Mesenchymal stem cell differentiation: Control by calcium-activated potassium channels
Abstract
Mesenchymal stem cells (MSCs) are widely used in modern medicine for which understanding the mechanisms controlling their differentiation is fundamental. Ion channels offer novel insights to this process because of their role in modulating membrane potential and intracellular milieu. Here, we evaluate the contribution of calcium-activated potassium (KCa) channels to the three main components of MSC differentiation: initiation, proliferation, and migration. First, we demonstrate the importance of the membrane potential (Vm) and the apparent association of hyperpolarization with differentiation. Of KCa subtypes, most evidence points to activity of big-conductance channels in inducing initiation. On the other hand, intermediate-conductance currents have been shown to promote progression through the cell cycle. While there is no information on the role of KCa channels in migration of MSCs, work from other stem cells and cancer cells suggest that intermediate-conductance and to a lesser extent big-conductance channels drive migration. In all cases, these effects depend on species, tissue origin and lineage. Finally, we present a conceptual model that demonstrates how KCa activity could influence differentiation by regulating Vm and intracellular Ca2+ oscillations. We conclude that KCa channels have significant involvement in MSC differentiation and could potentially enable novel tissue engineering approaches and therapies.
Abbreviations
-
- adMSC
-
- adipose-derived mesenchymal stem cell
-
- ALP
-
- alkaline phosphatase
-
- BK
-
- large-conductance KCa
-
- bmMSC
-
- bone marrow-derived mesenchymal stem cell
-
- BMP4
-
- bone morphogenetic protein 4
-
- BSP
-
- bone sialoprotein
-
- [Ca2+]i
-
- intracellular calcium concentration
-
- CLT
-
- clotrimazole
-
- CRAC
-
- calcium release activated channel
-
- EGF
-
- epidermal growth factor
-
- FGF
-
- fibroblast growth factor
-
- HGF
-
- hepatocype growth factor
-
- hMSC
-
- human mesenchymal stem cell
-
- IbTx
-
- iberiotoxin
-
- IK
-
- intermediate-conductance KCa
-
- InsP3R
-
- inositol triphosphate receptor
-
- KCa
-
- calcium-activated potassium channel
-
- LPL
-
- lipoprotein lipase
-
- MAPK
-
- mitogen-activated protein kinase
-
- mMSC
-
- mouse mesenchymal stem cell
-
- MSC
-
- mesenchymal stem cell
-
- NFAT
-
- nuclear factor of activated T cells
-
- NCX
-
- Na+/Ca2+ exchanger
-
- PPARG
-
- peroxisome proliferator activated receptor gamma
-
- pS
-
- picoSiemens
-
- rMSC
-
- rat mesenchymal stem cell
-
- RyR
-
- ryanodine receptor
-
- S1-S6
-
- segment 1 to 6 of a KCa channel
-
- SC
-
- stem cell
-
- SERCA
-
- sarco/endoplasmic reticulum Ca2+-ATPase
-
- SK
-
- small-conductance KCa
-
- TEA
-
- tetraethylammonium
-
- TGFβ
-
- transforming growth factor beta 1
-
- TRP
-
- transient receptor potential channel
-
- VGCC
-
- voltage gated calcium channel
-
- Vm
-
- membrane potential
-
- βNGF
-
- beta nerve growth factor
1 INTRODUCTION
All types of stem cell (SC), including embryonic and adult SCs, are characterized by their ability of self-renewal and asymmetrical division, the latter producing one cell which retains the stemness while the other daughter cell has reduced differentiation potential (Inaba, Venkei, & Yamashita, 2015; Muraglia, Cancedda, & Quarto, 2000). Adult SCs are tissue-specific and include several subtypes. A major subtype is the mesenchymal stem cell (MSC). These multipotent stromal cells are found mainly in bone marrow and can form either mesenchymal lineages (e.g., osteocytes, chondrocytes, adipocytes, muscle cells; Pittenger et al., 1999) or non-mesenchymal lineages (e.g., neuronal cells and hepatocytes; Johnstone, Liley, Dalby, & Barnett, 2015; Stock, Bruckner, Winkler, Dollinger, & Christ, 2014). MSCs are popular as model SCs as they can be extracted from a variety of accessible tissues including bone marrow, cord blood, and fat (Penfornis & Pochampally, 2011). Consequently, MSCs can potentially be harvested and used for repairing and engineering a wide range of tissues such as heart muscle, bone, cartilage, marrow stroma, and tendon (Caplan, 2007).
Differentiation of SCs, including MSCs, has multiple stages, ultimately generating new cell types with specific functional characteristics (Figure 1). Overall, we treat the differentiation process as consisting of three main components: initiation (or commitment), proliferation, and migration (Chen et al., 2015; Kim & Kino-oka, 2014). Apoptosis, which may be involved at all stages to ensure fidelity of differentiation, is outside the scope of this review. The initiation stage is responsible for changes in gene expression, leading to downregulation of SC markers and upregulation of cell-type-specific genes. Proliferation and migration can happen either before or after initiation, increasing the number of cells and directing them to specific sites in the body (Figure 1). Importantly, all three components involve additional cell-cell interactions and feedback effects, enabling dynamic “fine tuning” of the whole process (Chen, Xu, Huang, Cai, & Xi, 2016; Wang et al., 2007).
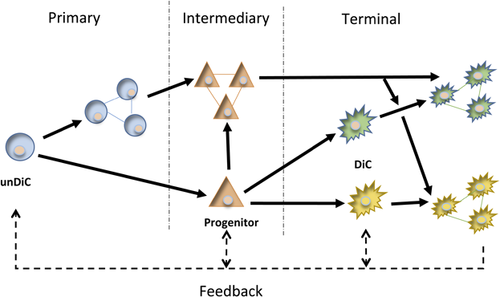
A range of chemical and physical factors can influence the differentiation process. These include “primary” chemical messengers, especially steroid hormones (e.g., estrogens, androgens, and glucocorticosteroids) and growth factors (e.g., EGF, FGF, TGF-β, activin-A, BMP-4, HGF, βNGF) (Hong, Zhang, Sultana, Yu, & Wei, 2011; Huang et al., 2013; Schuldiner, Yanuka, Itskovitz-Eldor, Melton, & Benvenisty, 2000). “Secondary” chemical factors include intracellular messengers, such as cyclic nucleotides (Tsai et al., 2015). In addition, post-transciptional regulators such as microRNAs (e.g., mi302/367, 9 and 124) are involved (Chen et al., 2015). Finally, physical properties of extracellular matrix and cell surface, such as topography and stiffness, can also influence MSC differentiation (Wang, Thissen, & Kingshott, 2016). In-depth information on such factors is outside the scope of this review and will be dealt with elsewhere.
Importantly, there is increasing evidence showing that changes in membrane potential and intracellular Ca2+ and pH can exert significant control over SC differentiation (Chiu et al., 2009; Ye et al., 2014). In fact, by regulating the intracellular milieu and membrane potential, ion channels can be involved in all three main components of the differentiation process. There are many types of ion channels previously described in SCs (Pillozzi & Becchetti, 2012). As regards MSCs, these include K+ channels (e.g., Kv1.4, Kv4.2, Kv4.3, KCa1.1/maxi-K, KCa3.1, KV10.1, Kir6.1, Kir6.2), Na+ channels (e.g., NaV1.7), Ca2+ channels (e.g., Cav1.3), as well as ionic regulatory mechanisms (e.g., Na+-Ca2+ exchanger, Na+-H+ exchanger, Ca2+-ATPase, Na+/K+-ATPase) (Deng et al., 2007; Li & Deng, 2011; Pillozzi & Becchetti, 2012; Tao, Lau, Tse, & Li, 2007).
In this review, we discuss the diverse involvement of Ca2+-activated K+ (KCa) channels in initiation, proliferation, and migration of adult MSCs. However, for completeness, we also refer to other SCs and, to a lesser extent, cancer and somatic cells. First, we give an account of the overall role played by the membrane potential (Vm) in the differentiation process.
2 MEMBRANE POTENTIAL
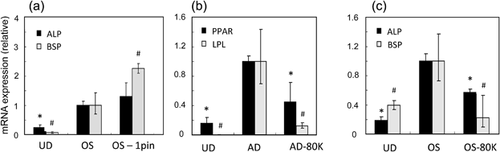
In all living cells, there is a potential difference across the plasma membrane, equivalent to some ca. I107 I V/m. This huge voltage gradient can vary dynamically depending on the functional status of the cell and can thus impact on the conformation of every protein in the membrane as well the intracellular environment. Generally, differentiated cells have hyperpolarized Vm's (ca. −60 to −70 mV), while cells with relatively depolarized Vm's (ca. −20 to −30 mV) tend to exhibit more SC-like properties (Sundelacruz, Levin, & Kaplan, 2008). Consistent with this pattern, increasing evidence obtained with a variety of technical approaches suggests that Vm hyperpolarization can promote, be necessary or even be sufficient for differentiation of a variety of SCs, including MSCs (Sundelacruz et al., 2008; van Vliet et al., 2010). Thus, electromagnetic field-induced membrane hyperpolarization of hMSCs (via voltage-dependent K+ currents accompanied by intracellular Ca2+ release) led to osteogenic differentiation (Kirkham et al., 2010; Petecchia et al., 2015). Using pharmacological agents, even short-term Vm hyperpolarization was found to lead to MSC differentiation into both osteogenic and adipogenic lineages, with significant upregulation of the respective biomarkers (Figure 2a) (Sundelacruz et al., 2008). Conversely, keeping the cells in a depolarized state, by increasing extracellular K+, raised expression of MSC biomarkers and decreased osteogenic and adipogenic differentiation (Figures 2b and 2c) (Sundelacruz et al., 2008) Furthermore, sufficient depolarization of a differentiated cell can induce reversal to a multi-potent progenitor (Sundelacruz et al., 2013). A landmark study on human lung adenocarcinoma (A549) stem cells employed an optogenetic (genetically coded voltage sensor) approach to manipulate Vm. It was thus found that facilitated anion transport down-regulated various stemness biomarkers (EpCAM, CD44, CD166) and induced differentiation (Soto-Cerrato et al., 2015). This effect occurred via Vm hyperpolarization and acidification of intracellular pH.
A further aspect of the role of Vm in differentiation is whether it could be “compartmentalized” so as to facilitate ultimately tissue patterning. It is not known if this occurs in SCs. However, dynamic variability of Vm, in association with “field potentials” has been found within the architecture of neurones (axons and dendritic spines) and polarized epithelia (Bloodgood, Giessel, & Sabatini, 2009; Chifflet & Hernandez, 2012). Such spatial differences of Vm can occur in electrically isolated patches of membrane in a given single cell. This property within a network of cells could enable generation of variable patterns of signaling among the neighboring cells. In addition, this could lead to a hierarchy of electrical interactions further influencing the integral output and the consequent differentiation process. Consistent with this notion, it was suggested that “prepatterning” in the form of Vm gradients can drive embryonic development (Levin, 2014).
In conclusion, Vm plays a significant role in maintaining cellular stemness and differentiation can be induced simply by hyperpolarizing the plasma membrane Vm. The available data raise the intriguing possibility, therefore, that small-molecule modulators or optogenetic manipulation of Vm could selectively target SCs and thus manipulate their differentiation status (Ono et al., 2017). Indeed, a high-throughput screening of selective inhibitors of cancer SC growth and survival identified the K+ ionophore salinomycin as such a promising drug candidate (Gupta et al., 2009). More work is needed, however, to determine whether Vm has such effects in vivo. Also, it will be necessary to take into account any donor variability in the hMSCs characteristics and, hence, the differentiation process, including lineage marker expression (Sundelacruz, Levin, & Kaplan, 2015).
3 Ca2+-ACTIVATED K+ CHANNELS AND Ca2+ SIGNALING
The contribution of KCa channels to MSC differentiation is dependent on many factors, including the channel subtype/splice variants, specific structural component(s) and the mechanism(s) regulating the intracellular Ca2+, the main activator. In the following, we give an overview of KCa's and then evaluate their role in the MSC differentiation process.
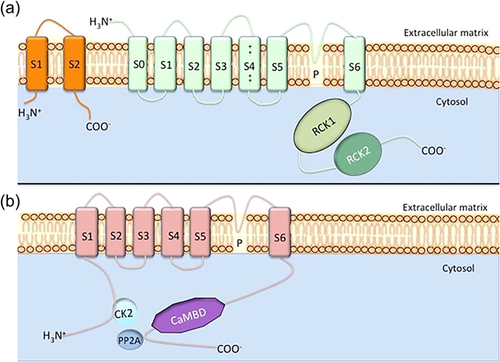
Based on the size of their conductance level, KCa channels can be subdivided into three groups (Table 1): BK (“big” conductance), IK (“intermediate” conductance), and SK (“small” conductance) (Chantome et al., 2009; Faouzi, Chopin, Ahidouch, & Ouadid-Ahidouch, 2010; Zhang et al., 2014). Although Ca2+ and to some extent Vm are the main gating mechanisms for KCa channels, a number of modulators (e.g., Mg2+) have also been identified (Horrigan & Ma, 2008). There is substantial structural homology between IK and SK, while BK appears to represent a different subfamily, related to some members of Na+-activated K+ channels, for example, Slo1 (Hage & Salkoff, 2012). Each KCa comprises α- and possible auxiliary β- and/or γ-subunit(s). The α-subunit is a pore-forming tetramer each with 7 (S0-S6) or 6 (S1-S6) transmembrane segments for BK and SK/IK channels, respectively (Figure 3). The auxiliary subunits regulate the α-subunit and thus affect nearly all aspects of the BK channel's biophysical and pharmacological properties, including voltage dependence and Ca2+ sensitivity (Li & Yan, 2016). Most work linking KCa's to SC differentiation has been done using pharmacological agents (Table 1).
| Channel subtype | Gene(s) | Voltage sensitivity | Conductance (pS) | Pharmacological inhibitors | Mode(s) of action |
|---|---|---|---|---|---|
| BK | Slo1 | High | 100-220 | Tetraethylammonium | Pore blocker |
| Iberiotoxin | Pore blocker | ||||
| Charybdotoxin | Pore blocker | ||||
| Paxilline | Allosteric stabilizer of S6 | ||||
| IK | KCNN4 | Low | 20-85 | Tetraethylammonium | Pore blocker |
| Charybdotoxin | Pore blocker | ||||
| Clotrimazole | Cytoplasmic side (S5-S6) binding | ||||
| TRAM-34 | Cytoplasmic side (S5-S6) binding | ||||
| SK | KCNN1 | Low | 2-20 | Tetraethylammonium | Pore blocker |
| KCNN2 | Apamin | Outer side (S5-S3/S4) binding | |||
| KCNN3 | UCL1684 | – |
Further diversity of KCa channels is achieved by alternative splicing and heteromerization of different subunits. BK channels are encoded by a single gene (KCNMA1, also known as Slo1) that can undergo extensive alternative splicing (Cunningham et al., 2015). The gene consists of 13 alternate exons the splicing of which is post-transcriptionally regulated by microRNAs, for example miR-9 (Pietrzykowski et al., 2008). A study on CNS found a specific BK variant with a stress-regulated exon insertion that had faster activation kinetics and was developmentally regulated in CNS (MacDonald, Ruth, Knaus, & Shipston, 2006). IK channels are also encoded by a single gene (KCNN4) which includes nine exons (Ghanshani et al., 1998). Alternative splicing generates nine variants with a complex pattern of tissue-specific expression and function (Cunningham et al., 2015). In particular, IKb was shown to interact with other transcripts in a dominant-negative manner (Ohya et al., 2011). SK channels are encoded by three genes: SK1/KCNN1 (4 splice variants), SK2/KCNN2 (7 splice variants), and SK3/KCNN3 (5 splice variants) (Cunningham et al., 2015). The SK2-ARK transcript was shown (i) to have an insertion of three amino acids (Alanine–Arginine–Lysine) that reduced its cell-surface expression and (ii) to be developmentally regulated in the cochlea (Scholl, Pirone, Cox, Duncan, & Jacob, 2014). Thus, the alternate transcripts generate considerable functional diversity by determining sensitivity to Ca2+ and Vm as well as domain interactions and subcellular localization (Johnson et al., 2011; Scholl et al., 2014).
A rise in [Ca2+]i can result from influx from extracellular environment and/or release from internal stores. Influx may occur through voltage-gated Ca2+ channels (VGCCs) and/or be due to a change in electrogenic Na+-Ca2+ exchanger (NCX) activity (Luckhoff & Busse, 1990). Intracellular Ca2+ stores (mainly the endoplasmic reticulum and mitochondria) may also contribute to the increase in [Ca2+]i, facilitated by InsP3 receptors (InsP3R's), Ca2+ pumps and Ca2+ release channels (CRACs)/ryanodine receptors (RyRs) (Kawano et al., 2002; Kawano, Otsu, Shoji, Yamagata, & Hiraoka, 2003). Several mechanisms are involved in returning [Ca2+]i to baseline, such as NCX, Ca2+-ATPases and Ca2+-binding proteins (Kawano et al., 2003).
Spatiotemporal characteristics of intracellular Ca2+ play a critical role in the differentiation process and its downstream effects (Berridge, 1990). Indeed, closure of CRAC channels by formation of nearby intracellular Ca2+ microdomains would result in decreased activation of transcription factor c-fos (Samanta & Parekh, 2016). Intracellular Ca2+ also affected Vm oscillations. Thus, in ventricular myocytes, a modeling study indicated that increasing [Ca2+]i would prolong the oscillation period at baseline SK currents but reduce it at higher current levels (Kennedy, Bers, Chiamvimonvat, & Sato, 2016). Taken together, the spatiotemporal characteristics of intracellular Ca2+ and Vm oscillations, dependent on the tissue specificity of the associated KCa current(s), could direct the resulting differentiation. In particular, the spatial profile of Ca2+ could be involved in the inherent directionality of the migratory component.
Importantly, although not yet studied extensively, the spatial aspect could also involve intercellular signaling leading to “network” interactions. Indeed, it has been shown that Ca2+ waves in neural progenitor cells are transmitted to adjacent cells via connexin 43 gap junctions (Malmersjo et al., 2013). Consistently, pharmacological blockage of connexin 43 in hMSCs resulted in downregulation of chondrogenesis markers (Schrobback, Klein, & Woodfield, 2015). These findings highlight the significance of cell-to-cell Ca2+ signaling and its “field effect” in the differentiation process.
As regards temporal characteristics, different patterns of electrostimulation of MSCs, leading to discrete profiles of intracellular Ca2+ release, resulted in two distinct (myogenic and neurogenic) MSC progeny lineages (Thrivikraman, Madras, & Basu, 2016). Oscillations of intracellular Ca2+ can occur in MSCs spontaneously and/or in response to extra- or intracellular stimuli (Smedler & Uhlén, 2014). Reducing or completely removing extracellular Ca2+ decreased the amplitude but did not eliminate the oscillations while the frequency remained constant (Kawano et al., 2003). The oscillations were driven mainly by Ca2+ release from the endoplasmic reticulum with the involvement of InsP3Rs and intracellular Ca2+ pump (SERCA) but not RyRs (Luckhoff & Busse, 1990).
On the whole, [Ca2+]i oscillations can control a wide range of cellular functions from the most fundamental (e.g., gene expression) to whole-cell (e.g., secretion, proliferation, and motility) (Dupont, Houart, & De Koninck, 2003; Ding, He, Shi, Wang, & Wang, 2010; Salazar, Politi, & Höfer, 2008; Smedler & Uhlén, 2014). Such functions depend on the frequency of the Ca2+ oscillations (Berridge, Bootman, & Roderick, 2003; Parkash & Asotra, 2012). In MSCs, the frequency of the oscillations was ∼3.3 mHz (Tao et al., 2011). From this, mainly NF-kB and, to a lesser extent, MAPK and NFAT were inferred as the main “decoders” (Smedler & Uhlén, 2014; Tao et al., 2011; Varga et al., 2011). Blockage of the oscillations resulted in suppression of nuclear translocation of NFAT leading to downregulation of lineage-specific markers (Varga et al., 2011). The location of the oscillations could result in further signal specificity. For example, NFAT1 activation depended on cytosolic Ca2+ alone, while NFAT4 activity was tightly controlled by two spatially segregated Ca2+ oscillations—cytosolic and nucleoplasmic (Kar, Mirams, Christian, & Parekh, 2016). Also, amplitude, duty cycle and propagation of Ca2+ oscillations can contribute to the encoding of cellular “information” (Berridge et al., 2003; Hannanta-anan & Chow, 2016; Song et al., 2012).
Generation of intracellular Ca2+ oscillations can involve KCa's (Kawano et al., 2003). Thus, inhibiting IK channels specifically with CLT suppressed cAMP ribose-initiated Ca2+ oscillations in hMSCs (Tao et al., 2011). In chicken MSCs, also, blocking K+ channels nonspecifically with TEA abolished Ca2+ oscillations and downregulated mRNA and protein levels of chondrogenesis markers (Varga et al., 2011). A similar role has also been seen in other cell types. For example, pharmacologically opening BK channels in human myometrial smooth muscle cells increased the rate of Ca2+ oscillations and vice versa (Wakle-Prabagaran et al., 2016). Importantly, the inter-dependence of KCa activity and Ca2+ oscillations can enable intrinsic “feed-back” effects thereby broadening the scope of the KCa channels in MSC differentiation. (Wakle-Prabagaran et al., 2016). Indeed, calmodulin (also a secondary regulator of IK and SK channels) was able to respond dynamically to the changes in Ca2+ oscillations profile (Samanta & Parekh, 2016). Thus, calmodilin could bidirectionally mediate the relationship between KCa activity and Ca2+ oscillations.
In conclusion, there is substantial evidence for functional association of intracellular Ca2+, Ca2+ oscillations, KCa channels, and differentiation of MSCs. However, further work is needed to determine the precise nature and mechanisms.
4 KCa'S IN MESENCHYMAL STEM CELLS AND THE DIFFERENTIATION PROCESS
On the whole, KCa channels are expressed widely in various SCs. Functional BK channels were found in all human embryonic SCs, while neuronal SCs exhibited mainly SK3-produced currents (Liebau et al., 2007; Wang et al., 2005). As regards MSCs, all main subtypes of KCa's were present, depending on tissue origin, species, and lineage. Cardiac-derived MSCs showed significantly higher IK and SK3 mRNA levels when compared to umbilical cord MSCs, highlighting the importance of source tissue (Subramani et al., 2016). In human adipose and bone-marrow MSCs, both IK and BK channels were found to be expressed commonly (Bai et al., 2007; Li, Sun, Deng, & Lau, 2005). Similarly, the KCa current in rMSCs was due to IK and BK (Deng et al., 2007). As regards SK, functional channels occurred mostly in fat-derived hMSCs (Bai et al., 2007; Li et al., 2005). On the other hand, in mouse bmMSCs no pharmacologically distinguishable SK current could be detected, although a general KCa current was present (Tao et al., 2007).
Functional KCa expression may be involved in all three components of the differentiation process: initiation, proliferation, and migration, as follows.
4.1 Initiation
Initiation is essentially the stage at which changes in gene expression occur whereby SC markers become downregulated and tissue-specific genes are upregulated. Throughout this process, the pluripotency of the cells declines. Initiation occurs in several stages: commitment, intermediate, and terminal differentiation (Huang et al., 2009; Wurm et al., 2015). The latter is likely to involve several sub-stages (with possible feedback effects), ultimately giving rise to mature, tissue-specific cell types.
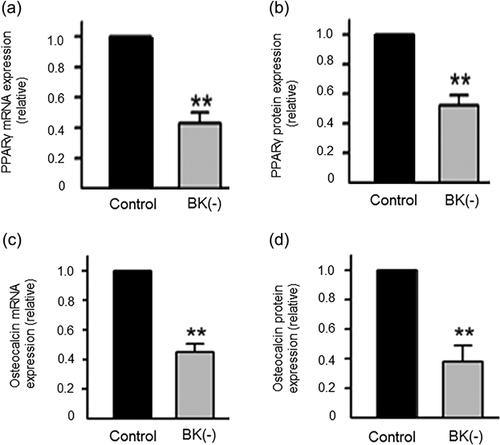
Stable suppression of BKs in hMSCs with shRNA significantly decreased both mRNA and protein levels of PPARγ and osteocalcin (intermediate and early biomarkers of adipogenesis and osteogenesis, respectively) (Figure 4) (Zhang et al., 2014). A recent study also showed that BK channel knockout in MSC-derived osteoblasts largely reduced osteocalcin, osteopontin and Runx2 expression (Hei et al., 2016). Similarly, KCa channels have been found to be involved in the initiation of other SCs. Thus, blocking the KCa activity non-specifically with TEA in endothelial SCs, known to highly express IK and BK mRNA and protein, upregulated the expression of the pluripotency marker, CD133 (Ye et al., 2014). Treatment of induced pluripotent SCs (iPSCs) with IbTx and apamin (specific blockers of BK and SK currents, respectively) had no effect on pluripotency markers (Jiang et al., 2010). This could indicate that there may be differences in the control of initiation by KCa (BK and SK) channels in induced SCs. BK channels were found to be involved in terminal differentiation of neural precursor cells. During differentiation to oligodendrocytes, functional BK channel expression declined with cell maturation and pharmacological blockage of the channels with IbTx suppressed the differentiation (Buttigieg, Eftekharpour, Karimi-Abdolrezaee, & Fehlings, 2011). Similarly, IK channel expression correlated inversely with myoblast differentiation, although its functional role in myogenesis is unknown (Fioretti, Pietrangelo, Catacuzzeno, & Franciolini, 2005). Importantly, the involvement of BK could extend to “fine tuning”. For example, asymmetry of the Caenorhabditis elegans olfactory neurone (AWC) pair was found to be determined by BK (i) coupling gap junctions to inhibition of Ca2+ signaling and (ii) regulating synaptic marker localization (Alqadah et al., 2016).
In conclusion, KCa channel activity is involved in the initiation process of a range of SCs including MSCs and there is some evidence that such a role may relate to terminal differentiation and specialization. However, further work is required to determine more precisely how KCa's control transcriptional initiation and progression of MSC differentiation.
4.2 Proliferation
Changes in Vm are well known to make significant contributions to progression through the cell cycle (Blackiston, McLaughlin, & Levin, 2009; Bregestovski, Medina, & Goyda, 1992). Blocking K+ channels non-specifically with TEA significantly decreased hMSCs’ proliferation (Ding et al., 2012). As regards KCa's, in rMSCs, significant decrease in cell cycle (G0/G1 to S) progression was observed after blockage of IK channel activity pharmacologically (using CLT) or siRNA (Figure 5) (Deng et al., 2007). These results agree with work on mMSCs which showed that IK inhibition or knockdown led to reduced cyclin D1 and E expression, and consequently, suppression of proliferation (Tao, Lau, Tse, & Li, 2008). In hMSCs, also, blocking BK activity with paxilline or shRNA decreased DNA synthesis and arrested the cell cycle at G0/G1 checkpoint (Zhang et al., 2014). Surprisingly, paxilline treatment in iPSC-derived hMSCs had the opposite effect, promoting proliferation (Zhao, Wei, Kong, Shim, & Zhang, 2013). This result, again, emphasises the importance of the origin, including the possible induced nature, of MSCs in determining their functional properties. Finally, although SK channels are also expressed in hMSCs, their possible role in proliferation remains unknown (Bai et al., 2007; Li et al., 2005). As regards mechanism(s), the fact that anti-proliferative effects were seen with pharmacological blockers would imply that channel conductance and resulting Vm hyperpolarization controlled cell cycle progression. We should note, however, that a study on HEK cells suggested a non-conducting role of IK in controlling proliferation (Millership et al., 2011). Further work is required to determine if a similar phenomenon applies to KCa channels in MSCs.
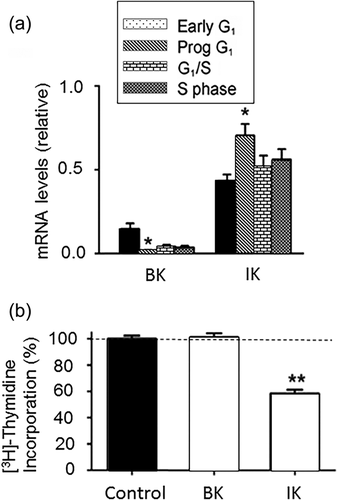
In conclusion, proliferation and cell cycle progression of MSCs are promoted by ionic currents generated by IK and BK channels. This is generally in line with other SCs and cancer cells (e.g., Zhang et al., 2015; Zhang, Yang et al., 2016). Although SK channel involvement in MSC proliferation has also been suggested, this is yet to be substantiated. Also, in all such cases, the underlying mechanism(s) is yet to be elucidated.
4.3 Migration
Stem cells need to migrate in order to reach (i) intermediary sites where additional signaling enables further progression through the differentiation process and/or (ii) their final, tissue-specific location where further fine tuning and functional specialization may take place (Figure 1). Although the importance of K+ currents generally in MSC migration has been demonstrated, at present there is no information on the possible involvement specifically of KCa's (Zhang, Li, Kang, & Neoh, 2016). Nevertheless, some evidence of BK and IK channel contribution to migration is available from other SCs. In particular, genetic and pharmacological silencing of BK channels in cardiac SCs resulted in decreased lateral and transverse cell migration demonstrated by wound healing and transwell assays, respectively (Figure 6a) (Zhang et al., 2015). IK channels have been shown to be involved in migration of cells from a wide range of tissues, including neuronal, cancer, and epithelial (Girault & Brochiero, 2014; Girault et al., 2015; Turner & Sontheimer, 2014; Yi, Dou, Lu, Yu, & Chen, 2016). In MSC-derived vascular smooth muscle cells blockage of IK activity with TRAM-34 significantly decreased migration (Su, Zhang, Yu, Wang, & Zhu, 2013; Zhao, Su, Wang, Li, & Deng, 2013) (Figure 6b). However, it was not clear if the IK channel originated from the MSCs or it arose and/or specialised during the differentiation process. Furthermore, there was a discrepancy between the effects of inhibiting IK using TRAM-34 or siRNA (Zhao, Su, et al., 2013). Such a discrepancy was also noted in a study on pancreatic ductal adenocarcinoma cell motility (Bonito, Sauter, Schwab, Djamgoz, & Novak, 2016). Possible reasons for this could include variable basal conditions and cell types.
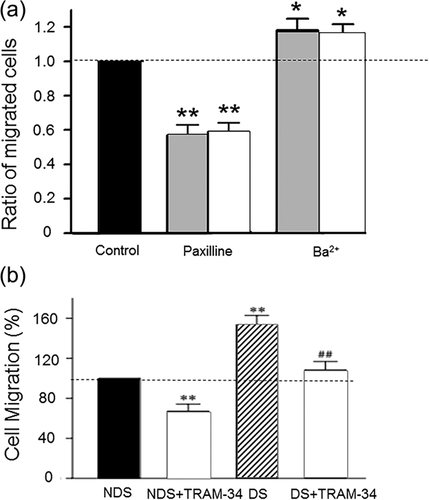
Migration of many other cancer cell types, which exhibit stem-like properties, also has been shown to involve KCa channels. Pharmacological blockage and gene silencing of IK resulted in decreased motility in triple negative breast cancer cells (Zhang, Yang et al., 2016). Treatment with TRAM-34 significantly reduced glioma cell motility (D'Alessandro et al., 2016; Ruggieri et al., 2012). Interestingly, blockage of IK in melanoma cells abolished secretion of melanoma inhibitory activity molecules, required for motility, highlighting a broader role of IK channels in metastasis (Schmidt, Friebel, Schönherr, Coppolino, & Bosserhoff, 2010). Downregulation of BK activity in malignant pleural mesothelioma using miRNA and siRNAs inhibited cell migration (Cheng et al., 2016). Conversely, BK activation by ionising radiation increased glioblastoma motility and this involved the calmodulin-binding domain (Steinle et al., 2011).
The functional role of the IK channel in cell motility has been formalized in the “hydrodynamic model”, initially proposed for neuroblasts (Figure 7) (Turner & Sontheimer, 2014). In this model, increase in [Ca2+]i induces actin elongation at the leading edge, resulting in cell protrusion. Concurrently, IK channels at the rear part of the cell become activated by intracellular Ca2+ waves, leading to K+ exit which, in turn, generates an osmotic force (alongside Cl- efflux). The resulting loss of water (through aquaporins) leads to volume decrease and retraction of the rear part of the cell, generating net movement. This model has been well supported by experimental evidence (Haas & Sontheimer, 2010; Watkins & Sontheimer, 2011). For example, treatment of mammalian neutrophils with TRAM-34 resulted in simultaneous impairment of cell volume regulation and decreased motility (Henríquez et al., 2016). Interestingly, work on neuroblasts showed that upon reaching their destination, migrated cells significantly downregulated IK expression (Turner & Sontheimer, 2014). This highlights further the dynamic nature of the overall differentiation process and the inherent role of the KCa channels.
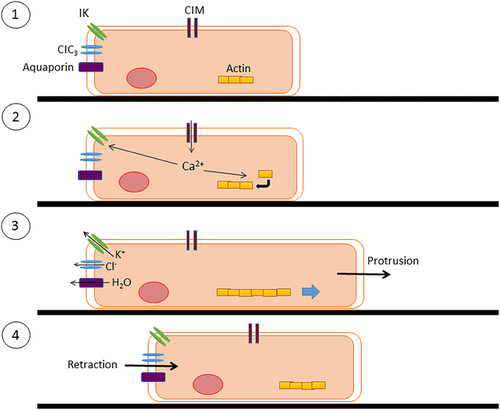
In conclusion, there is substantial evidence for functional role of KCa activity in promoting motility of a range of cell types, including SCs and cancer cells, in accordance with the hydrodynamic model. However, evidence for MSC migration is lacking and it remains also to be shown whether any such role of KCa's changes during differentiation progression.
5 A MODEL FOR CONTROL OF MSC DIFFERENTIATION BY KCa ACTIVITY
From the available information on MSCs, and SCs generally, that we have reviewed, we can propose a conceptual model of functional KCa involvement in differentiation (Figure 8). At the center of the model is [Ca2+]i, which is the main controller of KCa activity, and [Ca2+]i oscillations. At the relatively depolarized membrane potential of SCs/MSCs, [Ca2+]i increases due to the activity of a voltage-sensitive Ca2+ transporting mechanism (“X”). The raised [Ca2+]i activates KCa channel(s), leading to K+ efflux and Vm hyperpolarization. This reverses the effect on X, and [Ca2+]i decreases. As a result, KCa activity is suppressed and Vm depolarizes. The cycle is then repeated thus leading to [Ca2+]i oscillations (Figure 8). Consistently, KCa's have indeed been shown to play a major role in synchronizing both Vm and Ca2+ oscillations (Catacuzzeno, Fioretti, & Franciolini, 2012; Kawano et al., 2003; Reetz & Reiser, 1996).
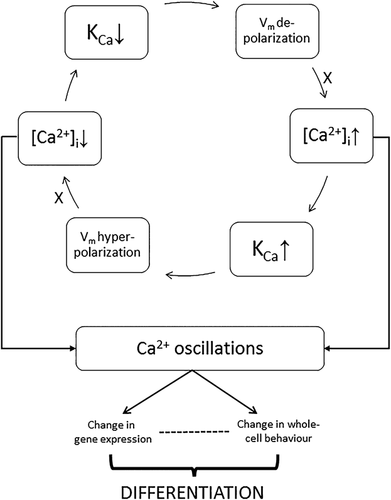
A number of mechanisms can be considered as “X” in the model. One possibility is a voltage-gated Ca2+ channel (VGCC), known to be expressed in MSCs (Ke et al., 2014). This can be ruled out, however, since although a depolarization-activated VGCC could lead to a rise in [Ca2+]i this would involve influx from outside while the [Ca2+]i oscillations have been shown to be independent of extracellular Ca2+ (Kawano et al., 2003). A likely mechanism is an electrogenic NCX typically extruding one Ca2+ for the import of three Na+ (Liu, Hsieh, & Eisenberg, 2016). Thus, at resting Vm, NCX would generate an inward current which will decrease with depolarization, that is, NCX will slow down thereby causing an increase of [Ca2+]i as required by the model (Figure 8). This will happen independently of extracellular Ca2+. Consistently, pharmacologically blocking NCX abolished [Ca2+]i oscillations (Kawano et al., 2003; Ye, 2010). Release of Ca2+ from intracellular stores may also contribute to the overall cytosolic Ca2+ concentration, for example, via Ca2+-induced Ca2+ release (Santini & Tyrrell, 2008). Further work is required to test the model in detail and, in particular, to determine the identity of “X.”
Such functional association of Vm, KCa(s), and [Ca2+]i can result in [Ca2+]i oscillations with mathematical modeling indicating that KCa activity plays a central role (Catacuzzeno et al., 2012). Indeed, in direct support of our model, rise in [Ca2+]i in adMSC hyperpolarized Vm via KCa activity (Catacuzzeno et al., 2012; Tarasov et al., 2017).
Thus, oscillation characteristics (e.g., frequency, amplitude, duty cycle, and duration) can vary dynamically depending on expression of particular KCa subtype(s) with differing Ca2+ dependence and conductance level, as well as tissue origin and developmental stage (Fioretti, Franciolini, & Catacuzzeno, 2005). Consequently, the resulting [Ca2+]i dynamics could regulate the range of cellular activities involved in the differentiation process (Berridge et al., 2003; Choi et al., 2016; Oh-hora, 2009; Smedler & Uhlén, 2014; Toth, Shum, & Prakriya, 2016).
6 CONCLUSION AND FUTURE PERSPECTIVES
In overall conclusion, KCa channels in MSCs, and most likely other SCs, play a significant role in all three main components of differentiation (initiation, proliferation, and migration). The underlying mechanisms are dynamic with feedback interactions and intercellular communication giving rise to possible field effects. Furthermore, chemical and physical factors in the cells’ microenvironment can impact upon the differentiation process. All three subtypes of KCa occur functionally in MSCs, expression being strongly dependent on lineage, tissue origin and species. Most available evidence relates to BK and IK channels, mainly in initiation and proliferation, respectively. At present there does not seem to be any study reporting KCa involvement in MSC motility despite a large volume of research on other SCs and cancer cells.
Although the evidence is substantial for the functional role of individual KCa's in MSC differentiation, more work remains to be done including, for example, the following. First, the possible contributions of SK channels to differentiation as a whole and KCa channels to motility of MSCs need to be explored. Second, the role of Vm itself and the precise mechanisms controlling intracellular Ca2+ dynamics (including its oscillations) in the differentiation process, inherent to the proposed model, needs further clarification. Here, application of optogenetic techniques or voltage-sensitive dyes would be welcome (Ono et al., 2017). Third, it would be interesting to know if blocking multiple KCa subtypes simultaneously would produce synergistic effects on differentiation. Fourth, how intracellular Ca2+ oscillations fundamentally code for the different cellular activities should be deciphered. Finally, fifth, the in vivo relevance of the phenomena described here and their underlying mechanisms should be tested and elucidated. Ultimately, our understanding could be enhanced by broadening the approach to other ion channels and SCs. A major advantage offered by ion channels is the available pharmacology and, thus, their “druggability” (Sampson, Botto-van Bemden, & Aufiero, 2015). Thus, as well as revealing a novel facet of MSC differentiation, targeting KCa channels could offer significant potential in tissue engineering and SC therapy.
ACKNOWLEDGMENTS
We are grateful to Dr. Cristina Lo Celso for reading and commenting on the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




