The expression and significance of p4E-BP1/4E-BP1 in prostate cancer
Funding information
This study was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LY20H050002 to Qi Ma), the Ningbo Science and Technology Planning Project (Grant No. 202002N3192 to Qi Ma), the Ningbo Natural Science Foundation (Grant No. 202003N4259 to Ke-jie Wang), and the Fund of Ningbo Clinical Research Center for Urological Disease (2019A21001)
Abstract
Background
Although the phosphorylation of 4E-BP1 that has been detected in high-grade prostate cancer has been reported in previous studies, overexpression of p4E-BP1 and 4EBP1 and their clinical significance in prostate cancer still remain unknown.
Methods
One hundred six samples of prostate tissues were collected and analyzed by immunohistochemistry with p4E-BP1 or 4E-BP1 specific antibodies. Everolimus was used to block the phosphorylation of p4E-BP1, and then flow cytometry, clone formation, transwell, and wound healing assays were performed to detect the survival and invasive ability of the prostate cancer cells.
Results
We found that the expression of 4E-BP1 and p4E-BP1 was higher in prostate cancer tissues than in normal tissues. Interestingly, the expression of p4E-BP1 was significantly associated with Gleason score and lymph node metastasis, but had no obvious correlation with PSA and the presence of bone or visceral metastasis. However, no evident correlation was found between the positive expression of 4E-BP1 and these clinical characteristics. In in vitro experiments, we found similar results as the clinical presentation that 4E-BP1 and p4E-BP1 were low expressed in normal prostate epithelial cells, but in prostate cancer cells, as the malignancy increasing, 4E-BP1 and p4E-BP1 expression also gradually increased. Then, we used Everolimus to inhibit the phosphorylation of 4E-BP1 and found that Everolimus effectively reduced cloning formation, inhibited cell migration, and promoted apoptosis in a dose-dependent manner in PC3 cells.
Conclusions
These findings suggest that p4E-BP1 is a potential biomarker and therapy target for prostate cancer, and patients with high expressions of p4E-BP1 may benefit from Everolimus treatment.
1 INTRODUCTION
Prostate cancer is the most common type of malignancy in men among developed countries,1 but in recent years, the incidence of prostate cancer has increased rapidly in China.2, 3 Currently, serum PSA testing is still the most widely used tool for prostate cancer diagnosis. However, an elevation in the level of serum PSA is not specific for prostate cancer, and the serum PSA level can be affected not only by iatrogenic treatments such as prostate biopsy and digital rectal examination but also by prostate volume, urinary tract infection, age and ejaculation, inserting the catheter, and other behaviors.4 Thus, there remains a need for a more sensitive and specific marker of prostate cancer.
Eukaryotic translation initiation factor 4E (eIF4E) is an important component of the transcription initiation complex, which can control mRNAs that generally encode proteins involved in cellular growth and survival.5 4E-BP1 is one of the inhibitory binding proteins, and it can combine with eIF4E to block its complex assembly and translation, which leads to inhibit the cell growth and survival.6 It has been reported that 4E-BP1 is a key regulatory element integrating the P3K/AKT and RAS/RAF/MEK/ERK pathways for oncogenic signaling, which can participate in tumor progress and metastasis.7 The phosphorylation of 4E-BP1 can block its binding to eIF4E, which leads to the release of eIF4E and promotes the translation of oncogenic mRNAs and eventually achieves tumorigenesis.8
Interestingly, it has been reported that the phosphorylation of 4E-BP1 resulting from the PI3K/AKT/mTOR pathway signaling occurs frequently in high-grade prostate cancers.9 Although the overexpression of p4E-BP1 in human prostate cancer tissue has been mentioned,10 the relationship between its expression and the clinical characteristics of prostate cancer patients is still unknown.
Herein, we detected the expression of 4E-BP1 and p4E-BP1 in samples of tissues collected from prostate cancer patients and investigated the correlation between the expression of these proteins and the clinical pathological characteristics of the patients with prostate cancer. Furthermore, we inhibited p4E-BP1 by the mTOR inhibitor, Everolimus, in prostate cancer cells and tested for the levels of apoptosis, tumorigenesis, and invasion and migration ability. Our study provides a further insight into the role of p4E-BP1 in prostate cancer, which might be developed as a new biomarker and therapeutic strategy to treat prostate cancer.
2 MATERIALS AND METHODS
2.1 Cell culture
Prostate cancer cells 22RV1, DU145, and PC3 and the normal prostate epithelial cells RWPE-1 in our study were obtained from the National Collection of Authenticated Cell Cultures, Shanghai, China. The cells were incubated in the corresponding medium supplemented with 10% heat-inactivated fetal bovine serum (PAN, Germany) in a humid environment containing 5% CO2 at 37℃.
2.2 Tissue samples
We have carried out immunohistochemical detection of the primary prostate tissue samples taken from 84 patients by surgery or biopsy between 2011 and 2017 at the Ningbo First Hospital (Zhejiang Province, China) with the approval of the institutional review board. Clinical data were obtained from the medical record system of the Ningbo First Hospital. Frozen prostate tissues were obtained from the Ningbo Pathology Center with the approval of the institutional review board.
2.3 Immunohistochemical staining and analyses
A total of 84 prostate cancer tissues and 22 precancerous tissues were obtained from the Ningbo Pathology Center of Zhejiang Province. Each case took 2–3 slides for experimentation. Slides were deparaffinized and hydrated at room temperature. Immunohistochemical staining was performed by following the manufacturer's directions of a specific kit (Absin, China). 4E-BP1 (dilution 1:100; Cell Signaling, US) and p4E-BP1 (dilution 1:100; Cell Signaling, US) were applied as the primary antibody at 37℃ for 12 h. For 4EBP1 or p4EBP1 scoring, the cytoplasmic staining intensity (4E-BP1 or p4E-BP1 typically stained the cytoplasmic of cancer cells) was rated as negative (no staining at all), weak (1+ staining intensity), moderate (2+ staining intensity), or strong (3+ staining intensity). In addition, it was stipulated that the product of the area percentage score of positive cells and the cell staining intensity score is regarded as a comprehensive staining score, with a score of ≥3 as positive, and a score of 0–2 as negative.
2.4 TCGA data analysis
Download the gene expression matrix of patients with prostate cancer in the TCGA database. The expression of 4EBP1 in prostate cancer was then evaluated by GraphPad Prism 8.0.
2.5 Western blot
Tumor tissues and cells were extracted with RIPA buffers containing 1% protease inhibitor mix (Solarbio, China) and 1% phosphatase inhibitor mix (Solarbio, China). The SDS-PAGE gel was used to isolate proteins, then the proteins were transferred to a PVDF membrane (Bio-RAD, USA), followed by blocking with 5% skim milk. Subsequently, the membranes were incubated with the corresponding primary antibodies for an overnight at 4℃. The primary antibodies 4E-BP1, p4E-BP1, and β-actin were all purchased from Cell Signaling Technology (USA). Finally, membranes were treated with HRP (horseradish peroxidase) labeled secondary antibody (Cell Signaling Technology, USA) at room temperature for 2–3 h and then detected by chemiluminescence.
2.6 Transwell assay
Cell transwell assays were performed using the 8 μM transwell chambers (Corning, USA) to evaluate the ability of cellular invasion. In brief, about 1 × 105 PC-3 cells in serum-free basal medium were seeded onto the upper compartment. A growth medium containing 10% FBS was filled into the lower chambers. After 48 h of incubation at 37℃, the noninvasive cells in the upper chamber were removed, and then, the invasive cells were fixed with 90% methanol and stained with 0.1% crystal violet (Sigma-Aldrich, Germany).
2.7 Wound healing assay
The ability of cellular migration was tested by performing the wound healing assay. Once the cells achieved 95% confluence in the 6-well plate after transfection, a 200 μl pipette tip was used in a lengthwise stripe was used to create a linear wound, and then the non-adherent cells were rinsed by PBS. In order to allow cells to move into the gap without being affected by cell growth, the medium containing 0.5% fetal bovine serum was added. Three different isometric points in the scratch region were photographed and imaged by a microscope (Olympus, Japan) at 0 h and 24 h, respectively. The migration rate was counted as the proportion of the initial scratch area in each sample, using the ratio of areas remaining cell-free between the two boundaries after cell migration.
2.8 Cell apoptosis assay
Annexin V-fluorescein/propidium iodide double-staining assay was performed to detect the cell apoptosis by using the corresponding kit (Lianke Biotechnology Corporate Limited, China). In short, the cells were harvested and resuspended in 500 μl 1 × binding buffer, and then they were stained with Annexin V-FITC and PI. After incubation in the dark at room temperature for 15 min, the number of apoptotic cells was detected by a FACScan flow cytometer (Becton Dickinson, USA). Data were analyzed with the FlowJo software (BD, USA).
2.9 Statistical analyses
All statistical analyses were done with the SPSS program. Tissues were divided into two groups: (a) prostate cancer, (b) paracancerous tissues. The difference of expression of 4E-BP1 and p4E-BP1 between these two groups was analyzed by using the T test. Correlation analysis between the expression of 4E-BP1/p4E-BP1 and the clinical and pathological data was performed using the χ2 test, Fisher's exact probability test, and the Spearman rank correlation analysis. The significant test level was calibrated to p < 0.05 (*p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001).
3 RESULTS
3.1 4E-BP1 is over-expression in prostate cancer tissues
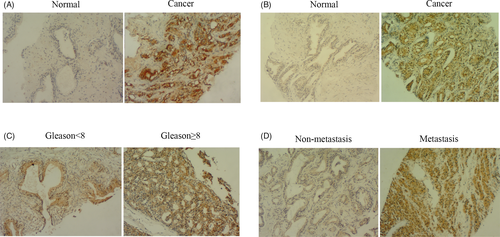
To explore the clinical significance of 4E-BP1 in prostate cancer, 106 pathological samples were detected using immunohistochemistry with a specific 4E-BP1 antibody. According to the staining results, the positive expression rate of 4E-BP1 in 84 prostate cancer samples reached 56.32%, while in 22 precancerous tissues, the positive rate was only 4.76% (p < 0.05, Table 1), which suggested that 4E-BP1 was expressed in higher levels in cancer tissues than in precancerous tissues (Figure 1A). However, there was no obvious correlation between the positive expression of 4E-BP1 and the Gleason score, PSA value, clinical stage, the presence of bone, lymph nodes, or visceral metastases in patients (p > 0.05, Table 2).
| Group | Cases | 4EBP1 | χ2 | p value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive rate | ||||
| PCa | 84 | 49 | 35 | 56.32% | 20.240 | 0.000* |
| Paracancer | 22 | 1 | 21 | 4.76% | ||
| Clinical data | Cases | 4EBP1 | χ2 or Fisher | p value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive rate | ||||
| Gleason | χ2 | |||||
| <8 | 35 | 21 | 14 | 60.00% | 0.200 | 0.412 |
| ≥8 | 49 | 27 | 22 | 55.10% | ||
| Pathology | ||||||
| Adenocarcinoma | 64 | 36 | 28 | 56.25% | 0.088 | 0.488 |
| Others | 20 | 12 | 8 | 60.00% | ||
| Lymph node metastasis | ||||||
| Yes | 30 | 18 | 12 | 60.00% | 0.156 | 0.436 |
| No | 54 | 30 | 24 | 55.56% | ||
| Bone or visceral metastasis | ||||||
| Yes | 40 | 23 | 17 | 57.50% | 0.004 | 0.950 |
| No | 44 | 25 | 19 | 56.82% | ||
| PSA | Fisher | |||||
| 0–10 | 9 | 5 | 4 | 55.56% | 0.630 | 0.470 |
| 10–50 | 36 | 22 | 14 | 61.11% | ||
| 50–100 | 14 | 8 | 6 | 57.14% | ||
| >100 | 25 | 13 | 12 | 52.00% | ||
| T staging | ||||||
| 2 | 55 | 31 | 24 | 56.36% | 2.163 | 0.378 |
| 3 | 19 | 13 | 6 | 68.42% | ||
| 4 | 10 | 4 | 6 | 40.00% | ||
3.2 p4E-BP1 is closely related to the malignant degree of prostate cancer
Although we have observed the abnormal expression of 4E-BP1 in prostate cancer tissues, we did not find its relationship with the clinical progression of the tumor. Therefore, we continued to explore the connection between p4E-BP1, the active form of 4E-BP1, and prostate cancer. Similar to the result of 4E-BP1, the positive expression rate of p4E-BP1 in prostate cancer tissue was 55.60%, while it was only 9.09% in precancerous tissues (Figure 1B) (p < 0.05, Table 3). Surprisingly, we found that the expression of p4E-BP1 was conspicuously related with the Gleason score (Figure 1C) and lymph node metastasis (Figure 1D) in patients with prostate cancer (Table 4), which suggested that p4EBP1 plays a key role in the progression of the cancer.
| Group | Cases | 4EBP1 | χ2 | p value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive rate | ||||
| PCa | 84 | 47 | 37 | 55.60% | 15.402 | 0.000* |
| Paracancer | 22 | 2 | 20 | 9.09% | ||
| Clinical data | Cases | 4EBP1 | χ2 or Fisher | p value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive rate | ||||
| Gleason | χ2 | |||||
| <8 | 35 | 15 | 20 | 42.86% | 4.185 | 0.041* |
| ≥8 | 49 | 32 | 17 | 65.31% | ||
| Pathology | ||||||
| Adenocarcinoma | 64 | 35 | 29 | 54.69% | 0.174 | 0.439 |
| Others | 20 | 12 | 8 | 60.00% | ||
| Lymph node metastasis | ||||||
| Yes | 30 | 12 | 18 | 40.00% | 4.819 | 0.028* |
| No | 54 | 35 | 19 | 64.81% | ||
| Bone or visceral metastasis | ||||||
| Yes | 40 | 20 | 20 | 50.00% | 1.098 | 0204 |
| No | 44 | 27 | 17 | 61.36% | ||
| PSA | Fisher | |||||
| 0–10 | 9 | 5 | 4 | 55.56% | 3.391 | 0.169 |
| 10–50 | 36 | 24 | 12 | 66.67% | ||
| 50–100 | 14 | 7 | 7 | 50.00% | ||
| >100 | 25 | 11 | 14 | 44.00% | ||
| T staging | ||||||
| 2 | 55 | 31 | 24 | 56.36% | 7.446 | 0.021* |
| 3 | 19 | 14 | 5 | 73.68% | ||
| 4 | 10 | 2 | 8 | 20.00% | ||
3.3 Inhibition the p4E-BP1 reduces cell cloning-formation, invasion, and migration and effectively promotes the apoptosis in PC3 cells
Using the TCGA and GTEx database search, we found that 4E-BP1 was highly expressed in prostate cancer tissues compared to normal prostate tissues (Figure 2A); however, we did not find the expression and corresponding clinical data analysis of p4E-BP1 in the database. As we found that p4E-BP1 was significantly associated with the Gleason score, lymph node metastasis, and clinical stage in patients with prostate cancer by immunochemistry, we further tested for the expression of 4E-BP1 and p4E-BP1 in various prostate cancer cell lines. We could see that the expression of 4E-BP1 and p4E-BP1 were lower in normal prostate epithelial cells (RWPE-1). While in prostate cancer cells, as the malignant degree of cancer cells increased (cell malignancy rank 2RV1 < DU145 < PC3), the expressions of 4E-BP1 and p4E-BP1 also gradually elevated (Figure 2B). As the PC3 cell line showed the highest expression of p4E-BP1, we chose the PC3 cells for the following experiments.
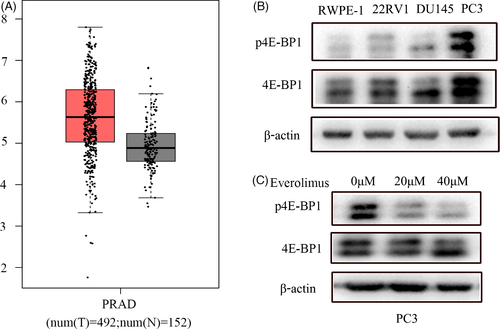
From the results of the clinical data analysis, we believe that 4E-BP1 played a relevant role in prostate cancer through its phosphorylated form (p4E-BP1). To verify this point, we treated PC3 cells with different concentrations of Everolimus, which is the inhibitor that can suppress the phosphorylation of mTOR, which is the upstream molecule of 4E-BP1, for 24 h. We found that Everolimus could indeed significantly inhibit the phosphorylation level of 4E-BP1 (Figure 2C).
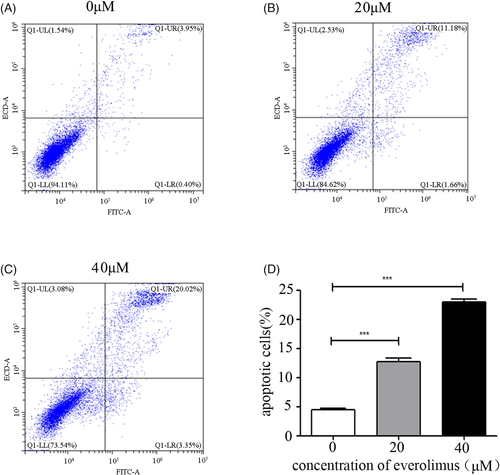
Next, we treated PC3 cells with different concentrations of Everolimus for 24 h and detected the apoptosis level by flow cytometry (Figure 3A–C) and found that inhibition of the phosphorylation level of 4E-BP1 could obviously increase the apoptosis of prostate cancer cells in a dose-dependent manner (Figure 3D).
Then, we estimated the clone-formation ability of prostate cancer cells after Everolimus treatment (Figure 4A), and found that when the phosphorylation level of 4E-BP1 was inhibited, the cloning ability of PC3 cells was significantly reduced.
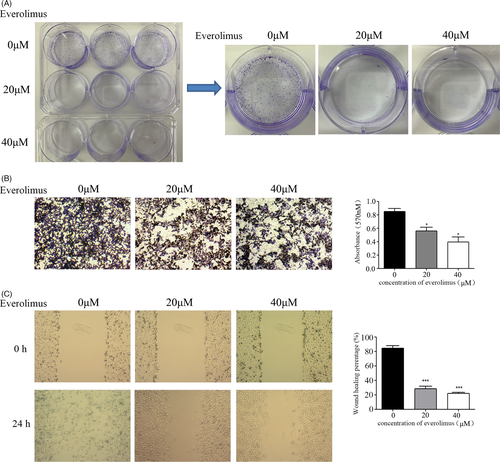
In addition, we observed that the expression of p4E-BP1 was related to tumor metastasis from the analysis of our clinicopathological data; therefore, we further tested the changes in the invasion and migration ability of prostate cancer cells after treatment with Everolimus (Figure 4B, C). The results showed that the invasion and migration ability of PC3 was significantly reduced after the inhibition of p4E-BP1.
4 DISCUSSION
According to the latest statistics from the American Cancer Society (ACS), prostate cancer is a malignant tumor with the highest incidence and is the second leading cause of high mortality rate among European and American men, especially in elderly men.1
Mammalian target of rapamycin (mTOR) is part of the PI3K/AKT/mTOR pathway, which plays a key intracellular regulatory effect in terms of cell growth, protein synthesis, cell cycle regulation, and cell movement.11-14 Previous literature believe that phosphorylation-activated mTOR further regulates the downstream signaling pathway of 4E-BP1 and plays a regulatory role in mRNA translation.8, 15, 16
In this study, we first measured the expression of 4E-BP1 in 106 prostate tissue samples.
and found that 4E-BP1 had higher expression levels in cancer tissues than in precancerous tissues (Figure 1A, Table 1). However, we did not observe any connection between its expression level and clinicopathologic data (Table 2). Therefore, we continued to explore the connection between p4E-BP1, the active form of 4E-BP1, and the clinical characteristics in prostate cancer patients. Similar to the result of 4E-BP1, the expression of p4E-BP1 proteins was significantly increased in prostate cancer tissues (Figure 1A, Table 3). However, we found that p4E-BP1 was closely related to the Gleason score (Figure 1C) and lymph node metastasis (Figure 1D) of prostate cancer (Table 4), which suggested that 4E-BP1 played an important role in prostate cancer in its phosphorylated form.
Next, we used TCGA database to analyze the expression of 4E-BP1 and P-4E-BP1 in prostate cancer. Unfortunately, we only found the data about 4E-BP1(Figure 2A), but not p4E-BP1. In order to further explore the role of p4E-BP1 in prostate cancer, we tested the expression of 4E-BP1 and p4E-BP1 in various prostate cancer cell lines and found that the expression of 4E-BP1 and p4E-BP1 were low in normal prostate epithelial cells (RWPE-1). While in prostate cancer cells, as the malignant degree of cancer cells increased (22RV1 < DU145 < PC3), the expressions of 4E-BP1 and p4E-BP1 also gradually elevated (Figure 2B). Furthermore, we inhibited the phosphorylation of 4E-BP1 by the mTOR inhibitor Everolimus in prostate cancer cell lines (Figure 2C) and observed that p4E-BP1 inhibition could inhibit the cell clonal formation, migration, invasion (Figure 4) and increase the apoptosis of PC3 cells (Figure 3).
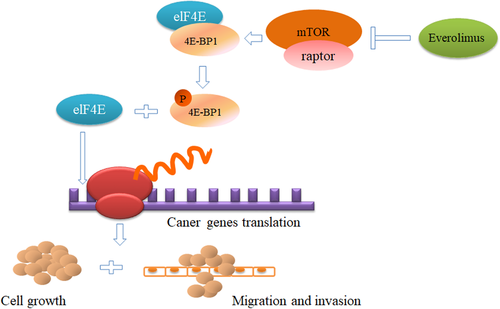
Collectively, we studied the expression of 4E-BP1 and p4E-BP1 at the tissue level and revealed the correlation between the expression and clinicopathological characteristics of patients with prostate cancer. At the in vitro level, we confirmed that inhibition of the phosphorylation of 4E-BP1could effectively suppress the tumorigenesis, migration and invasion capabilities of prostate cancer cells, and promote the cell apoptosis (Figure 5). Our results provide a further insight into the role of p4E-BP1 in prostate cancer, which might be developed as a new biomarker and therapy strategy to treat prostate cancer.
However, there are still some flaws in this study. First, the number of samples included in this study is relatively small (especially the samples with the PSA value in the gray zone), and the collection of clinical data is also limited; second, this study does not conduct more in-depth molecular mechanism research and explore how p4E-BP1 affects the development of prostate cancer by regulating gene expression.
In conclusion, we have confirmed the important role of phosphorylated 4E-BP1 in prostate cancer. Also, inhibition of p4E-BP1 could inhibit the tumorigenesis, migration, and invasion capabilities of prostate cancer cells. More basic and clinical studies should be performed to benefit prostate cancer patients.
CONFLICT OF INTEREST
The authors hereby declare no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding authors upon request.




