CRISPR base editors to knock out genes by changing single nucleotides to create stop codons. CRISPR-STOP-mediated targeted screening demonstrates comparable efficiency to WT Cas9, which indicates the suitability of our approach for genome-wide functional screenings (Kuscu et al., 2017). Recently, efficient, footprint-free conditional genome editing was achieve by encapsulating Cas9 gene on a piggyBac transposon (Wang et al., 2017). Additionally, CRISPR/Cas9 has been used human in preimplantation embryos to edit human genetic disease associated gene- the endogenous β-globin gene (HBB), but off-targets and mosaic by-product were all found in those edited embryos, which highlight the pressing need to further improve the fidelity and specificity of the CRISPR/Cas9 platform, a prerequisite for any clinical applications of CRSIPR/Cas9-mediated editing (Liang et al., 2015). Impressively, the other independent group has cured human hypertrophic cardiomyopathy through editing mutated MYBPC3 in embryos nowadays. And the low rate of mosaics, the unusually high efficiency and no detected off-target of gene editing make the study stand out, which put a stake in the exercisable ground that this technology.
Besides those above impressive developments and applications of CRISPR based technologies in biological and biomedical investigations, ethical and regulatory guidelines must also be thoughtfully developed to ensure a balance between realizing the enormous potential of these tools to benefit mankind and minimizing the risk of their misuse.




