Exploring the Molecular Interaction Between NR2E3 and NR1D1 in Retinitis Pigmentosa: A Docking and Molecular Dynamics Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Background and Aims
Retinitis pigmentosa (RP) is a hereditary retinal disorder that gradually leads to vision loss due to photoreceptor cell degeneration. This study aims to investigate the clinical features and genetic underpinnings of RP within a large Iranian family. Our focus centered on mutations in the NR2E3 gene, which plays a critical role in the development and maintenance of the retina.
Methods
Twenty-five family members showed symptoms of RP, and fourteen of them underwent clinical examinations conducted by geneticists and ophthalmologists. The DNA samples of five individuals diagnosed with RP from the family were subjected to whole-exome sequencing (WES) as part of the study. The candidate variant identified through WES was subsequently confirmed using bidirectional sequencing in additional family members. Additionally, in silico analysis, including molecular modeling, protein–protein docking, and molecular dynamics simulation (MD), was employed to assess potential pathogenic effects associated with the candidate variants.
Results
Ophthalmic examination revealed night blindness, which is a common symptom among affected individuals. Genetic analysis identified a homozygous missense variant (c.934G>A/p.R311Q) in NR2E3 exon 6, which co-segregates with other affected family members. Furthermore, molecular docking analysis indicated potential disruption in the binding affinity between NR2E3 and NR1D1 proteins. In-depth, molecular dynamics analysis, considering parameters such as RMSD, RMSF, and hydrogen bonding, revealed notable differences between normal and mutant protein complexes.
Conclusion
Exploring the molecular interaction between NR2E3 and NR1D1 provides new insights into the pathogenic mechanism of the p.R311Q mutation in RP.
1 Introduction
Inherited Retina Degeneration (IRD) is a group of progressive and degenerative diseases that profoundly impact an individual's visual function, especially those of working age [1]. Among the various forms of IRD, Retinitis Pigmentosa (RP; OMIM # 268000) stands out as the most common type, responsible for irreversible blindness due to fundus disease [2]. It affects approximately one in every 3500–5000 individuals worldwide, accounting for half of all IRD cases, and significantly disrupts patient's health and quality of life [3]. RP can manifest in syndromic or non-syndromic forms and is characterized by the gradual loss of rod and cone photoreceptors, leading to severe visual impairment in both eyes [4]. Night blindness, typically beginning in adolescence, is followed by a gradual decline in peripheral and central vision, which are the clinical hallmarks of RP [5].
RP presents a multifaceted challenge for both geneticists and physicians due to its significant genetic, clinical, and penetrance variability. Moreover, this challenge is compounded by the fact that RP shares overlapping driver genes with other inherited retinal disorders (IRDs), further emphasizing the complex nature of the disease [6]. RP is a hereditary condition that follows three classic Mendelian inheritance patterns: autosomal dominant (30%–40%), autosomal-recessive (50%–60%), or X-linked (5%–15%). Understanding the genetic base of RP is crucial for effective management and treatment. However, non-Mendelian inheritance patterns, such as digenic and maternal inheritances, have also been reported in a small proportion of cases, highlighting the need for a comprehensive understanding of the genetic factors involved [7, 8]. To date, researchers have discovered over 70 genes and loci associated with RP. However, there are still unidentified RP genes awaiting identification [9, 10]. The discovering of novel RP genes would significantly enhance diagnosis, prevention, and treatment strategies for this challenging disease [11].
NR2E3, also called RP37, is a gene intricately involved in retinal development and function. It encodes a transcription factor that regulates genes crucial for vision. Mutations in NR2E3 disrupt its normal function, leading to retinal abnormalities. This disruption can result in impaired rod cell development, dysfunction of both rod and cone photoreceptors, and altered signaling within the retina. Numerous types of mutations in NR2E3 are associated with various related diseases. Research conducted in Arab countries has identified two specific mutations in NR2E3: a frameshift deletion (c.951del; p.(Thr318Argfs*6)) and a point mutation (c.932G>A; p.(Arg311Gln)). Both of these mutations are linked to rod-cone dystrophy [12]. The R311Q mutation, in particular, stands out as the most commonly reported variant in NR2E3. It is associated not only with enhanced S-cone syndrome (ESCS) but also with other forms of retinal degeneration. Patients who are homozygous for this mutation typically experience significant visual impairment and exhibit distinctive retinal changes, such as clumped pigment.
In addition to NR2E3, other factors play crucial roles in rod differentiation, including CRX, NRL, and NR1D1. These transcription factors, along with NR2E3, interact and form multi-protein transcriptional regulatory complexes, providing evidence of their collaborative role in gene regulation during rod development.
The Nuclear Receptor Ligand-Binding Domain (NR_LBD) of NR2E3 is pivotal for ligand binding, gene expression regulation, and protein–protein interactions. It plays a central role in the function of NR2E3 as a transcription factor and contributes to the fine-tuning gene expression in various biological processes. Mutations within the NR_LBD domain of NR2E3 have been linked to various retinal disorders, including RP and enhanced S-cone syndrome.
The complexity of RP arises from multiple factors. Notably, different mutations within the same RP-associated gene can lead to diverse disease manifestations. Additionally, clinical variability exists in both inter- and intra-familial cases with identical mutations, which may be attributed to modifier genes [13].
The advent of next-generation sequencing (NGS) technologies, including whole-exome sequencing (WES) and panel-based NGS, has revolutionized the molecular genetic diagnosis of IRDs such as RP. WES, which targets the entire protein-coding region of the genome, has emerged as a powerful tool for identifying causative genes of retinal diseases. Particularly, in populations with high consanguinity rates and large family sizes, such as the Iranian population, genetic analysis of hereditary diseases is particularly effective [13-15]. In this study, we utilized WES to identify disease-causing variants (DCVs) associated with RP, comparing potential deleterious variants across affected and unaffected families to establish genotype–phenotype correlations.
2 Methods
2.1 Ethical Approval
The present research, which was authorized by the Research Ethics Committee of Birjand University of Medical Sciences (IR.BUM.REC1400.4.9), required written informed consent from all probands and their family members. If the family members were minors, consent was obtained from their guardians.
2.2 Clinical Data and Sample Collection
We recruited four pedigrees with a confirmed diagnosis of RP and a clear family history of at least two affected members in Nehbandan city (South Khorasan province, Iran) between January 2022 and May 2022.
- Patient History and Fundus Examination: A thorough assessment of the patient's medical history and a detailed examination of the fundus.
- Family History: Consideration of any family history of night blindness or low vision.
- Abnormal Static Perimetry Results: Objective assessment of visual fields through static perimetry testing.
RP within families was determined based on symptoms reported by probands and their relatives, such as vision problems and difficulty seeing at night. These reports were then verified through clinical assessments. To confirm retinal pathology, we employed digital fundus photography, focusing on conditions such as bone spicule pigmentation and optic disc pallor.
All patients underwent comprehensive ophthalmic examinations, and genetic counseling sessions were conducted with the probands' parents, incorporating the expertise of a senior human geneticist. We meticulously documented family history, including any notable occurrence of this condition in historical records (Table 1). Figure 1 displays the pedigree chart generated using Evagene software (https://www.evagene.com/). Venous blood samples were collected from both affected and unaffected family members using EDTA vacutainers.
| Patient ID | Patient Gender | Diagnosis | Age | Age of Onset | RP Fundus Feature | Visual Acuity OD OS | |
|---|---|---|---|---|---|---|---|
| III11 | Female | RP | 28 | 9 | Yes | 0.7 | 0.2 |
| II4 | male | RP- cataract | 58 | 13 | Yes | HM | CF at 3 m |
| IV7 | Female | RP | 8 | 4 | Yes | CF at 5 m | CF at 2 m |
| III6 | Male | RP- cataract- High myopia | 26 | 10 | Yes | CF at 5 m | 0.1 |
| III25 | Male |
RP Photophobia |
45 | 12 | Yes | CF at 1 m | CF at 1 m |
| III4 | Female | RP | 39 | 11 | Yes | 0.5 | 0.5 |
| III19 | Male | RP | 34 | 8 | Yes | CF at 0.5 m | CF at 1 m |
| IV13 | Female | RP | 9 | NA | Yes | 0.5 | 0.5 |
| III41 | Male | RP | 43 | 10 | Yes | CF at 1 m | CF at 1 m |
| III15 | Male |
RP Photophobia |
10 | 7 | Yes | CF at 0.5 m | CF at 0.5 m |
| III30 | Male | RP-Strabismus | 28 | 11 | Yes | 0.1 | 0.6 |
| IV21 | Male | RP | 30 | 12 | Yes | CF at 5 m | CF at 5 m |
| IV15 | Male | RP- Strabismus- Tongue tie | 34 | 15 | Yes | CF at 1 m | CF at 1 m |
| III31 | Female | RP | 50 | 12 | Yes | CF at 5 m | CF at 5 m |
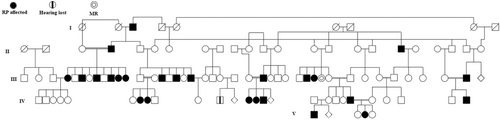
2.3 Whole-Exome Sequencing (WES)
In this study, we employed WES to uncover genetic variants associated with RP. genomic DNA was isolated from the patient's blood using the QIAamp DNA Blood Mini-Kit (QIAGEN, Germany) and evaluated the quality and quantity of the extracted DNA using a NanoDrop spectrophotometer (Epoch, Bio-Tek Inc., VT, USA). The methods employed for our genetic analysis were previously outlined [16]. Briefly, we selectively captured the protein-coding regions of the genome using the SureSelect Human All Exon V6 kit from Agilent. The captured DNA fragments underwent sequencing using the HiSeq2500 platform from Illumina (San Diego, USA), generating paired-end reads of 150 base pairs. Standard bioinformatics pipelines were used to process the resulting sequence data. We aligned sequences to the reference genome using Burrows-Wheeler Aligner (BWA) software. Duplicate reads were remove using Picard tools, and base quality scores were adjusted using the Genome Analysis Toolkit (GATK). Variant calling was performed using GATK's Haplotype Caller tool [17]. Identified variants were annotated using ANNOVAR software [17]. Variants with a minor allele frequency (MAF) greater than 1% in genetic databases (such as ExAC_EAS, 1000 Genomes Project, and Iranome) (http://iranome.ir/) were filtered out. The remaining variants were further filtered based on functional annotation, predicted pathogenicity, and known association with RP. We evaluated the clinical significance of the variant using various genetic mutation databases, including ClinVar, Online Mendelian Inheritance in Man (OMIM), and the Human Gene Mutation Database (HGMD). The variant was then classified and interpreted according to the guidelines provided by the American College of Medical Genetics (ACMG) [18]. Sanger sequencing confirm the identified variants in affected individuals and tested for segregation in family members. The PCR primers are designed for a 377 base-pair fragment: Forward (5′-CATTCTCGGGTCCCAGGACAGCA-3′) and Reverse (5′-ACTCAGTTACCTGGCTTGAAGAGG-3′). Sequencing was conducted on an ABI 3730XL sequence analyzer (Applied Biosystems, USA).
2.4 Protein Modeling
The amino acid sequences for NR2E3 and NR1D1 were retrieved from the NCBI protein database, and the LBD of NR2E3, containing the identified mutations, was modeled using homology modeling techniques. In our study, we utilized Iterative Threading Assembly Refinement (I-TASSER) as a computational tool for predicting the three-dimensional structure of the mutant protein. This approach provided valuable insights into the structural features and potential functional implications of the mutated variant, deepening our understanding of its molecular properties. The quality of the I-TASSER model was assessed using the confidence score (C-score), which ranges from −5 to 2. The C-score serves as a measure of the accuracy and reliability of the predicted protein structures. Subsequently, the model underwent assessment using Molprobity. This allowed us to assess the geometric and energetic properties of both the mutant and normal protein structures. Furthermore, we constructed Ramachandran plots using MolProbity to compare the quality of the structures.
2.5 Protein–Protein Docking
Computational tools have become invaluable for detecting pathogenic variants and assessing their impact on protein function. In this study, our primary objective was to explore the potential effects of mutations occurring in the LBD of NR2E3 on its interaction with NR1D1. To achieve this, we employed protein–protein docking simulations to investigate the dynamics between these two proteins. The docking process was initiated using the ClusPro 2.0 protein–protein docking server (https://cluspro.bu.edu/) to dock the crystal structure of NR1D1 and NR2E3 onto the LBD of NR1D1. This approach generated various complex structures representing potential binding configurations between NR2E3 and NR1D1. To assess and gain insights into these docked complexes, we used the PyMOL v2.4 (Schrödinger, USA) molecular graphics system. Leveraging its powerful visualization capabilities, we meticulously examined and analyzed feasible interactions and structural models resulting from the protein–protein docking simulations. This step provided valuable information on potential binding modes and the stability of the NR2E3-NR1D1 complexes. By combining these techniques, our study aimed to illuminate the interplay between mutations in the LBD of NR2E3 and their interaction with NR1D1, offering insights into the molecular mechanisms underlying these interactions and their potential implications.
2.6 Molecular Dynamics Simulation
In our investigation, we employed GROMACS 2021.4 for molecular dynamics (MD) simulations, utilizing CHARMM27 force field parameters to accurately capture the behavior of both mutant and normal proteins. To ensure system fidelity, we carefully selected simulation boxes with appropriate dimensions, applying periodic boundary conditions. Placing the protein complexes approximately 1 nm away from the carriers' surface within each box effectively mitigated undesired interactions between neighboring components, preserving system integrity.
After placing the protein complexes, we solvated the simulation boxes meticulously using explicitly treated water molecules, adopting the widely-accepted TIP3P model. This choice allowed us to accurately mimic the behavior of water molecules, creating a realistic environment for the proteins within the simulation. To maintain system neutrality and establish a biologically relevant context, we strategically introduced Na and Cl ions by replacing water molecules. This crucial step balanced any charge imbalances, ensuring system stability and physiological accuracy throughout the simulation. Subsequently, we conducted energy minimization to relax the system, followed by a series of equilibration steps to gradually bring the system to the desired temperature and pressure. These equilibration procedures allowed the system to reach equilibrium, enabling the proteins to adjust and settle into their normal conformations. Executing these methodologies within GROMACS, our simulations aimed to unravel the intricate dynamic behavior, conformational changes, and intermolecular interactions exhibited by the mutant and normal protein complexes. This comprehensive exploration provides valuable insights into the structural stability, flexibility, and functional implications of the proteins under investigation, advancing our understanding of their behavior within a simulated biological context. The simulations allowed us to calculate several important parameters, such as Root Mean Square Deviation (RMSD), Root Mean Square Fluctuation (RMSF), and hydrogen bond (HB) interactions. RMSD provides information about the structural stability and conformational changes of proteins during the simulation. It quantifies the overall structural similarity or dissimilarity over time. RMSF allows us to examine the flexibility and fluctuations of individual residues within the proteins. By analyzing RMSF, we gain insights into specific regions that experience significant conformational changes or exhibit higher mobility during the simulation. HBs play a crucial role in protein stability and function. Studying HB interactions during MD simulations helps us understand how these bonds contribute to maintaining the protein's structure and stability.
Analyzing these parameters obtained from the MD simulations deepens our understanding of the dynamic behavior and structural properties of both mutant and normal proteins.
3 Results
3.1 Clinical Finding
Among the twenty-five members of a large family with definite or suspected clinical diagnoses of RP, fourteen individuals (II4, III4, III6, III11, III15, III19, III25, III30, III31, III41, IV7, IV13, IV15, and IV21) underwent clinical examinations performed by geneticists and ophthalmologists. Within this specific subgroup, DNA samples from five individuals (III4, III15, III30, IV7, and IV21) diagnosed with RP were analyzed using WES. In the affected family members with RP, a variety of symptoms were observed, including tunnel vision, photophobia, color vision abnormalities, and visual disturbances. Additionally, they exhibited accompanying ocular conditions such as cataracts, strabismus, and myopia. Figure 2 provides fundus images from four different patients, illustrating the diverse range of retinal findings. These valuable observations are summarized in Table 1.
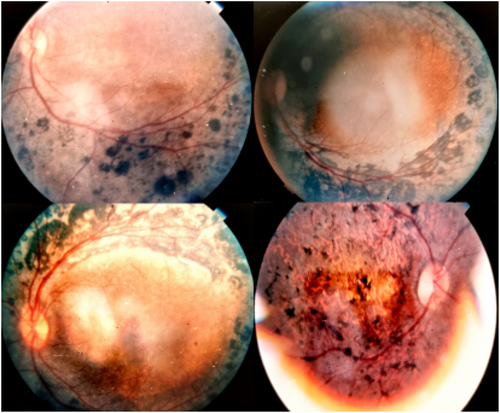
3.2 Genetic and Bioinformatics Findings
Using WES, we identified a pathogenic missense variant (c.932G>A, p.R311Q; rs28937873) in exon 6 of the NR2E3 gene (GenBank NM_014249.4). This variant leads to the substitution of arginine (codon: CGG) with glutamine (codon: CAG) at position 311 of the NR2E3 protein. The NR2E3 transcript consists of eight exons and is characterized by seven domains and features. Notably, this variant is located within the NR_LBD. Following the standards and guidelines of the American College of Medical Genetics and Genomics (ACMG), we rigorously assessed the pathogenicity of the identified variant. Among the sequencing results, only the NR2E3 gene was associated with RP, and no other genes related to eye disorders were identified. To understand the variant's frequency in various human populations, we consulted databases such as TOPMED, GnomAD, ALFA, 1000 Genomes (1000G), and ExAC. The observed frequencies were as follows: 0.000468, 0.000321, 0.000338, 0.0008, and 0.000342, respectively. Based on the information extracted from HGMD and ClinVar, this mutation is firmly established as a pathogenic variant. Direct sequencing confirmed the presence of the c.932G>A, p.R311Q variant in a homozygous state in all five patients included in the WES analysis, consistent with autosomal recessive inheritance (Figure 3). Taken together, these results strongly support the notion that the NR2E3 variant fully segregates with RP in this family, suggesting it is likely the causal mutation underlying their condition.
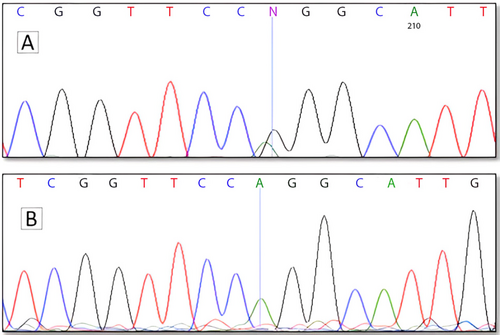
3.3 Molecular Docking Findings
To gain a more comprehensive understanding of the molecular mechanics and structural implications arising from the p.R311Q mutation in NR2E3, we utilized online I-TASSER and SWISS MODEL servers to generate models of the mutant protein. The computational models allow us to visualize how the mutation might affect the protein's three-dimensional structure. The quality of the modeled protein was rigorously assessed using multiple methods, including analysis of the Ramachandran plot, ERRAT, utilization of the MolProbity tools, and examination with Bioluminate software (Release 2023-1, Schrödinger, USA) based on the protein reliability report (Figure 4).

To examine how the mutation impacts the binding site of NR2E3, we conducted molecular docking analyses using BioLuminate (Release 2023-1, Schrödinger, USA). Our target was the structural complex formed by NR2E3-NR1D1. To accomplish this, we generated combined individual crystal structures of NR2E3 and NR1D1 to create potential models. These models represent the interaction between the two proteins. We then compared these models with the docking complex formed by the mutant NR2E3 and NR1D1. This allowed us to pinpoint any differences caused by the p.R311Q mutation. Figure 5 illustrates the docking of both the wild-type and mutant NR2E3 with NR1D1. Notably, the docking results revealed significant changes in the interaction between the mutated NR2E3 and NR1D1 when compared to the wild type. Furthermore, our structure refinement analysis indicated a substantial total energy change of 141.85 kcal/mol upon substituting the arginine residue with glutamine in the NR2E3 amino acid sequence (Table 2).

| Total energies | |
|---|---|
| Normal NR2E3 and NR1D1 complex | −25737.12 |
| Mutant NR2E3 and NR1D1 complex |
−25595.26 |
- Note: Total energy = COV_TOT + NB_TOT + OTHER—SGB_14|SGB_TOR. COV is covalent energies, NB is No bonded (intramolecular) energies, SGB stands for “Surface Generalized Born” model. The SGB model is a solvation model used to calculate the solvation energy of proteins, TOR is Torsional energy.
3.4 Molecular Dynamics Simulation Findings
Following our docking analysis results, we delved into the dynamic characteristics of both the normal and mutant complexes. To initiate the MD simulation, we selected the docking conformation with the most negative binding energy, as obtained from BioLuminate software. The RMSD plots (Figure 6) revealed that the normal complex achieved a stable RMSD value of approximately 0.6 nm after the initial 75 ns, indicating a conserved overall structure throughout the simulation. On the other hand, the mutant complex exhibited slightly increased fluctuations, with an RMSD reaching 0.7 nm. This implies a certain level of conformational flexibility in the mutant variant. To assess residue-specific fluctuations, we examined the RMSF profiles (Figure 6). In the normal complex, residues 50–130 and 125–250 exhibited increased RMSF values, indicating higher flexibility in these regions. Interestingly, in the mutant complex, residues 120–150 displayed even higher RMSF values, suggesting a potential impact of the mutation on local dynamics. Furthermore, we investigated the HB interactions in both complexes. This indicates that the mutation introduced in the protein did not have a substantial impact on the HB network within and between the complexes. The HB interactions remained relatively stable and consistent between the normal and mutant variants, suggesting that the mutation did not disrupt the crucial HB interactions involved in the complex formation. This finding implies that the mutation may not directly affect the binding affinity or stability of the complex through alterations in HB interactions.
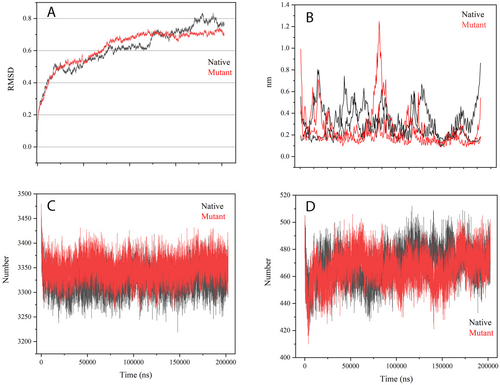
4 Discussion
RP is a hereditary disorder that profoundly affects the retina and often leads to vision loss and eventual blindness. The underlying cause lies in disruptions in rod differentiation. The process of rod differentiation is influenced by a number of critical elements, such as NR2E3, NR1D1, NRL, and CRX, which collaborate to establish intricate transcriptional regulatory complexes [19]. The combined action of NR2E3 and NR1D1 leads to an enhancement of rhodopsin promoter activity, and the intricate nature of these interactions contributes to the diverse range of observed phenotypes in both patients and animal disease models. Co-immunoprecipitation studies have demonstrated the existence of a multi-protein complex containing NR2E3, NR1D1, NRL, and CRX in the retinal nuclear extract of bovine, suggesting their involvement in regulating gene expression in vivo [20, 21].
Understanding the genetic and molecular mechanisms underlying RP is crucial for identifying its causes and potential therapeutic targets. In this study, we investigated the molecular and structural implications of the p.R311Q mutation within the NR2E3 gene. This mutation has been associated with disruptions in the regulatory network of transcription factors, including both NR2E3 and NR1D1. The identification of NR1D1 as an interacting protein with NR2E3 adds a novel perspective to the involvement of nuclear receptors in retinal development.
Despite its well-established role in the circadian rhythm and cellular metabolism, the function of the NR1D1 gene in the retina has remained elusive. To unravel its significance, extensive research has explored how the absence of NR1D1 impacts retinal development and how it interacts with the photoreceptor-specific nuclear receptor gene NR2E3 in both developing and mature retinas [22, 23]. Studies have uncovered a novel role for NR1D1, in collaboration with its cofactor NR2E3, in the precise regulation of transcriptional networks essential for photoreceptors development and proper functioning [24]. Therefore, to gain a comprehensive understanding of the interactions and relationships between NR2E3 and NR1D1, as well as their implications in retinal development and function, further molecular and in silico studies are warranted.
To initiate molecular studies involving protein–protein interactions and molecular dynamics simulations of the complex, it is crucial to establish a dependable model for the mutant NR2E3. This will facilitate a thorough investigation of the interactions and functional implications of association with the mutant NR2E3. Researchers can achieve this by utilizing a combination of tools, including the Ramachandran plot, ERRAT, MolProbity, and analysis with Schrodinger's Bioluminate software. These tools allow for systematic evaluation of various models to enabling research to select the most accurate and reliable structure for the modeled protein. Additionally, they provide valuable insights into the conformational quality, overall geometry, and stereochemical properties of the protein model, empowering researchers to make well-informed decisions regarding its suitability for subsequent molecular studies [25, 26].
Several in vivo studies have demonstrated the impact of NR2E3 LBD variants on the function of the CRX/NRL/NR2E3 complex [27]. The wild-type NR2E3 protein was found to enhance NRL/CRX-mediated transactivation of the rhodopsin promoter. In this context, various variations, including p.A256E, p.L336P, and p.L353V, were studied, and their effects were potentiated in the presence of p.R311Q and p.R385P variants. Consequently, it became imperative to investigate the effect of the p.R311Q variant on the interaction of the NR2E3 and NR1D1 protein complex [28]. This exploration will contribute to a more comprehensive understanding of the molecular mechanisms underlying retinal development and function.
The identified mutation has been discovered to affect the dynamic behavior and local dynamics of the NR2E3 and NR1D1 protein complex, while still maintaining crucial HB interactions. These observations make a substantial contribution to our comprehension of the structural and dynamic repercussions of the mutation and offer valuable insights for delving further into the functional implications of the complex. To affirm and broaden these findings, it is crucial to carry out additional comparative studies with other pertinent articles [29].
The validation of the protein–protein complex structures through RMSD, RMSF, and HB analysis is a crucial step to ensure the reliability and accuracy of our in silico interpretations [30]. However, it's important to note that computational needs to be complemented by biochemical and in vivo investigations. Therefore, further studies involving biochemical assays and in vivo experiments are necessary to validate the observed structural and dynamic consequences of the mutation. These experimental validations will provide essential insights into the underlying molecular mechanisms and help bridge the gap between in silico predictions and real-world observations. By gaining a deeper understanding of the NR2E3 and NR1D1 protein complex and its altered dynamics due to the mutation, we can pave the way for the development of new therapeutic strategies for more efficient treatment of RP. The insights obtained from this study will guide future experimental research aimed at identifying potential targets within the complex for therapeutic interventions. Ultimately, such investigations hold promise for the development of novel therapies that can effectively address the challenges associated with RP.
In conclusion, the process of rod differentiation involves the interplay of several key transcription factors, including NR2E3, NR1D1, NRL, and CRX. These factors come together to form multi-protein transcriptional regulatory complexes. Specifically, NR2E3 and NR1D1 synergistically enhance the activity of the rhodopsin promoter.
The identification of the p.R311Q mutation in the NR2E3 gene as a potential cause of RP underscores the importance of genetic testing and counseling, especially in families with a history of consanguineous marriage and retinal disorders. Computational techniques offer valuable insights into the structural and dynamic consequences of this mutation, deepening our understanding of the underlying mechanisms.
To validate and expand upon these findings, it is crucial to perform experimental validations through functional assays and in vivo studies. Additionally, non-invasive imaging techniques, such as fundus photography, play a significant role in the diagnosis, monitoring, and management of retinal disorders.
By integrating genetic analysis, computational modeling, and experimental investigations, a comprehensive approach can be developed. This integrated approach holds great potential for advancing targeted therapies and improving outcomes for individuals affected by RP. Continued research and collaboration across different disciplines will contribute to the development of effective treatments and interventions for this condition.
Author Contributions
Farzane Vafaeie: genetic analysis, figure preparation, molecular dynamics simulation, and manuscript writing. Mojtaba Mohammadpour, Malihe Nikandish: clinical examination, and data collection. Shokoofeh Etesam and Shahnaz Zarifi: genetic counseling, and data collection. Abolfazl Yari: in silico analysis and manuscript writing. Hassan Hashemzadeh: molecular dynamics simulation. Mohammad Reza Hajiabadi: manuscript writing. Ebrahim Miri-Moghaddam: was involved in study design, genetic counseling, and review of the final draft. All authors have seen and agree with the contents of the manuscript.
Acknowledgments
The authors thank the patient's family for their invaluable participation in this study and for providing consent to publish their data.
Ethics Statement
In adherence to the guidelines established in the Declaration of Helsinki, our investigation was conducted, and we acquired approval from the Ethics Committee of Birjand University of Medical Sciences (Ethics code: IR.BUMS.REC1400.4.9) prior to commencing the study.
Consent
Participation: To engage in the study, adult participants provided consent through the completion of consent documents. For participants under the age of 18, written informed consent was obtained from their parents, guardians, or next of kin.
Publication: For the publication of specific details regarding the medical care of patients and any accompanying images within this manuscript, written informed consent was obtained from the parents or legal guardians of the patients.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are accessible upon request from the corresponding author. Due to privacy or ethical restrictions, the data cannot be publicly disclosed.




