Sestrin2 and Beclin1 levels in Polycystic Ovary Syndrome
Funding information
This research was supported by grant NO 98-3-70-16167 from Iran University of Medical Sciences
Abstract
Background
Sestrin2 and beclin1 are two newly found proteins that have essential roles in autophagy. This study attempted to evaluate the plasma concentrations of sestrin2 and beclin1 in women with polycystic ovary syndrome (PCOS) and healthy controls and to explore the clinical value of these proteins as novel biomarkers for PCOS.
Methods
In this case-control study, plasma levels of sestrin2 and beclin1, fasting blood sugar (FBS), lipid profile, insulin, and androgens were evaluated in 63 women (31 patients and 32 controls). Sestrin2 and beclin1 levels were determined using enzyme-linked immunosorbent assay (ELISA). Descriptive statistics, correlation coefficients, logistic regression, and ROC curve analyses were used in this study.
Results
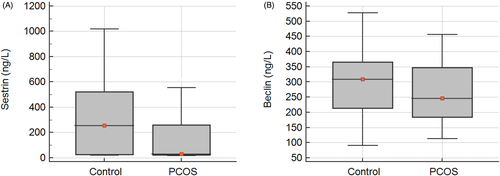
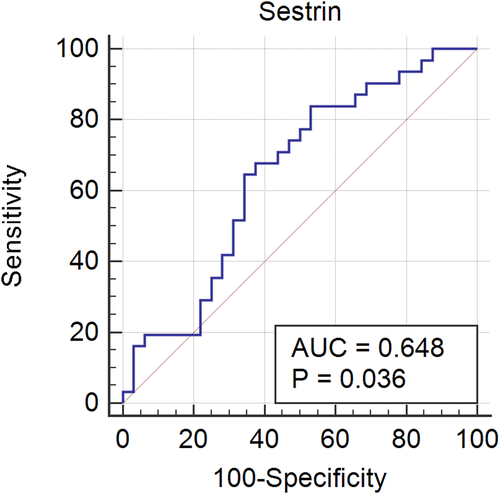
Plasma sestrin2 levels of the subjects with PCOS (40.74 [24.39–257.70]) were significantly lower than those of healthy subjects (255.78 [25.46–528.66]; p-value = 0.040). ROC curve analysis showed that a cutoff value of 420.5 ng/L had an appropriate sensitivity (83.87%) and specificity (46.88%) for discriminating individuals with and without PCOS, with the area under the curve (95% CI) of 0.648 (0.518 to 0.764), p = 0.036. There were no statistically significant differences between the two groups concerning plasma levels of beclin1, biochemical parameters, blood pressure, and anthropometric features.
Conclusion
Our findings highlight the dysregulation of sestrin2 as a marker of autophagy in PCOS and its potential usefulness as a novel biomarker for PCOS. Further research is needed to better understand the role of this protein in the pathophysiology of PCOS and its value as a diagnostic tool for the evaluation of PCOS patients.
1 INTRODUCTION
Polycystic ovary syndrome (PCOS) is a heterogenous, prevalent endocrinopathy affecting nearly 6%–10% of women of childbearing age.1 PCOS is associated with significant metabolic and reproductive disturbances including oligo/anovulatory cycles, infertility, obesity, insulin resistance, dyslipidemia, and type 2 diabetes.2 Although PCOS has significant and diverse clinical implications, the exact pathophysiological mechanism leading to this syndrome is still unclear.3 Although some researchers have described it as mainly an intrinsic ovarian disorder, this disorder is now more precisely referred to as an adrenal abnormality, resulting from hypothalamic-pituitary dysfunction.4 Moreover, the theory of insulin resistance in the pathogenesis of PCOS has recently gained much attention.5 Insulin resistance is seen in 50%–80% of patients with PCOS, and it appears to be independent of obesity.6 Alterations of diverse cytokines such as neuregulin-4 and omentin-1 have been reported to be associated with insulin resistance and chronic inflammation in PCOS.7, 8 Aberrant levels of the aforementioned cytokines have been demonstrated in other metabolic and inflammatory disorders like type 2 diabetes,9 diabetic microvascular complications,10 and insulin resistance in pregnancy;11 appendicitis;12 chronic pancreatitis;13 and chronic kidney disease.14
Over the past few years, it has been proposed that dysregulation of autophagy may underlie the impaired insulin sensitivity in metabolic disorders such as type 2 diabetes, obesity, and nonalcoholic steatohepatitis.15 Autophagy, a stress-induced lysosome-mediated degradation process, has a key function in the breakdown of potentially toxic cytosolic proteins and damaged organelles for the production of cellular energy.16 Interestingly, an increasing number of studies have found that autophagy also plays a vital part in regulating the endometrial and ovarian functions. Dysregulation of autophagy has been observed in the endometrium and ovarian granulosa cells of women with PCOS.17, 18
The complex process of autophagy is regulated by several proteins including sestrin2.19, 20 It has been suggested that sestrin2, a highly evolutionarily conserved protein, attenuates insulin resistance through the regulation of glucose and lipid homeostasis.21 At the least, two mechanisms have been identified to contribute to the protective function of sestrin2. First, sestrin2 acts as an antioxidant to eliminate excessive amounts of reactive oxygen species; second, p53-induced sestrin2 activates AMP-activated protein kinase (AMPK), causing inhibition of the mammalian target of rapamycin complex 1 (mTORC1) and subsequently autophagy induction under stressful conditions.22 Previous studies have reported that systemic deficiency of sestrin2 results in insulin resistance and abnormal glucose and lipid metabolism. This may be due to downregulation of AMPK and overactivation of mTORC1.21, 23 Sestrin2 has also been reported to be reduced in patients with obesity at an early age.24 Although a considerable amount of the literature has been published on the altered expression of sestrin2 in various human diseases, including cardiovascular, lung, liver, kidney, neurological, and immunological diseases,25-28 no previous study has investigated the circulating level of sestrin2 and its clinical implications in patients with PCOS.
Beclin1 is another key regulator of autophagy. Beclin1 binds to the class III phosphatidylinositol-3 kinase (PI3KC3)/vacuolar protein sorting 34 (Vps34), enabling the recruitment of the other autophagy proteins involved in the nucleation of autophagosome.29 Altered levels of beclin1 protein expression have been proposed to have a crucial role in the pathogenesis of various diseases, including malignancies, neurological disorders, and metabolic disorders.30, 31 Nevertheless, little is known about the clinical value of plasma levels of beclin1 protein in patients with PCOS.32 Therefore, to find a relationship between autophagy and PCOS and also to clarify the value of plasma levels of sestrin2 and beclin1 as biomarkers or therapeutic targets for this syndrome, we evaluated the plasma sestrin2 and beclin1 levels in patients with PCOS and healthy controls.
2 MATERIALS AND METHODS
2.1 Study design
This case-control study was carried out at the Endocrinology Outpatient Clinic of H. Aliasghar Hospital, an affiliate of Iran University of Medical Sciences, Tehran, Iran, from November 2019 to August 2020.
2.2 Study participants
Patients with PCOS were diagnosed according to the Androgen Excess and PCOS Society (AE-PCOS) criteria, that is, (a) biochemical or clinical evidence of androgen excess; (b) evidence of ovarian dysfunction (oligo-ovulation and/or polycystic ovaries); and (c) exclusion of relevant etiologies.32 Data from the history, physical examination, laboratory tests, and the results of ultrasonography were used to confirm the diagnosis of PCOS.
The exclusion criteria were (a) the use of hormonal or insulin-modifying therapy such as drugs containing steroids, anti-androgens, hormonal contraceptives, aspirin, statins, or any other medications that could interfere with androgen or insulin levels, up to three months prior to the study; (b) other causes of hyperandrogenism (eg, androgen-secreting tumors and non-classic congenital adrenal hyperplasia); and (c) other underlying disorders such as cardiovascular diseases, neurodegenerative disorders, and malignancies. The control group consisted of healthy women with regular menses and normal ovarian morphology identified by ultrasound.
2.3 Measurements
Weight and height were measured for all participants, and body mass index (BMI) was calculated as weight/height² (kg/m²). Systolic (SBP) and diastolic (DBP) blood pressures were examined on the right arm after five minutes of rest.
Venous blood collection was performed early in the morning after an overnight fasting of about 12 h. For plasma separation, blood samples were immediately kept on ice and separated in a refrigerated centrifuge to prevent any loss of analytes. All plasma samples were stored at −80°C. Fasting blood sugar (FBS) and lipid profile including triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL-C) were measured using colorimetric kits (Pars Azmoon). Plasma levels of testosterone, and dehydroepiandrosterone (DHEA) were measured using an electrochemiluminescence (ECL) kit (Abbott, Ireland). Measurements of androstenedione and dihydrotestosterone (DHT) levels were performed based on an enzyme-linked immunosorbent assay (ELISA) method (LDN, Germany). Insulin levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Monobind, USA) with intra- and inter-assay coefficients of variation (CV) of 8.0% and 6.8%, respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by the formula FPG (mg/dl) × fasting insulin (µU/ml)/405 and was used as an index of insulin resistance. Plasma sestrin2 and beclin1 levels were determined by using ELISA kits (Crystal Day, China). The intra- and inter-assay CV of the ELISA kits were <8% and 10%, respectively.
2.4 Ethics statement
Ethical approval was obtained based on the principles of the World Medical Association Declaration of Helsinki. This study was approved by the Institutional Review Board of Iran University of Medical Sciences (Ref No: IR.IUMS.FMD.REC.1398.1386). All patients signed informed consent statements prior to the enrollment.
2.5 Statistical analysis
SPSS software version 25.0 (SPSS, Inc.) was used for statistical analyses. The normal distribution of the variables was examined using the Kolmogorov-Smirnov test. The data with normal distribution were expressed as mean ± SD, and those without normal distribution were presented as median (interquartile range). The Mann–Whitney U test (nonparametric) and Student's t test (parametric) were used to analyze the differences between the two groups. The correlations between biochemical and anthropometric parameters were measured by Spearman's and Pearson's correlation tests for nonparametric and parametric variables, respectively. Logistic regression was used to identify whether sestrin2 and beclin1 were potentially relevant predictors for PCOS. Receiver operating characteristic (ROC) curve analysis was carried out, and the area under the curve was calculated to appraise discrimination between those with and without PCOS. Differences were considered statistically significant if p < 0.05.
3 RESULTS
Thirty-one female patients with PCOS and 32 healthy controls were enrolled in the study. The anthropometric and biochemical characteristics of the participants are presented in Table 1. There were no significant differences in height, weight, BMI, SBP, and DBP between the two studied groups. Furthermore, there were no significant differences in FPG, TG, total cholesterol, HDL-C, and LDL-C. Insulin levels were slightly higher in patients with PCOS, but the differences were not significant. However, HOMA-IR levels were significantly higher in patients than in healthy subjects, indicating increased insulin resistance in the patients with PCOS. Taking into account the recommended optimal threshold of HOMA-IR for diagnosis of insulin resistance in Iranian population of 1.8,33 80% of patients were classified as being insulin-resistant. Both sestrin2 and beclin1 levels were compared between the subjects with or without insulin resistance, and no significant values were obtained (p = 0.663 for beclin1 and p = 0.971 for sestrin2).
| Characteristic | PCOS group (n = 31) | Control group (n = 32) | p-value |
|---|---|---|---|
| Age | 25.54 ± 7.72 | 32.58 ± 7.42 | 0.001 |
| Height (cm) | 160.79 ± 5.01 | 161.09 ± 6.29 | 0.83 |
| Weight (kg) | 63.75 ± 8.17 | 62.52 ± 8.80 | 0.57 |
| BMI (kg/m2) | 24.71 ± 2.66 | 23.98 ± 2.87 | 0.31 |
| SBP (mmHg) | 111.42 ± 8.90 | 109.69 ± 9.75 | 0.47 |
| DBP (mmHg) | 75.53 ± 6.28 | 71.51 ± 8.96 | 0.51 |
| FPG (mg/dl) | 90.76 ± 8.29 | 90.42 ± 7.56 | 0.87 |
| TC (mg/dl) | 163.11 ± 31.71 | 154.94 ± 28.08 | 0.34 |
| TG (mg/dl) | 87.39 ± 56.17 | 70.21 ± 24.89 | 0.23 |
| HDL-C (mg/dl) | 50.59 ± 12.36 | 51.06 ± 12.72 | 0.90 |
| LDL-C (mg/dl) | 93.77 ± 25.97 | 89.84 ± 26.59 | 0.62 |
| Insulin (µIU/ml) | 11.8 (7.8–21.4) | 10.3 (7.2–12.4) | 0.50 |
| HOMA-IR | 3.06 (2.0–4.7) | 2.36 (1.5–2.9) | 0.033 |
| Testosterone (ng/ml) | 0.56 ± 0.56 | 0.26 ± 0.09 | 0.004 |
| DHEA (µmol/L) | 235.41 ± 88.76 | 211.30 ± 113.40 | 0.37 |
| Androstenedione (ng/ml) | 3.24 ± 1.86 | 2.41 ± 1.01 | 0.01 |
| DHT (pg/ml) | 412.50 ± 206.77 | 286.55 ± 122.78 | 0.005 |
| Beclin1 (ng/L) | 258.66 ± 102.7 | 296.46 ± 107.4 | 0.170 |
| Sestrin2 (ng/L) | 40.74 (24.4–257.7) | 255.78 (25.5–528.7) | 0.042 |
- The data are presented as Mean ± SD for parametric and median (interquartile range) for non-parametric variables.
- Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone; FPG, fasting plasma glucose; HDL-C, high density lipoprotein cholesterol; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
Plasma levels of sestrin2 in patients with PCOS were significantly lower than those in the control subjects (U = 311, p = 0.042; Table 1 & Figure 1A). No significant differences were observed between the study groups with regard to plasma beclin1 levels, t (58) = 1.38, p = 0.170 (Table 1 & Figure 1B).
Direct logistic regression was employed to evaluate the effect of sestrin2 and beclin1 as the independent variables on the likelihood of PCOS. The model correctly classified 69.8% of cases. As shown in Table 2, sestrin2 made a statistically significant contribution to the model, with an odds ratio of 0.995, indicating that higher sestrin2 levels were associated with less likelihood of PCOS.
| B | SE | Wald | df | p | Odds Ratio | 95% CI for Odds Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Sestrin2 | −0.005 | 0.002 | 5.79 | 1 | 0.016 | 0.995 | 0.991 | 0.999 |
| Beclin1 | 0.006 | 0.004 | 2.07 | 1 | 0.150 | 1.006 | 0.998 | 1.015 |
The correlations between sestrin2, beclin1, and metabolic parameters are shown in Table 3. The results showed that there were no significant correlations between plasma levels of these proteins and indices of metabolic health, including lipid and glycemic indices, which signifies an independent relationship between sestrin2 and PCOS. Interestingly, a significant positive relationship was found between beclin1 and sestrin2 (r = 0.810, p < 0.001), highlighting their related biological role.
| Variable | Sestrin2 | Beclin1 | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| BMI | −0.001 | 0.99 | 0.37 | 0.77 |
| SBP (mg/dl) | 0.14 | 0.25 | 0.08 | 0.50 |
| DBP (mmHg) | 0.09 | 0.45 | 0.01 | 0.90 |
| FPG (mg/dl) | 0.01 | 0.93 | 0.08 | 0.50 |
| TC (mg/dl) | 0.09 | 0.51 | −0.004 | 0.98 |
| TG (mg/dl) | −0.02 | 0.86 | 0.17 | 0.22 |
| HDL-C (mg/dl) | 0.20 | 0.15 | 0.17 | 0.23 |
| LDL-C (mg/dl) | 0.10 | 0.48 | −0.004 | 0.97 |
| Insulin (µIU/ml) | 0.08 | 0.57 | 0.07 | 0.62 |
| HOMA-IR | 0.148 | 0.273 | 0.184 | 0.171 |
- Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
The correlations of beclin1 and sestrrin2 with androgens were also evaluated in this study. As it is presented in Table 4, apart from a single significant correlation between sestrin2 and testosterone levels, other androgens did not show any significant correlation with either beclin1 or sestrin2.
| Variable | Sestrin2 | Beclin1 | ||
|---|---|---|---|---|
| r | p-value | R | p-value | |
| Testosterone (ng/ml) | −0.280 | 0.032 | −0.222 | 0.091 |
| DHEA (µmol/L) | −0.143 | 0.279 | −0.053 | 0.688 |
| Androstenedione (ng/ml) | −0.144 | 0.262 | 0.061 | 0.637 |
| DHT (pg/ml) | −0.181 | 0.166 | −0.106 | 0.419 |
- Abbreviations: DHEA, dehydroepiandrosterone; DHT, dihydrotestosterone.
According to the significant difference of sestrin2 levels in subjects with PCOS, ROC curve analysis was used for the assessment of its value as a marker for discriminating patients with PCOS. ROC curve analysis showed that a cutoff value of 420.5 ng/L had an appropriate sensitivity (83.87%) and specificity (46.88%) for discriminating patients with PCOS from healthy controls, with the area under curve (95% confidence interval) of 0.648 (0.518 to 0.764), p = 0.036 (Figure 2).
4 DISCUSSION
Autophagy has recently been recognized as a contributing factor in the pathogenesis of several disorders such as obesity, type 2 diabetes, and cardiovascular disorders. The up- and downregulations of autophagy-related genes have been reported in PCOS.30 In this study, we evaluated the plasma levels of sestrin2 and beclin1, as two autophagy-related factors, in patients with PCOS and healthy subjects. The results indicated that plasma levels of sestrin2 in patients with PCOS were significantly lower than those of the healthy controls.
Sestrin2 plays an important role against oxidative stress by diverse mechanism including the stimulation of nuclear factor E2-related factor 2 (Nrf2) antioxidant pathway.25 There are numerous studies that report oxidative stress as the central player in the pathophysiology of PCOS.25, 34 Therefore, reduced levels of sestrin2 might be considered among the factors that increase the susceptibility of patients with PCOS against detrimental stimuli, especially oxidative stress. Additionally, sestrin2 plays a critical role in metabolic homeostasis via activation of the key energy sensor AMPK and inhibition of mTORC1.35 mTORC1 is one of the main signaling nodes linking metabolic and environmental signals to the control of cell growth and metabolism.36 The experimental data about the relationship between PCOS and mTORC1 signaling are rather controversial.37, 38 There is, however, general agreement that mTORC1 plays an important role in ovarian follicular growth and development. Dysregulation of mTORC1 signaling can result in abnormal folliculogenesis.36 Another possible link between PCOS and mTORC1 signaling is the metabolic derangements during PCOS.39 It seems possible that the insufficient level of sestrin2 leads to dysregulated mTORC1 signaling, which finally results in diverse reproductive and metabolic pathologies associated with PCOS.
A number of previous studies have examined circulating concentrations of sestrin2 in several human diseases,40, 41 but to date, none has evaluated the plasma levels of this protein in patients with PCOS. Lee et al. found that sestrin2 deficiency enhances insulin resistance, glucose intolerance, oxidative stress, and progression of type 2 diabetes in obese mice kept on high-fat diet. However, in their study, setrin2-deficient mice did not differ in weight gain from control mice.42 In 2017, Nourbakhsh et al.24 showed that the concentration of sestrin2 was significantly lower in obese children than in normal-weight children. They reported that sestrin2 levels were negatively correlated with BMI, and positively correlated with HDL-C. Similarly, a recent study by Mohany and Al Rugaie found significantly low levels of serum sestrin2 in patients with type 2 diabetes mellitus compared with the healthy controls. Their results showed significant negative correlations between serum sestrin2 levels and BMI, glycosylated hemoglobin%, and serum glucose.43
On the other hand, in the study by El-Ashmawy et al.,44 serum sestrin2 levels indicated significant positive correlations with HOMA-IR, TG, total cholesterol, LDL-C, and SBP in patients with type 2 diabetes. Likewise, in the study by Chung et al., serum sestrin2 concentration showed an increasing trend in individuals with metabolic syndrome. Their results showed that serum sestrin2 concentrations have significant positive correlations with BMI and HOMA-IR in patients with type 2 diabetes. Meanwhile, they found no significant correlations between serum sestrin2 levels and BMI as well as SBP, DBP, total cholesterol, LDL-C, HDL-C, FBS, and HbA1c in individuals with normal glucose tolerance.45 A possible explanation for this paradoxical increase in serum sestrin2 in subjects with metabolic syndrome might be a compensatory mechanism to overcome the metabolic stress or resistance to sestrin2.41, 45 In the current study, we did not find any significant correlation between sestrin2 and anthropometric characteristics including BMI, indicating that the reduced level of sestrin2 may not be a consequence of obesity. Additionally, in the present study, none of the investigated metabolic parameters were correlated with sestrin2, suggesting that the attenuation of sestrin2 in PCOS does not occur exclusively as a consequence to metabolic derangements and different mechanisms can affect the expression of the sestrin2 gene.
As mentioned before, we found that plasma levels of sestrin2 in patients with PCOS were significantly lower than those in the healthy controls. Interestingly, this finding contradicts some of the previous research on cardiovascular and pulmonary diseases, cancer, and neurodegenerative disorders which found an increase in the circulating sesrin2 levels in the patients.40, 41 It has been suggested that induction of sestrin2 in these diseases protects against damage associated with different stress conditions.41 For example, plasma sestrin2 levels were shown to be significantly increased in patients with coronary artery disease (CAD) and correlated with the severity of stenosis. This effect was suggested to be a protective response against the progression of CAD.46 Additionally, it has recently been proven that high plasma concentrations of sestrin2 in patients with chronic heart failure significantly increase the occurrence of major adverse cardiac events and predict poor outcome.47 Levels of plasma sestrin2 have also been reported to be considerably high in patients with obstructive sleep apnea,48 as well as those with asthma,49 which again may be a compensatory mechanism to combat chronic hypoxia.48, 49 Elevated plasma levels of sestrin2 in different malignancies such as lung and colorectal cancers suggest a tumor-suppressive role for this protein.50, 51 Moreover, significantly increased serum levels of sestrin2 have been demonstrated in Parkinson's disease and Alzheimer's disease, with a significant negative correlation with the mental and cognitive state.41, 52, 53 Considering the role of oxidative stress and the accumulation of aberrantly processed or misfolded proteins in the pathogenesis of neurodegenerative disorders, the protective enhancement of sestrin2 is a plausible mechanism due to its role in antioxidative defense and promotion of autophagy.41, 54 The reason for the difference between the findings of this research and previous studies is not clear, but it may have something to do with the dynamic expression and compensatory function of sesrin2 in different pathological conditions. Several potential upstream regulators and downstream pathways of sesrin2 can lead to variation in its plasma concentrations.25
Another important finding of this research was the significant negative correlation of sestrin2 with testosterone. A plausible mechanism for this finding is Nrf2 that might serve as a link between testosterone and sestrin2. Testosterone has been shown to be able to downregulate Nrf2, which is a redox-sensitive transcription factor that activates the expression of many key antioxidant genes, including sestrin2.55
Beclin1 was also assessed in the patients with PCOS. No statistically significant differences were found between study groups regarding plasma beclin1 levels. We also investigated whether there were any correlations between the plasma levels of beclin1 and biochemical parameters of patients with PCOS. No significant correlations were found between plasma levels of these proteins and other clinical variables of the study participants.
Studies on the beclin1 protein levels in patients with PCOS are relatively sparse and offer contradictory findings. In 2018, Li et al. noted that autophagy was enhanced in the ovarian tissue of patients with PCOS, and there was a small increase in the beclin1 gene expression.56 Similarly, in an investigation into protective effects of melatonin against mitochondrial injury in the granulosa cells of patients with PCOS, Yi et al.30 found that the increased expression level of beclin1 is linked to PCOS pathogenesis. On the other hand, Sumarac et al.18 reported that endometrial mRNA expression of beclin 1 is significantly reduced in patients with anovulatory PCOS compared with the healthy endometrium. In the present study, we did not observe any significant differences in the circulating concentrations of beclin1 between patients and healthy controls, which indicates that in contrast to tissue expression of beclin1, its circulating levels are not different from healthy controls.
The current investigation was limited by its small sample size; however, our careful selection of cases makes the results reliable in their own context. The results of this study might pave the way for further research on diagnostic and therapeutic potentials of sestrin2 in PCOS.
5 CONCLUSION
Overall, our findings suggest that plasma sestrin2 protein is differently expressed in PCOS patients and healthy women. Therefore, sestrin2 can be considered as a novel diagnostic biomarker for PCOS and a suitable therapeutic target for the management of this disorder. Further research is required to determine the exact role of this protein in the pathophysiology of PCOS.
ACKNOWLEDGMENTS
The authors would like to thank all the participants of this study.
CONFLICT OF INTERESTS
No competing financial interests exist.
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.




