Effect of Docosahexaenoic Acid on Cell Cycle Pathways in Breast Cell Lines With Different Transformation Degree
Abstract
n-3 polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), abundant in fish, have been shown to affect development and progression of some types of cancer, including breast cancer. The aim of our study was to further analyze and clarify the effects of these nutrients on the molecular mechanisms underlying breast cancer. Following treatments with DHA we examined cell viability, death, cell cycle, and some molecular effects in breast cell lines with different transformation, phenotypic, and biochemical characteristics (MCF-10A, MCF-7, SK-BR-3, ZR-75-1). These investigations showed that DHA is able to affect cell viability, proliferation, and cell cycle progression in a different way in each assayed breast cell line. The activation of ERK1/2 and STAT3 pathways and the expression and/or activation of molecules involved in cell cycle regulation such as p21Waf1/Cip1 and p53, are very differently regulated by DHA treatments in each cell model. DHA selectively: (i) arrests non tumoral MCF-10A breast cells in G0/G1 cycle phase, activating p21Waf1/Cip1, and p53, (ii) induces to death highly transformed breast cells SK-BR-3, reducing ERK1/2 and STAT3 phosphorylation and (iii) only slightly affects each analyzed process in MCF-7 breast cell line with transformation degree lower than SK-BR-3 cells. These findings suggest a more relevant inhibitory role of DHA within early development and late progression of breast cancer cell transformation and a variable effect in the other phases, depending on individual molecular properties and degree of malignancy of each clinical case. J. Cell. Physiol. 231: 1226–1236, 2016. © 2015 Wiley Periodicals, Inc.
The close relationship between diet and development and/or prevention of diseases is well known from a long time (Willett, 1994). Diet must provide the correct intake of nutritional factors, such as glucides, lipids, proteins, vitamins, and oligoelements, but also bioactive nutrients have a relevant role. These “chemopreventive” compounds have specific biological activities and thus result able to affect etiological mechanisms of many pathologies, including cancer (Sung et al., 2011). Breast cancer is one of the most common neoplastic diseases among women in the world (Siegel et al., 2013; Malvezzi et al., 2014; Youlden et al., 2014). Both genetic and environmental factors, including diet, are believed to play a role in the onset and evolvement of this kind of neoplasia (Mahoney et al., 2008; Brennan et al., 2010). Dietary fats are one of the most interesting nutritional factors within studies concerning the association between intake quality and risk of developing breast cancer (Saadatian-Elahi et al., 2004; Kim et al., 2006; MacLean et al., 2006).
Among subtypes of dietary fats, n-3 polyunsaturated fatty acids (n-3 PUFAs), and more specifically docosahexaenoic acid (DHA, 22:6, n-3) and eicosapentaenoic acid (EPA, 20:5, n-3), present at high levels in fish, have been shown to affect significantly the course of many pathological conditions of wide relevance. In addition to their well-known cardiovascular beneficial effects (Das, 2015), n-3 PUFAs have proven a role in fetal development, immune function, and neurodegenerative diseases (Ruxton et al., 2005; Swanson et al., 2012). Several molecular and cellular activities promoted by these compounds suggested also their potential ability to affect carcinogenesis and tumor progression. These activities include: (i) alteration of cell membrane dynamics and phospholipids composition, (ii) inhibition of arachidonic acid (AA) metabolism and consequent decrease of AA derived eicosanoids, (iii) expression and function modulation of numerous receptors, transcription factors and lipid derived signaling molecules and (iv) increase of cellular oxidative stress (Cockbain et al., 2012; Abel et al., 2014; Liu and Ma, 2014).
Epidemiological studies regarding the association between intake or plasma concentrations of n-3 PUFAs and cancer risk reduction, provide limited and sometime contrasting data (Dahm et al., 2012; Zheng et al., 2013; Crowe et al., ; Noel et al., 2014). Nevertheless, in a meta-analysis and systematic review of prospective cohort studies, dietary intake of fish derived n-3 PUFAs, such as DHA and EPA, but not shorter alpha linolenic acid (ALA), was associated with a lower risk of breast cancer (Zheng et al., 2013). Furthermore, many in vivo and in vitro investigations indicate that these nutrients may have anticancer properties (Giros et al., 2009; Hu et al., 2010; Jing et al., 2011; Cockbain et al., 2012; Piazzi et al., 2014), even if mechanistic bases have not yet been completely clarified and may be different in each type of studied neoplasia (Giros et al., 2009; Hu et al., 2010; Liu and Ma, 2014). In breast cancer, n-3 PUFAs have shown to inhibit or limit carcinogenesis and reduce risk in both rodent (Karmali et al., 1984; Zou et al., 2013) and cellular (Schley et al., 2005; Liu and Ma, 2014) models. Despite the abundance of these evidences, the molecular mechanisms by which n-3 PUFAs appear to inhibit breast carcinogenesis are still to be completely understood.
Several in vitro studies have shown a variable n3-PUFA-mediated effect on viability and proliferation of different cancer cell lines (Bégin et al., 1986; Finstad et al., 1998; Ding et al., 2004). Therefore, the difficulty of identifying a definite and univocal mechanism of n-3 PUFAs action in carcinogenesis and tumor progression processes may be due to different cell-specific activities of these compounds. This specificity may depend on the phenotypic or biochemical/molecular characteristics of the different cell line models, as derived from tumoral cells caused from different transformation processes (Anel et al., 1992; Maehle et al., 1995; Ding et al., 2004; Ding and Lind, 2007). To clarify these concepts and thus deepen the underlying mechanisms of the anticancer action of n-3 PUFAs, the present study compares the responses to DHA treatments in four breast cell lines: MCF-10A, MCF-7, SK-BR-3, and ZR-75-1 (Soule et al., 1973, 1990; Trempe, 1976; Engel et al., 1978).
We found differential (in some cases even opposite) DHA-induced effects on proliferation, death, and cell cycle progression. Involved mechanisms resulted differentiated among several cell lines, although all derived from mammary epithelium, but at the same time with different biochemical, molecular, and transformation degree characteristics.
Materials And Methods
Reagents
cis-4,7,10,13,16,19-Docosahexaenoic acid, bovine serum albumin-fatty acid free and all other chemicals were purchased from Sigma–Aldrich (St. Louis, MO). Cell culture reagents were purchased from Lonza group (Basel, Switzerland). Nitrocellulose, hyperfilms, and ECL Western blot system were provided by GE Healthcare Europe GmbH (Milan, Italy). Protein assay reagent and protein molecular weight standards were from Bio-Rad Laboratories (Hercules, CA). Antibodies against ERK 1/2, pERK 1/2, STAT-3, p21Waf1/Cip1, phospho-p53 (Ser15) were from Cell Signaling Technology (Danvers, Massachusetts); antibody against phospho-STAT3 (Ser727), p53, GAPDH, and normal rabbit IgG were from Santa Cruz Biotechnologies (Heidelberg, Germany); peroxidase-conjugated anti-mouse antibody from Jackson ImmunoResearch (West Grove, PA). DNase I and SYBR Green I Master-Mix were from Roche Applied Science (Mannheim, Germany). Trizol Reagent and SuperScript® II Reverse Transcriptase kit were from Invitrogen (Carlsbad, CA). Primers were custom synthesized by Primm (Milan, Italy).
Cell cultures and treatments
Human breast cancer cell lines MCF-7, ZR-75-1 (American Type Culture Collection, ATCC) and SK-BR-3 (Interlab Cell Line Collection, ICLC, Genova, Italy) were cultured in Dulbecco's modified minimum essential medium supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Human breast endothelial line (MCF-10A) was purchased from ATCC and maintained in 1:1 mixture of DMEM and Ham's F12 medium supplemented with 5% horse serum, L-glutamine, human recombinant epidermal growth factor (20 ng/ml), insulin (10 μg/ml), cholera toxin (100 ng/ml) and hydrocortisone (5 μg/ml). Cells were routinely grown in culture dishes (Corning, Corning, NY) in an environment containing 5% CO2 at 37°C and splitted every 3 days.
For experiments, cells were seeded at a density of 8 × 105 per 100-mm dish and allowed to adhere for 24 h. Medium was then replaced with 5% serum fresh medium with 100 and 300 µM DHA bound to fatty acid-free bovine serum albumin. DHA was dissolved in ethanol and mixed with bovine serum albumin at 2:1 molar ratios before addition to medium. As negative control, cells were treated with vehicle containing the same amounts of ethanol mixed with bovine serum albumin.
Cell viability assay
The number of viable cells was quantified by MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]) assay. Cells (6–8 × 103) were plated in each well of 96-well tissue culture plate in 100 μl medium. After 24 h, the medium was replaced with 100 μl of fresh medium containing DHA or only vehicles and cells were grown for designed amounts of time. After each incubation time, medium was removed and cells were stained with 100 µl of MTT dye (1 mg/ml) for 2 h. The MTT crystals were dissolved in DMSO and the absorbance (490 nm) was detected on a Multiskan™ GO Microplate Spectrophotometer (Thermo Scientific, Waltham, MA). Test was carried out on at least five technical and three biological replicates.
Flow cytometry analysis
Cellular DNA content was evaluated by propidium iodide (PI) staining method as previously described (Riccardi and Nicoletti, 2006). Cells were analyzed with a FACSCalibur™ (Becton Dickinson, San Jose, CA). Percentages were calculated using the CellQuest and MODFIT software. Analysis were performed on at least three technical and biological replicates.
Cell protein extraction and Western blot analyses
After treatments, cells were washed and lysed as previously described (Caputo et al., 2014). Thirty micrograms of total proteins from each extract were separated by 8% or 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes in a cooling system at 100 V for 1 h. Membranes were saturated for 1 h at room temperature with 0.1% Tween-20, 5% dry milk or BSA in PBS. Membranes were then incubated with antibodies against the appropriate human primary antibody diluted 1:500 or 1:1,000, overnight at 4°C, washed several times and incubated with peroxidase-conjugated secondary antibodies (anti-mouse or anti-rabbit diluted 1:10,000) for 1 h at room temperature. Specific bands were then detected by ECL Western blot system. Antibody against GAPDH were used as normalizing control. Densitometry of bands was performed with ImageJ software (http://rsbweb.nih.gov/ij/download.html). Molecular sizes were evaluated referring to protein molecular weight standards. Each treatment was performed at least in biological triplicate.
RNA extraction and reverse-transcription
Total RNA was isolated from treated cells as previously described (Caputo et al., 2014).
Real-time PCR
Real-time PCR was performed with Light-Cycler® 480 (Roche Diagnostics GmbH, Mannheim, Germany) using SYBR Green detection in a total volume of 20 µl with 1 µl of forward and reverse primers (10 mM) and 10 µl of SYBR Green I Master-Mix. Reactions included an initial cycle at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 sec, annealing at 56°C for 5 sec, extension at 72°C for 15 sec.
To analyze p21Waf1/Cip1 expression level the following primer sets were used: forward p21Waf1/Cip1 5′-GAA CTT CGA CTT TGT CAC CG-3′; reverse p21Waf1/Cip1 5′-GCA CAA GGG TAC AAG ACA GT-3′; forward 18S 5′-CGA TGC TCT TAG CTG AGT GT-3′; reverse 18S 5′-GGT CCA AGA ATT TCA CCT CT-3′.
Fold change of induction was determined by calculating the ratio between control and treatment normalized signals. Differences between mean values were evaluated for statistical significance using the unpaired Student t-test. Each treatment was performed at least in four biological replicates.
Statistical analysis
Data are presented as mean ± standard deviation. Differences between treatment groups were analyzed by Student t-test. Differences were considered significant when P < 0.05.
Results
DHA effects on cell viability of human breast cell lines
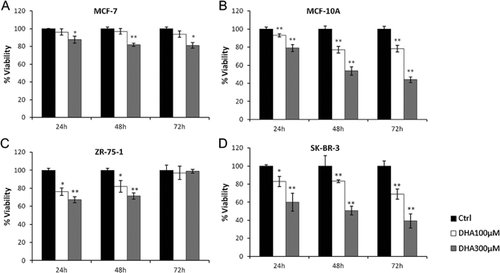
We first examined the effects of two DHA concentrations (100 μM and 300 μM) on the viability of four selected human breast cell lines (MCF-10A, MCF-7, SK-BR-3, ZR-75-1) by MTT assays. Each cell line was incubated with both doses of DHA and the numbers of viable cells were determined after 24, 48, and 72 h, comparing them with those of respective controls. As shown in Figure 1, treatments with DHA induced only a slight reduction of viability of MCF-7 and ZR-75-1 (A and C) compared to control values. In MCF-7, small (<20%) but still significant effects on viability, were observed only with the highest concentration of this compound (A). The small and early decreases in the number of vital ZR-75-1 cells disappear at 72 h (C). Conversely, DHA appears to exert a strong inhibitory action (time and dose dependent) on MCF-10A and SK-BR-3 growth (Fig. 1B and D). In particular, we found a 60% reduction in the number of viable MCF-10A cells after a 72 h treatment with 300 μM DHA (B). Panel D shows a similar pattern obtained with the SK-BR-3 cell line.
DHA effects on cell cycle
To assess if the reductions of cells number, observed with MTT assays, were due to a cell cycle arrest or to an induction of cell death, DNA content was measured by flow cytometry in control cells and in cells treated for 24, 48, and 72 h with DHA 100 and 300 μM.
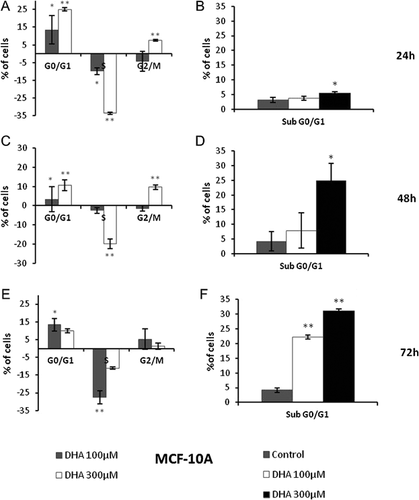
Graphics in figures 2A and B show that DHA treatments are able to produce a significant increase of number of MCF-10A cells in the G0/G1 phase of cell cycle and a smaller one in the G2/M phase, after 24 h. These increases of G0/G1 and G2/M cell populations result concomitant to a decrease of cells in S phase and an unchanged percentage of sub G0/G1 cells. After 48 and 72 h (Fig. 2C–F), we found a reduction of G0/G1 arrest caused by DHA treatments and a significant increase of hypodiploid and G2/M arrested cells.
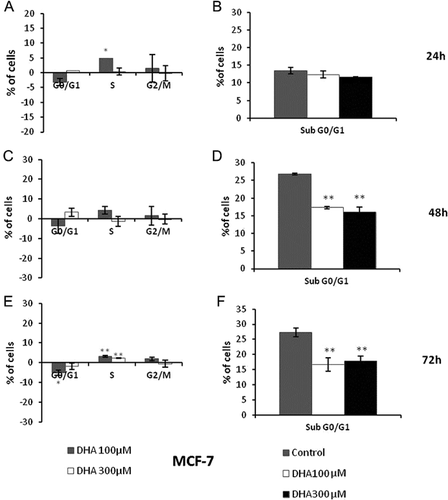
In MCF-7 cell model, treatments with DHA for 24 h do not cause any prominent variation of both proportion of apoptotic/necrotic cells and progression of cell cycle (Fig. 3A, B). We found an unexpected reduction of hypodiploid percentage compared to control and no alterations of cell cycle after 48 and 72 h (Fig. 3C–F).
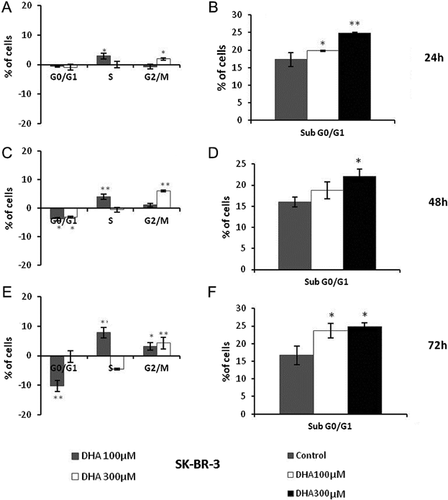
DHA treatments increase SK-BR-3 population in sub G0/G1 phase already after 24 h, without relevant changes of cells distributions in cell cycle phases (Fig. 4A, B). These changes began more significant with longer treatment times (48 and 72 h) (Fig. 4C–F).
DHA effects on ERK 1/2 and STAT3 activation
To define molecular mechanisms underlying the differential actions of DHA on each analyzed breast cell line, we evaluated its ability to affect two pathways typically involved in proliferation and cell cycle regulation. We analyzed phosphorylation levels of ERK 1/2 kinases and of STAT3 transcription factor after 2, 16 and 24 h incubation with DHA.
Western blot analysis showed that DHA treatment significantly increased phosphorylation levels of ERK 1/2 after 2 h and until 24 h in MCF-10A cell line (Fig. 5). These phosphorylation levels were weakly affected in MCF-7 cells and reduced only at 24 h. Conversely, in SK-BR-3 cell line, treatment produced a significant inhibition of ERK 1/2 activation in all assayed times.
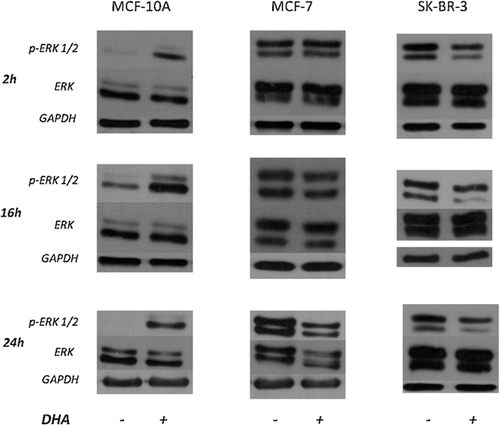
Figure 6 shows STAT3 phosphorylation (Ser 727) which resulted significantly and rapidly increased by DHA treatments in MCF-10A cells. STAT3 phosphorylation was decreased (about 50%) in MCF-7 cell line 24 h after DHA addition. Treatment decreased phosphorylation after 16 and 24 h also in SK-BR-3 cells (about 30% and 60%, respectively).
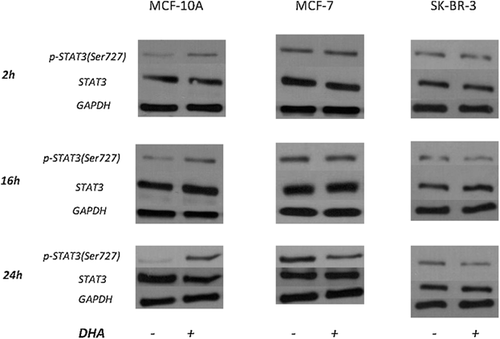
Neither treatment affected the expression of ERK 1/2 and STAT3 total forms.
DHA effects on p21Waf1/Cip1expression
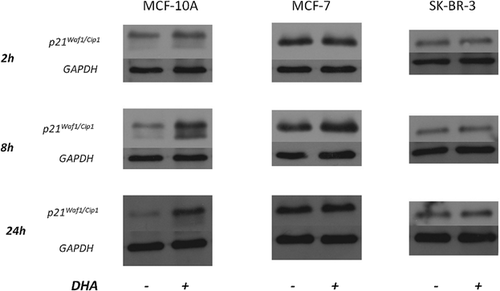
Protein levels of p21Waf1/Cip1 were analyzed in all three cell lines treated or not with DHA for 2, 8 and 24 h. Figure 7 shows that p21Waf1/Cip1 protein expression was increased by DHA administration in MCF-10A cells from 2 h and even more after 8 and 24 h. No significant changes of p21Waf1/Cip1 protein level in both MCF-7 and SK-BR-3 cell lines were observed.
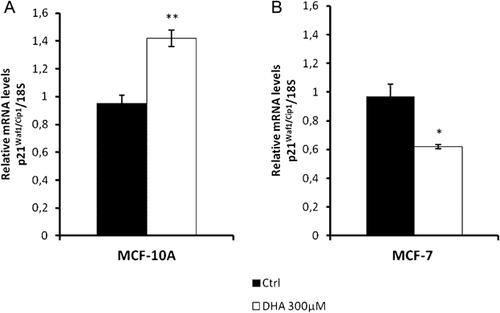
p21Waf1/Cip1 mRNA expression under the same previous experimental conditions was assayed by real-time PCR. In this case the two cell lines (MCF-10A and MCF-7) showing the most different sensitivity to DHA treatments were selected. In MCF-10A cells treatment up-regulates p21Waf1/Cip1 mRNA level, with an increase of about 50% (Fig. 8A). However this level was significantly reduced in MCF-7 cells (Fig. 8B).
DHA effects on p53 expression and activation
To verify its possible role in our experimental conditions, p53 was also analyzed. Figure 9 shows that p53 phosphorylation at serine 15 increases overtime (mostly at 24 h) in MCF-10A cells exposed to DHA, although decreases in SK-BR-3 cell line. MCF-7 cells do not express phophorylated p53 and DHA treatments do not change this activation.
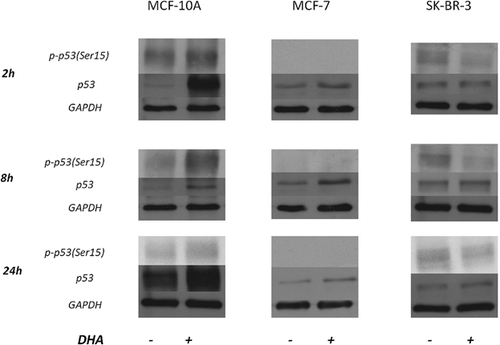
The analysis of total p53 showed a different trend (Fig. 9). In MCF-10A cells there was a quick increase of p53 (already after 2 h of DHA treatment), stronger than that of phosphorylated form but decreasing over time. DHA did not affect p53 expression in SK-BR-3 cell line. A weak over-expression of p53 in treated MCF-7 cells was also observed.
Discussion
The present study was conducted to give additional insight on the preventive and therapeutic effects reported for n-3 PUFAs and more specifically for DHA (Ruxton et al., 2005; Cockbain et al., 2012; Swanson et al., 2012; Abel et al., 2014). We used an investigative approach based on the comparison of DHA effects on several breast cell models. We selected four breast cell lines: MCF-10A, MCF-7, SK-BR-3, ZR-75-1, which are widely considered valid as models for the study of molecular mechanisms of breast carcinogenesis (Lacroix and Leclercq, 2004). These cells are all derived from mammary epithelium, but with differential transformation degrees and biochemical characteristics. MCF-10A breast cells are an immortalized but non-malignant cell line, expressing many specific antigens of breast tissue (Soule et al., 1990). MCF-7, SK-BR-3, and ZR-75-1 cells are breast cancer cell lines; the first two being derived from breast adenocarcinoma, although the third from ductal breast carcinoma (Soule et al., 1973; Davis et al., 1985; Tamm et al., 1994; Osmak et al., 1997). MCF-7 and ZR-75-1 cells retain typical characteristics of mammary differentiated epithelium and express estrogen receptor (ER+) (Brooks et al., 1973; Engel et al., 1978). SK-BR-3 cell line over-expresses HER2/c-erb-2 gene, does not have estrogen receptor and presents a less differentiated and more invasive phenotype compared to the other two lines (Trempe, 1976; Davis et al., 1985; Järvinen et al., 2000).
Several epidemiological and experimental studies suggest diet fatty acids, above all n-3 EPA and DHA, as capable to affect the onset and the later evolution of breast cancer (Zheng et al., 2013; Zou et al., 2013; Liu and Ma, 2014). Beside the numerous in vitro and in vivo evidences of the direct anti-tumor activities of n-3 PUFAs, there are less studies investigating the same effects in patients with primary or metastatic carcinoma. Cockbain et al. (2014) showed n-3 EPA anti-colorectal cancer activities in patients awaiting surgery for colorectal cancer liver metastases. n-3 PUFAs were investigated in the prevention of colorectal cancer (Courtney et al., 2007; West et al., 2010) and as adjuvants with traditional chemoradiotherapy of breast cancer (Bougnoux et al., 2009). This latter phase II study evaluated addition of 1.8 g DHA daily to an anthracycline-based chemotherapy regimen for metastatic breast cancer, revealing that the treatment may enhance the outcome in patients with high DHA-incorporation in plasma phospholipids (Bougnoux et al., 2009). The DHA concentrations used in our experiments are close to the serum levels reported in a number of patients with different nutritional status (Zirpoli et al., 2012).
Viability of all different cell lines, although derived from the same type of tissue, was differentially affected by DHA treatments. This allowed to distinguish two cell lines with the highest sensitivity to DHA, MCF-10A and SK-BR-3, although MCF-7 and ZR-75-1 were those less affected. The nutrient induced the highest cytotoxicity in the non-transformed MCF-10A cell line and in the cell line with the highest degree of transformation (SK-BR-3), although slightly affected the growth of the two other cell lines with lower transformation degree (MCF-7 and ZR-75-1). Therefore, it is not possible to correlate DHA effects on viability with cell transformation degree, but it could be hypothesized that there is an intermediate phase of the transformation process, in which cells have biochemical and/or phenotypic characteristics making them more resistant to DHA effects. Since MCF-7 and ZR-75-1 cell lines have similar transformation degrees and properties, we decided to select only the first one for further investigations, although we decided to analyze both the two most DHA sensitive cell lines, MCF-10A and SK-BR-3, because they have quite different characteristics.
Cell cycle analyses showed that DHA inhibits cell proliferation through different mechanisms. In MCF-10A cell line, DHA first (at 24 h) affects cell cycle progression, leading a strong G0/G1 arrest and then induces cell death. However, in SK-BR-3 cells the compound exerts its antiproliferative effect predominantly through a cell death induction. In agreement with viability assay results, MCF-7 cells did not show any significant alteration of both cell cycle and death levels. In this line, the observed reductions of sub G0/G1 cells (above all at 48 and 72 h) may suggest a DHA ability “to switch on” specific cell survival mechanisms.
We found different DHA-mediated modulations of ERK1/2 and STAT3 phosphorylation levels in each cell model. Activation of these factors is commonly coupled with an increase of cell survival and proliferation (Pearson et al., 2001; Johnston and Grandis, 2011). Thus their inhibition in SK-BR-3 cells by DHA results in agreement with its antiproliferative effects which was observed previously and also results in our data. This is also true for the small phosphorylation reductions of these proteins which were observed in MCF-7 cells (only after 24 h). Conversely, in MCF-10A cell line DHA-mediated growth inhibitions matched with an activation increase of these proliferative factors. However, only in this cell line expression of p21Waf1/Cip1, which is a cell cycle inhibitor (Abbas and Dutta, 2009), was increased by DHA at mRNA and protein level. In addition the activation of the pathways involving ERK 1/2 and STAT3 was also reported as responsible of antiproliferative effects (Giraud et al., 2004; Cagnol and Chambard, 2010). For example, STAT3 acts as transcriptional factor up-regulating growth promoting genes such as c-myc, pim-1 or cyclin D1, but also inducing pro-apoptotic activities and p21Waf1/Cip1 expression (Giraud et al., 2002, 2004). Therefore, in some cases STAT3 may be able to arrest cell cycle progression and to prevent an abnormal cell proliferation. It was reported that STAT3-mediated inhibition of cell growth through induction of p21Waf1/Cip1 expression is lost during tumor progression (Flørenes et al., 1999). Thus, STAT3 activation promoted by DHA in MCF-10A cells is consistent with their G0/G1 arrest through an increase of p21Waf1/Cip1 expression. Indeed, in this cell line after DHA treatment, we observed a significant up-regulation of both p21Waf1/Cip1 mRNA and protein, although no changes of the protein were found in MCF-7 cells, even with an actual reduction of the mRNA. Moreover, it is known that STAT3 phosphorylation on Ser727 is required for its gene regulation function (Wen et al., 1995). Several studies have shown that ERK MAP kinases are the main effectors of this phosphorylation (Chung et al., 1997). This is in agreement with the increase of ERK1/2 phosphorylation level observed in MCF-10A cells after DHA treatment.
Finally, DHA showed a differential action also on p53 expression and phosphorylation. This protein is a key cell cycle inhibitor and may act as transcription factor for p21Waf1/Cip1 (Prives and Hall, 1999; el-Deiry et al., 1993). Our results suggest that also this factor may be involved in DHA-mediated up-regulation of p21Waf1/Cip1 in MCF-10A cells. In fact, in this cell line, we found a rapid and strong increase of p53 expression (2 h after DHA addition) and a later stimulation of its phosphorylation (above all at 24 h). In particular, we analyzed p53 phosphorylation on Ser15, which is important to induce its maximal transcriptional activation (Lambert et al., 1998). In the same experimental conditions, in MCF-7 cell line, a similar increase of total p53 expression is not matched by any increase of p53 phosphorylation and therefore of its active state. Nevertheless, this cell line completely lacks basal p53 phosphorylation. In SK-BR-3 cells DHA induces a reduction of Ser15 p53 phosphorylation, which corresponds to a decrease of its nuclear accumulation and transcriptional activity (Lambert et al., 1998). Therefore, it is conceivable to hypothesize that mechanisms independent from p21Waf1/Cip1 and p53 activities are involved in the effects induced by DHA on this cell line viability.
A different PUFAs sensitivity in several tumor cells was reported (Finstad et al., 1998; Ding et al., 2004), but underlying involved mechanisms were not clarified. This study shows that DHA is able to induce specifically different effects both on cells derived from non neoplastic breast tissue and on cell lines extracted from various cancer specimens from the same type of tissue, suggesting that the action of this nutrient is dependent on the individual biological characteristics of each single neoplasia. In fact, DHA appeared to affect breast cell viability through different (in some cases opposite) mechanisms. DHA showed to inhibit the growth of a breast cancer cell line with the highest transformation degree (SK-BR-3) among the three examined, through a cell death induction mechanism likely mediated by the inhibition of proliferative pathways involving ERK 1/2 and STAT3. A rapid and substantial antiproliferative effect appeared also in MCF-10A cell line after DHA treatments. This is a non tumor breast cell line, but in any case immortalized and possibly also comparable to a breast cell in a early transformation stage. Nevertheless, in this case the growth inhibitory action of DHA is mediated through different mechanisms, such as an early G0/G1 cell cycle arrest, the activation of both ERK and STAT3 factors and the induction of p21Waf1/Cip1 and p53. On MCF-7 cells DHA showed just weak effects on cell viability, cell cycle progression, ERK 1/2 and STAT3 phosphorylation. However, in this frankly transformed cell line, differently from SK-BR-3 cell line, we observed, on DHA stimulation, a significant down-regulation of p21Waf1/Cip1 mRNA, although without reduction of its protein level and effects on p53 phosphorylation level.
Several molecular and cellular activities, promoted by n-3 PUFAs, were proposed as the most important players of their anti-tumor action (Cockbain et al., 2012; Abel et al., 2014; Liu and Ma, 2014) . Our data show that mechanisms, underlying the variable DHA effects, are depending on the molecular status of each transformed cell line, even if originating from the same tissue. The further perspectives of this study will have to include also the analysis of the effect of other PUFAs, as well as the assessment of fatty acid profiles, both inside and outside treated cells, and also of free radicals and peroxides, since literature contains relevant data about their involvement within these processes (Das and Madhavi, 2011; Zhang et al., 2015). The developments of this study will allow to better define the biochemical “microenvironments” making cancer cells more sensitive to n-3 fatty acids action in order to more appropriately employ them in both chemoprevention and as adjuvants in therapeutic treatments of neoplastic diseases.
Acknowledgments
This work was supported by University of Salerno (FARB Grants 2013 and 2014) and MIUR (Ministero della Istruzione, Università e Ricerca, PRIN 200832E9J9_003).




