Modulation of 17β-Estradiol Signaling on Cellular Proliferation by Caveolin-2
Abstract
The sex hormone 17β-estradiol (E2) exerts pleiotropic effects by binding to the ligand-activated transcription factor estrogen receptor α (ERα). The E2:ERα complex regulates several physiological processes, including cell survival and proliferation, through transcriptional effects (i.e., estrogen responsive element [ERE]-based gene transcription) and non-transcriptional membrane-initiated effects (i.e., the activation of extra-nuclear signaling cascades), which derive from the activation of the pool of ERα that is localized to plasma membrane caveolae. Caveolae are ω-shaped membrane sub-domains that are composed of scaffold proteins named caveolins (i.e., caveolin-1, caveolin-2, and caveolin-3). Although caveolin-3 is exclusively expressed in muscles, caveolin-1 and caveolin-2 are co-expressed in all human tissues. From a functional point of view, caveolin-2 can operate both dependently on and independently of caveolin-1, which is the main coat component of caveolae. Interestingly, while a functional interplay between caveolin-1 and ERα has been reported in the control of E2-induced physiological effects, the role of caveolin-2 in E2:ERα signaling within the cell remains poorly understood. This study shows that siRNA-mediated caveolin-2 depletion in breast ductal carcinoma cells (MCF-7) reduces E2-induced ERα phosphorylation at serine residue 118 (S118), controls intracellular receptor levels, precludes ERα-mediated extra-nuclear activation of signaling pathways, reduces ERα transcriptional activity, and prevents cellular proliferation. Meanwhile, the impact of caveolin-1 depletion on ERα signaling in MCF-7 cells is shown to be similar to that elicited by siRNA-mediated caveolin-2 depletion. Altogether, these data demonstrate that caveolin-2 expression is necessary for the control of E2-dependent cellular proliferation. J. Cell. Physiol. 231: 1219–1225, 2016. © 2015 Wiley Periodicals, Inc.
17β-estradiol (E2), the most effective form of female estrogen, is a general regulator of integrated physiology and body homeostasis. At the cellular level, E2 controls diverse basic processes, including cellular proliferation (Ascenzi et al., 2006; Hammes and Levin, 2007; Pallottini et al., 2008). The molecular mechanisms that are used by E2 to drive cellular proliferation via its interaction with estrogen receptor α (ERα) depend on the sub-cellular localization of the receptor. Indeed, ERα is a ligand-activated transcription factor whose function is to control E2-dependent gene expression. However, ERα is also localized to the plasma membrane, from which it drives the E2-dependent activation of extra-nuclear signaling pathways (e.g., ERK/MAPK; PI3K/AKT) (Ascenzi et al., 2006; Acconcia and Marino, 2011). Plasma membrane ERα-dependent signaling is necessary and sufficient for carrying out E2-mediated cellular effects, including cellular proliferation both in cell lines and in vivo. Finally, ERα in the membrane is localized to caveolae (Schlegel et al., 2001; Razandi et al., 2002; Acconcia et al., 2005; Pedram et al., 2012, 2014, 2007; La Rosa et al., 2012; Adlanmerini et al., 2014).
Caveolae are specialized plasma membrane sub-domains that are coated by caveolins (i.e., caveolin-1, caveolin-2, and caveolin-3). Caveolins are differentially distributed in human tissues, with caveolin-1 and caveolin-2 being ubiquitous and caveolin-3 expressed only in muscles. However, while caveolin-1 and caveolin-3 are required for caveolae formation, caveolin-2 contributes to caveolae structure by heterodimerizing with caveolin-1. However, caveolin-1-independent functions of caveolin-2 have also been reported (Le and Kurzchalia, 2005). Caveolins control organ physiology, including cardiovascular, pulmonary, and mammary gland functions, by directing the caveolar localization of multiple signaling molecules (Le and Kurzchalia, 2005; Stan, 2005). In turn, a role for caveolins in cancer has also been proposed, but at the present, the impact of caveolins in the regulation of cancer cell proliferation remains controversial (Yang et al., 1999; Kato et al., 2002; Le and Kurzchalia, 2005; Elsheikh et al., 2008; Urra et al., 2012; Wang et al., 2014).
In addition to the localization of ERα to caveolae, there is functional cross-talk between caveolins and ERα. Indeed, several lines of evidence clearly indicate that caveolin-1 contributes both to the plasma membrane localization of the receptor and to the activation of E2-mediated extra-nuclear signaling (Schlegel et al., 2001; Razandi et al., 2002; Acconcia et al., 2005; Park et al., 2009; La Rosa et al., 2012; Totta et al., 2015). However, much less information is available about the interplay between caveolin-2 and ERα (Razandi et al., 2002; Park et al., 2009). In particular, at the present, whether caveolin-2 plays a role in the E2:ERα-dependent cellular effects that control cellular proliferation is completely unknown.
This study reports that caveolin-2 expression is necessary for the E2:ERα signaling that drives breast cancer cells (MCF-7 and T47D-1) to proliferation.
Materials and Methods
Cell culture and reagents
17β-estradiol, DMEM (with and without phenol red), and fetal calf serum were purchased from Sigma–Aldrich (St. Louis, MO). Bradford protein assays was obtained from Bio-Rad (Hercules, CA). Antibodies against ERα (HC-20 rabbit), cyclin D1 (H-295 rabbit), phospho-ERK1/2 (E4 mouse), ERK2 (C14 rabbit), Bcl-2 (C2 mouse), progesterone receptor (C20 rabbit), cathepsin D (H75 rabbit), caveolin-3 (A3 mouse), and caveolin-1 (N20 rabbit) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); anti-vinculin and anti-tubulin antibodies were purchased from Sigma–Aldrich (St. Louis, MO). Anti-phospho-AKT, anti-AKT, anti-phospho-S118 ERα, anti-caveolin-2 were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Chemiluminescence reagent for Western blotting was obtained from BioRad Laboratories (Hercules, CA). All the other products were from Sigma–Aldrich. Analytical- or reagent-grade products, without further purification, were used. The identities of all of the cell lines that were used (i.e., human breast carcinoma cells [MCF-7 and T47D-1]) were verified by STR analysis (BMR Genomics, Italy).
RNA isolation and qPCR analysis
The sequences for gene-specific forward and reverse primers, RNA extraction and qPCR procedures have been described in Totta et al. (2015).
Cellular and biochemical assays
Cells were grown in 1% charcoal-stripped fetal calf serum medium for 24 h and then stimulated with E2 (10 nM) at the indicated time points. Assays were performed as described in Totta et al. (2014, 2015). For the cell number analysis, the CyQUANT® Cell Proliferation Assay (Life Technologies, Carlsbad, CA) was used according to manufacturer's instructions. In detail, MCF-7 cells were treated with or without siRNA, as described below. After two rounds of transfection, cells were counted, and 3,000 cells were plated in 96-well plates in quintuplicate. After 4 h, the CyQUANT® assay was performed at time 0 (i.e., plated cells). In other 96-well plates, E2 (10 nM) was administered to MCF-7 cells in 1% charcoal-stripped fetal calf serum medium, and after 24 h, the CyQUANT® assay was performed. Western blot analyses were performed as in Totta et al. (2015). Proteins were transferred onto pre-casted nitrocellulose or PVDF membranes using the trans-blot turbo transfer system (Biorad Laboratories, Hercules, CA) for 10 min at room temperature. Band acquisition was performed using the C-Digit® Blot Scanner (Li-Cor Lincon, NE).
RNA interference experiments and siRNA membrane trafficking library
The silencing of caveolin-1 and caveolin-2 in MCF-7 cells was performed by transient transfection of siRNA oligos (RIBOXX), as previously reported (Totta et al., 2015).
Four different RNAi oligos were used to target caveolin-1 in MCF-7 cells:
Oligo 1: 5′-AUUAUGAAGUGAAUGGCAGCCCCC-3′
Oligo 2: 5′-ACAUAACAAAUAUUCAGGCCCCC-3′
Oligo 3: 5′-UACUUGUACACUCCAUGGCCCCC-3′
Oligo 4: 5′-AGUUCUUCAUAUUUCUCUCCCCC-3′
Only oligonucleotides #1 and #3 effectively silenced caveolin-1 protein expression. Experiments were conducted for both oligonucleotides and gave similar results. The results from oligonucleotide #1 are shown in the figures.
Four different RNAi oligos were used to target caveolin-2 in MCF-7 cells:
Oligo 1: 5′-AUUCAUUUGAUUUGGUCCCCC-3′
Oligo 2: 5′-UUUAUAUUCACCAGUCCUGCCCCC-3′
Oligo 3: 5′-ACUUACUGUAUAAUUCUGGCCCCC-3′
Oligo 4: 5′-UACAUAAUCAGAACAGUGCCCCC-3′
Only oligonucleotide #4 effectively silenced caveolin-2 protein expression.
Statistical analysis
A statistical analysis was performed using 1-way ANOVA (analysis of variance) and Tukey's post-hoc tests with the InStat version 3 software system (GraphPad Software Inc., San Diego, CA). Densitometric analyses were performed using the freeware software Image J by quantifying the band intensity of the protein of interest with respect to the intensity of the relative loading control band (i.e., vinculin or tubulin). In all analyses, P values <0.01 were considered to be significant, except for in densitometric analyses, where P was <0.05.
Results
Caveolin-2 influences E2-induced ERα degradation and extra-nuclear signaling
The pattern of caveolin expression was assessed in MCF-7 cells, and as was expected based on the tissue distribution of caveolin, breast cancer cells express caveolin-1 and caveolin-2 (Fig. 1A, A′, B, and B′) but not caveolin-3 (data not shown). The role of caveolin-2 in E2:ERα signaling was then evaluated by transiently reducing caveolin-2 cellular levels in MCF-7 cells using antisense siRNA oligonucleotides. Because caveolin-2 functions occur either together with or independently of caveolin-1 (Le and Kurzchalia, 2005), the effect of caveolin-1 depletion on E2-induced signal transduction was also examined in MCF-7 cells. As expected (Le and Kurzchalia, 2005), while the reduction in caveolin-2 intracellular levels did not change caveolin-1 expression (Fig. 1A and A′), the depletion of caveolin-1 induced a reduction in caveolin-2 intracellular levels (Fig. 1B and B′).
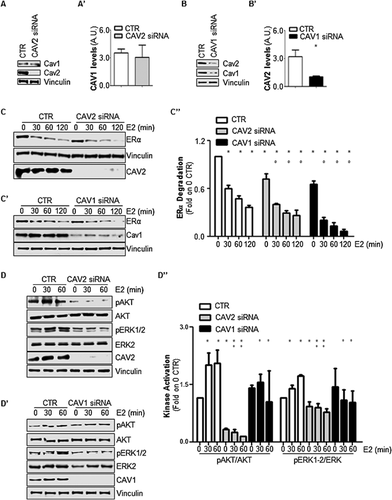
Because E2-dependent control of ERα levels is necessary for E2 signaling-mediated cellular proliferation (Leclercq et al., 2006; Totta et al., 2014, 2015), initial experiments were performed to evaluate the ability of E2 to control intracellular levels of the receptor both in the presence and in the absence of either caveolin-2 or caveolin-1. The depletion of caveolin-2 and caveolin-1 from MCF-7 cells accelerated E2-induced ERα degradation over a period of 2 h with respect to MCF-7 control cells. Remarkably, a small but significant reduction in basal receptor intracellular levels was also detected in both caveolin-2- and caveolin-1-depleted cells (Fig. 1C, C′, and C″).
It has been previously demonstrated that the regulation of ERα content in different breast cancer cell lines is under the control of E2-induced extra-nuclear signaling (i.e., PI3K/AKT activation) (La Rosa et al., 2012; Pesiri et al., 2014; Totta et al., 2014, 2015). Thus, the ability of E2 to activate ERα extra-nuclear signaling in caveolin-2- or caveolin-1-depleted MCF-7 cells was tested. As expected, in MCF-7 control cells, E2 induced a rapid increase (30–60 min) in both AKT and ERK1/2 phosphorylation (Fig. 1D, D′, and 1D″) while the hormone failed to trigger this event when either caveolin-2 (Fig. 1D and D″) or caveolin-1 (Fig. 1D′ and 1D″) expression was reduced. Notably, while caveolin-1 depletion did not change the basal AKT and ERK1/2 phosphorylation levels, a reduction in caveolin-2 decreased the basal activation states of these two signaling kinases (Fig. 1D, D′, and 1D″).
Altogether, these data indicate that caveolin-2 depletion, as well as caveolin-1 depletion, dysregulates both the control of ERα intracellular levels and E2-induced ERα-mediated AKT and ERK activation.
Caveolin-2 influences E2-induced ERα transcriptional activity
E2-depedent extra-nuclear signaling activation controls ERα phosphorylation at serine 118 (S118) (Medunjanin et al., 2005; Acconcia et al., 2006; Totta et al., 2015), which is a post-translational modification that protects the receptor from degradation (La Rosa et al., 2012). According to the previous observations (Fig. 1), E2 was less able to induce ERα S118 phosphorylation in either caveolin-2 or caveolin-1 knockdown cells than in MCF-7 control cells (Fig. 2A, A′, and A″).
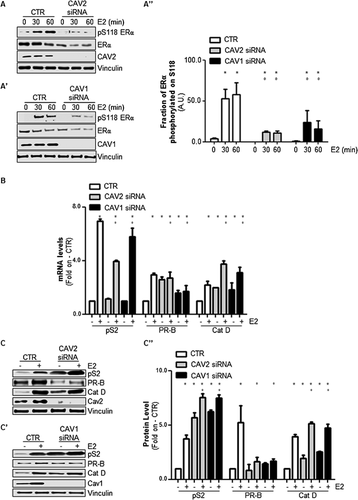
E2-induced ERα S118 phosphorylation potentiates receptor-dependent transcriptional activity (Ali et al., 1993) by favoring the association of the receptor with estrogen responsive elements (ERE) within target gene promoters (Weitsman et al., 2006). In turn, the ability of ERα to regulate the expression of three well-known ERE-containing genes (i.e., presenelin2/TIFF [pS2], progesterone receptor [PR], and cathepsin D [Cat D]) was tested under caveolin-2- or caveolin-1-ablated conditions. As shown in Figure 2B, the E2-induced accumulation of pS2, PR, and Cat D mRNA levels was significantly reduced in MCF-7 cells that had been treated with either caveolin-2 or caveolin-1 siRNA antisense oligonucleotides when compared with MCF-7 control cells. Consistent with these results, the E2-dependent increase in pS2, PR, and Cat D protein levels was also reduced in MCF-7 cells under caveolin-2- or caveolin-1-depleted conditions (Fig. 2C, C′, and C″). On the contrary, basal pS2, PR, and Cat D protein and mRNA levels were differentially increased in caveolin-2 and caveolin-1 knockdown (KD) MCF-7 cells with respect to control MCF-7 cells (Fig. 2B, C, C′, and C″), most likely because of a direct effect of caveolin-1 and caveolin-2 on promoter regulation. Notably, E2 also increased the amount of both caveolin-2 and caveolin-1 in MCF-7 control cells (Figs. 2C, 2C', 3A, and 3A'), which also occurs in other cell lines (Razandi et al., 2002; Park et al., 2009).
Thus, these data indicate that caveolin-2 and caveolin-1 depletion interferes with the ability of E2 to induce pS2, PR, and Cat D gene expression.
Caveolin-2 influences E2-induced cellular proliferation
ERα transcriptional activity also occurs on genes that do not contain an ERE sequence within their promoters. Such an indirect mechanism is mediated by the association of the receptor with other transcription factors (e.g., AP-1 and Sp-1) that are activated as a result of E2-induced extra-nuclear signaling (Ascenzi et al., 2006). Thus, MCF-7 cells were used to study the impact of either caveolin-2 or caveolin-1 depletion on the ability of E2 to control the intracellular levels of cyclin D1 and B cell lymphoma 2 (Bcl-2), two recognized E2 target genes whose transcription is indirectly regulated by ERα extra-nuclear signaling (Castoria et al., 2001; Marino et al., 2002; Acconcia et al., 2005). As is shown in Figure 3A, A′, and A″, the E2-dependent induction of cyclin D1 and Bcl-2 protein levels was reduced in caveolin-depleted MCF-7 cells.
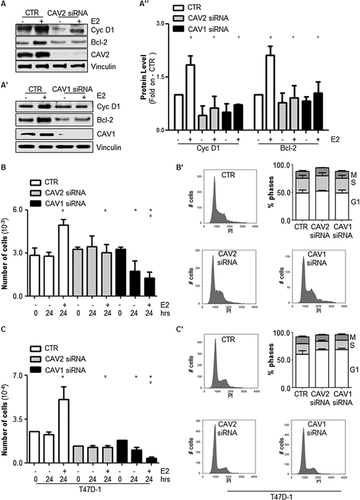
As E2-dependent transcription of both cyclin D1 and Bcl-2 contributes to the E2-dependent induction of cell cycle progression (Castoria et al., 2001; Marino et al., 2002; Acconcia et al., 2005; Pesiri et al., 2014), the ability of E2 to induce cellular proliferation in MCF-7 cells in which either caveolin-2 or caveolin-1 expression was reduced was then evaluated. As expected, E2 treatment increased the number of MCF-7 control cells but not that of MCF-7 caveolin-2- and caveolin-1-KD cells (Fig. 3B). Interestingly, the depletion of caveolin-1 in MCF-7 cells partially reduced the basal cell number (Fig. 3B and Supplementary Fig. S1A and A′). Thus, the potential impact of caveolin-2 and caveolin-1 on the cell cycle was next explored. Figure 3B' shows that the depletion of either caveolin-2 or caveolin-1 changed neither the cell cycle profiles nor the percentage of cells in the G1, S, or M phases. Remarkably, similar results have been obtained in other breast cancer cell lines (i.e., T47D-1) (Fig. 3C, 3C′, and Supplementary Fig. S1B and B′).
Taken together, these data demonstrate that a reduction in intracellular caveolin levels interferes with the ability of E2 to induce cellular proliferation.
Discussion
The primary aim of the present work was to evaluate the involvement of caveolin-2 in E2-activated ERα-mediated signals to induce cellular proliferation. In human cells, there are three different isoforms of caveolin (i.e., caveolin-1, caveolin-2, and caveolin-3) that play distinct roles in physiology (Le and Kurzchalia, 2005; Stan, 2005). This study focused on caveolin-2 because little information was available on the relationship between this membrane protein and ERα. Indeed, ERα has been shown to immunoprecipitate with caveolin-2, and caveolin-2 expression is negatively modulated by E2 (Razandi et al., 2002; Park et al., 2009).
This study confirms that E2 is a physiological inducer of caveolin-2 and further demonstrates that the presence of caveolin-2 is necessary for E2-induced cellular proliferation in breast carcinoma cells (MCF-7 and T47D-1). Indeed, siRNA-mediated depletion of caveolin-2 prevents MCF-7 cell proliferation by blocking the E2-induced up-regulation of the cell cycle-regulating protein cyclin D1 and the anti-apoptotic, pro-survival protein Bcl-2, which coordinates cell cycle progression (Castoria et al., 2001; Marino et al., 2002; Acconcia et al., 2005). Remarkably, cell cycle analysis revealed no changes in the sub-G1 phase of the cell cycle. Thus, in the experimental conditions that were used in this study, no increase in apoptosis was detected. In turn, the greater reduction in cell numbers under caveolin-1 KD conditions relative to caveolin-2 KD conditions, both in the presence and absence of E2, could be ascribed to the fact that caveolin-1 KD is actually a double knockdown for both caveolins (Fig. 1 and see also below).
Consequently, E2-dependent proliferative stimuli are impaired when caveolin-2 expression is reduced because caveolin-2 depletion blocks the ability of E2 to induce extra-nuclear PI3K/AKT and ERK/MAPK pathway activation and to trigger the PI3K/AKT-dependent phosphorylation of serine (S) 118 on ERα (Medunjanin et al., 2005; La Rosa et al., 2012). The lack of phosphorylation on this receptor residue, as caused by caveolin-2 ablation, reduces the ability of the receptor to mediate a nuclear E2-dependent increase in ERE- (i.e., pS2, PR, Cat D) and non-ERE- (i.e., cyclin D1, Bcl-2) containing genes because S118 phosphorylation is required for full ERα transcriptional activity (Ali et al., 1993). Although nuclear and extra-nuclear effects of ERα can be experimentally separable, it is now clear that these mechanisms refer to a single pathway where they are activated by the same receptor protein, which functions both at the plasma membrane and in the nucleus. Indeed, it has been clearly reported that these two mechanisms are deeply intertwined (Pedram et al., 2002; La Rosa et al., 2012). Moreover, a previous study indicated that a lack of ERα plasma membrane localization prevents E2-induced PI3K/AKT and ERK/MAPK pathway activation, ERα promoter occupancy, E2 target gene transcription, and E2-regulated physiological processes, including cellular proliferation (La Rosa et al., 2012). Because all of these E2-mediated effects require caveolin-2, a plasma membrane protein, these data strongly support the concept that caveolin-2 contributes to the integration of both the extra-nuclear and nuclear ERα signaling that is required for the E2-dependent induction of cellular proliferation.
The cellular functions of caveolin-2 can occur either in conjunction with or irrespective of the presence of caveolin-1, which is the principal structural coat protein that is required for caveolae formation (Le and Kurzchalia, 2005). Thus, in the attempt to dissect whether the role of caveolin-2 in ERα signaling depends on caveolae or non-caveolae functions of caveolin-2, the impact of caveolin-1 depletion in MCF-7 cells on E2-mediated cellular proliferation signaling was also evaluated. Interestingly, the effects of caveolin-1 depletion in MCF-7 cells phenocopy those of caveolin-2 KD on ERα signaling. This observation, together with the fact that caveolin-1 depletion also reduces caveolin-2 intracellular levels, strongly suggests that the influence of caveolin-2 on E2-dependent cellular proliferation could be ascribed to the plasma membrane functions of caveolin-2 (i.e., at caveolae), possibly in association with caveolin-1 (i.e., CAV2:CAV1 hetero-oligomers). In this respect, E2 induces ERα dissociation from caveolin-1 (Schlegel et al., 2001; Razandi et al., 2002; Acconcia et al., 2005), which is followed by an association of the receptor with other membrane partner proteins (i.e., insulin-like growth factor 1 receptor - IGF1-R) (Song et al., 2004) and by the activation of E2-dependent extra-nuclear signaling (Song et al., 2004; La Rosa et al., 2012; Totta et al., 2015). Thus, it is tempting to speculate that the lack of E2-mediated extra-nuclear signaling activation that is observed under caveolin-2 depletion conditions could depend on a de-regulated ability of the receptor to re-associate with other membrane partner proteins (e.g., IGF1-R).
Another interesting finding that is reported here is that caveolin-2 and caveolin-1 depletion accelerates the basal and E2-induced control of ERα intracellular levels. Interestingly, it was recently reported that either chemical inhibition of palmitoyl-transferases (PATs) (La Rosa et al., 2012) or genetic ablation of both clathrin heavy chain (CHC) and adaptin-2 (AP2) (Totta et al., 2015) accelerate E2-induced ERα degradation. This effect is due to the lack of PI3K/AKT pathway-dependent ERα S118 phosphorylation, which renders the receptor more prone to proteolytic breakdown (La Rosa et al., 2012). Thus, the effect of caveolin-2 and caveolin-1 depletion on receptor levels could be attributed to the lack of E2-dependent PI3K/AKT-induced ERα S118 phosphorylation. Moreover, these data further suggest the existence of a unique pathway that includes PATs, caveolin-2 and caveolin-1, CHC and AP2; impinges on the control of ERα degradation; and most likely begins at the plasma membrane.
Therefore, these findings support the concept that the ERα plasma membrane localization and association with caveolin-1 and caveolin-2 (Razandi et al., 2002; Acconcia et al., 2005; Pedram et al., 2007) are pivotal for E2-dependent physiological effects (e.g., cellular proliferation) at both the cellular and organismal levels (Adlanmerini et al., 2014; Pedram et al., 2014) and further introduce caveolin-2 in the array of proteins that regulate E2:ERα-mediated physiological functions.
Acknowledgments
This study was supported by grants from AIRC-Associazione Italiana Ricerca sul Cancro (MFAG12756) to F.A. and Ateneo Roma Tre to F.A. The Authors wish to thank Dr Stefano Leone for technical assistance.




