Reference interval and the role of plasma oligomeric beta amyloid in screening of risk groups for cognitive dysfunction at health checkups
Funding information
This research received no specific grant from any funding agency in the public or commercial sectors
Abstract
Background
Alzheimer's disease (AD) has a prolonged preclinical stage characterized by cognitive dysfunction. Simple, reliable, and noninvasive biomarkers reflecting the pathogenesis of AD are needed for screening cognitive dysfunction in primary health care. The aims of this study were to determine (1) the potential utility of the Multimer Detection System-Oligomeric Amyloid-β (MDS-OAβ) value in cognitive assessments and (2) the reference interval (RI) of plasma MDS-OAβ values in the general population.
Methods
This prospective study consecutively recruited 1,594 participants who underwent health checkups including cognitive function examination at 16 health-promotion centers in Korea between December 2020 and January 2021. The inBloodTM OAβ test (PeopleBio, Gyeonggi-do, Republic of Korea) was utilized to quantify MDS-OAβ values in plasma. The reference subjects were obtained among those with normal general cognition on cognitive screening tools. RIs were established according to the CLSI C28-A3 guidelines.
Results
The median MDS-OAβ value was higher in subjects with Korean Dementia Screening Questionnaire-Cognition (KDSQ-C) scores ≥8 than in those with KDSQ-C scores of 6–7 (P = 0.013). The median MDS-OAβ value was higher in subjects with Mini-Mental State Examination for Dementia Screening (MMSE-DS) scores of 21–26 than in those with MMSE-DS scores ≥27 (P = 0.011). The RI (one-side upper 95th percentile) of the MDS-OAβ value was 0.80 ng/mL (95% confidence interval = 0.78–0.82) in those aged ≥50 years.
Conclusions
The plasma MDS-OAβ value reflects cognitive function as assessed using the KDSQ-C and MMSE-DS. RIs obtained from a large and cognitively healthy community-based sample are presented.
1 INTRODUCTION
The rapid increase in the aging population is resulting in the prevalence of Alzheimer's disease (AD), one of the most common types of dementia, also increasing in Korea.1 As AD has a prolonged preclinical stage characterized by cognitive dysfunction,2 periodic screening for cognitive dysfunction is needed before performing clinical diagnosis in the community. Several screening tools for cognitive dysfunction have been applied in primary health care in the community. The current screening tools for cognitive dysfunction are questionnaires designed to be completed by the participant or an informant. Although cognitive screening using questionnaires for evaluating dementia is a fundamental healthcare procedure for evaluating cognitive function, this approach has limitations such as being subjective and yielding different outcomes when completed by participants and their informants.3 Therefore, simple, reliable, and noninvasive biomarkers that reflect the pathogenesis of AD are needed for screening cognitive dysfunction in primary health care.
Traditionally validated biomarkers for AD are cerebrospinal fluid (CSF) Aβ42 and amyloid positron emission tomography (PET).4 However, these approaches not only have their own limitations, such as the risk of CSF sampling, high cost, and unavailability in primary healthcare settings, but also are not well accepted by patients. There have been some researches into developing blood-based biomarkers for AD.5-7 Beta amyloid (Aβ) is derived from beta-amyloid precursor protein (APP), which is cleaved by beta- and gamma-secretase. APP mutations result in the increased production of all forms of Aβ, which self-aggregate into oligomeric forms, and promote plaque formation. Oligomerized Aβ (OAβ) is the most toxic8, 9 and is strongly associated with the pathogenesis of AD.10, 11 The Multimer Detection System-Oligomeric Aβ (MDS-OAβ) was introduced to measure the oligomerization dynamics of Aβ in a plasma sample. This technique measures the level of OAβ after spiking plasma samples with purified synthetic Aβ.12 A validation study showed that MDS-OAβ to measure plasma Aβ oligomerization was a valuable blood-based biomarker for clinical diagnosis in differentiating AD from normal cognition.13 However, there have been few studies of the potential utilization of MDS-OAβ in screening people with cognitive dysfunction in primary healthcare settings.
The aims of this study were to determine (1) the potential utility of MDS-OAβ in cognitive assessments through screening the plasma MDS-OAβ value at health checkups and (2) the reference interval (RI) of plasma MDS-OAβ values in the general population.
2 MATERIALS AND METHODS
2.1 Study subjects
This prospective study consecutively recruited 1,594 participants who underwent health checkups including cognitive function examinations and agreed to undergo additional blood sampling for MDS-OAβ at 16 health-promotion centers in Korea between December 2020 and January 2021. Individuals younger than 50 years or with a history of head injury, stroke, brain tumor, or depression were excluded. The RI for plasma MDS-OAβ values was calculated for the reference subjects, who were defined as follows: (1) Korean Dementia Screening Questionnaire-Cognition (KDSQ-C) score <6, (2) Mini-Mental State Examination-Dementia Screening (MMSE-DS) score >26, and (3) the absence of depression (Korean version of Patient Health Questionnaire-9 [PHQ-9] score <10 (Figure 1).
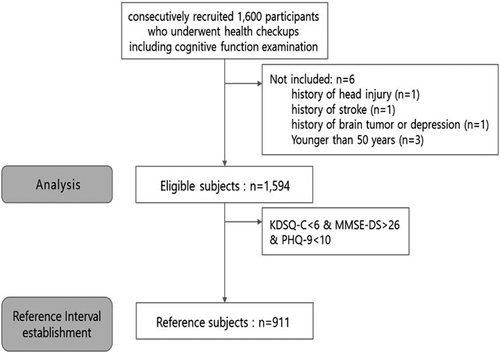
This study was approved by the Institutional Review Board of the Korea Association of Health Promotion (approval no. 130750–202009-HR-014).
2.2 Clinical assessment
All subjects were evaluated for their medical history and underwent a physical examination and neuropsychological batteries comprising the KDSQ-C and MMSE-DS. The KDSQ-C is a semi-structured questionnaire that includes 15 questions assessing memory impairment, other cognitive impairments including language impairments, and the ability to perform complex tasks in daily life. The KDSQ-C has answer options of “no,” “sometimes,” and “often,” which are scored as 0, 1, and 2, respectively, with a higher score indicating worse function or a higher frequency. A KDSQ-C score of 6 is considered a valid threshold for identifying dementia.14
The MMSE is a widely used instrument that estimates global cognitive function and screens for dementia. The MMSE-DS is a Korean version of the MMSE that contains 19 items and has a maximum score of 30 points (10 for orientation, 6 for verbal memory, 5 for concentration and calculation, 5 for language, 3 for praxis, and 1 for visuospatial construction), with a lower score indicating worse cognitive function.15 Depression was assessed using PHQ-9.16
2.3 Measurement of oligomerization of Aβ in plasma
Venous blood was collected in 10-mL sodium-heparin-containing tubes and immediately centrifuged at 1500 x g for 10 minutes. Aliquots of plasma samples were stored at −70℃ until they were analyzed. The inBloodTM OAβ test (PeopleBio, Gyeonggi-do, Republic of Korea) was used to quantify the level of MDS-OAβ in plasma. This test utilizes the Multimer Detection System, which is a modified atypical sandwich enzyme immunoassay that uses capture antibodies and epitope-overlapping detection antibodies to preferentially detect oligomers or multimers from monomers through competitive bindings to specific epitopes. The epitopes for the 6E10 and W0-2HRP antibodies overlap at the N terminus of Aβ, and mouse monoclonal anti-6E10 (BioLegend, San Diego, CA, USA) and ant-W0-2-HRP antibodies (Absolute Antibody, Oxford, UK) were used to capture and detect OAβ, respectively. The relative luminescence unit (RLU) signal was detected using the luminometer. Dilutions providing signals within the linear range of the standard curves were used for the converting RLU values into OAβ concentrations. The cutoff MDS-OAβ values for borderline and high risks were 0.78 and 0.93, respectively.13
2.4 Brain volume measurement
Brain volume analysis was performed using NeuroQuant software version 3.0 (CorTechs Labs, San Diego, CA, USA) in 300 subjects who chose MRI examination voluntarily during their health checkups. This software detects areas of atrophy or shrinkage by measuring the hippocampus, superior lateral ventricles, and inferior lateral ventricles in the brain. It utilizes a dynamic probabilistic neuroanatomical atlas, with age and sex specificity based on the MRI image intensity. NeuroQuant compares a subject's results to an FDA-approved database of normal healthy brains at the subject's particular age. Details of the dynamic atlas are available at https://www.cortechslabs.com/whitepapers/.
2.5 Statistical analysis and calculation of RIs
Data are presented as mean ± standard deviation or frequency (percentage) values. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). Differences in the characteristics of the study subjects were analyzed according to the plasma OAβ level using ANOVA or the chi-square test. Differences in the median plasma MDS-OAβ values were analyzed according to categorical groups of KDSQ-C scores, and MMSE-DS scores using the Wilcoxon rank-sum test. A P value of <0.05 was considered statistically significant.
The RI for plasma MDS-OAβ values was calculated for the 911 reference subjects. The plasma MDS-OAβ values were analyzed according to the CLSI C28-A3 guidelines.17 Scatter and distribution plots were used to inspect the data. The data in each partition were transformed using the Box-Cox transformation method. The Tukey test was used to remove outliers. RIs for age-groups were calculated using non-parametric methods (one-side upper 95th percentile). Variations in MDS-OAβ values were analyzed according to age grouping into the following age-groups: 50–59, 60–69, 70–79, and ≥80 years. The Wilcoxon rank-sum test was used to compare the MDS-OAβ values according to age-groups.
3 RESULTS
This study analyzed 1,594 subjects (714 males and 880 females) with a mean age of 68.5 years (age range 50–87 years) and an MDS-OAβ value of 0.45 ± 0.22 ng/mL.
3.1 Demographic and clinical characteristics of the study subjects according to the plasma MDS-OAβ values
The characteristics of the study subjects are presented in Table 1. Based on the cutoff MDS-OAβ values, 105 (6.6%) and 18 (1.1%) subjects were categorized into the borderline and high-risk groups of plasma OAβ level. Age, sex, and education period did not differ significantly among the three groups. There are more people with MMSE-DS scores <27 in the high-risk group of plasma MDS-OAβ values than in the low-risk and borderline-risk groups (P = 0.008). However, KDSQ-C values did not differ among the three groups. The NeuroQuant findings representing brain atrophy also did not differ significantly among the three groups.
| Variables | Plasma MDS-OAβ value | P | ||
|---|---|---|---|---|
| Low risk (<0.78 ng/mL) (N = 1,471) | Borderline (0.78–0.92 ng/mL) (N = 105) | High risk (≥0.93 ng/mL) (N = 18) | ||
| Age, years | 68.4 ± 6.4 | 69.6 ± 5.7 | 70.4 ± 7.0 | 0.094 |
| Sex, male | 664 (45.1) | 41 (39.0) | 9 (50.0) | 0.434 |
| Education, ≤12yrs | 1,196 (81.3) | 87 (82.9) | 14 (77.8) | 0.856 |
| KDSQ-C score | 2.4 ± 2.8 | 2.5 ± 3.0 | 2.1 ± 2.7 | 0.775 |
| <6 | 1,313 (89.3) | 94 (89.5) | 17 (94.4) | 0.776 |
| ≥6 | 158 (10.7) | 11 (10.5) | 1 (5.6) | |
| MMSE-DS score | 26.8 ± 2.8 | 26.8 ± 2.9 | 25.4 ± 2.5 | 0.092 |
| >26 | 932 (63.4) | 66 (62.9) | 5 (27.8) | 0.008 |
| ≤26 | 539 (36.6) | 39 (37.1) | 13 (72.2) | |
| NeuroQuant | ||||
| HOC percentiles 1–4 | 18/276 (6.5) | 3/24 (12.5) | - (0.0) | 0.448 |
| Hippocampi percentiles 1–4 | 5/276 (1.8) | 1/24 (4.2) | - (0.0) | 0.692 |
| SLV percentiles 96–99 | 10/276 (3.6) | - (0.0) | - (0.0) | 0.581 |
| ILV percentiles 96–99 | 20/276 (7.2) | 3/24 (12.5) | - (0.0) | 0.525 |
Note
- Data are mean ± standard deviation or N (%) values.
- P value are from chi-square test or ANOVA.
- Abbreviations: HOC, hippocampal occupancy score; SLV, superior lateral ventricles; IVL, inferior lateral ventricles.
3.2 MDS-OAβ values according to (A) KDSQ-C and (B) MMSE-DS categorical values
The median MDS-OAβ value was higher in subjects with KDSQ-C scores ≥8 than in those with KDSQ-C scores of 6–7 (P = 0.013). The median MDS-OAβ value was higher in subjects with MMSE-DS scores of 21–26 than in those with MMSE-DS scores ≥27 (P = 0.010) (Figure 2).
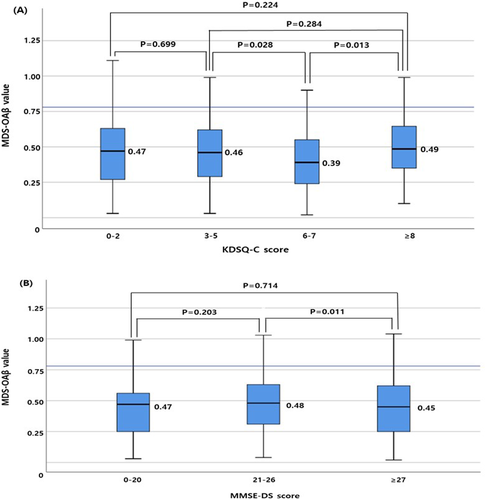
3.3 RIs of MDS-OAβ values
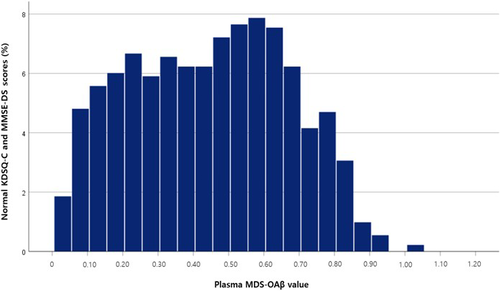
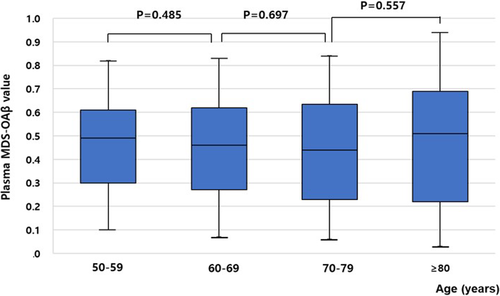
A non-normal distribution of MDS-OAβ values was evident in the reference subjects from both visual inspection and the Shapiro-Wilk test (P < 0.05) (Figure 3). To analyze the variations in MDS-OAβ values according to age, box plots of MDS-OAβ values were drawn for the following age-groups: 50–59, 60–69, 70–79, and ≥80 years. The median MDS-OAβ values did not different among these age-groups (Figure 4). The RIs for MDS-OAβ values are presented in Table 2. The overall one-side upper 95th percentiles of the MDS-OAβ value presented 0.80 ng/mL (95% confidence interval [CI] = 0.78–0.82), and 0.78 ng/mL (95% CI = 0.77–0.82), 0.81 ng/mL (95% CI = 0.79–0.84), and 0.91 ng/mL (95% CI = 0.77–0.94) in those aged <70, 70–79, and ≥80 years, respectively, with no significant differences among these age-groups.
| Age-group | N | Percentile (95%CI) | ||||
|---|---|---|---|---|---|---|
| 5th (95% CI) | 25th (95% CI) | 50th (95% CI) | 75th (95% CI) | 95th (95% CI) | ||
| KDSQ-C score <6 & MMSE-DS score >26 | ||||||
| Total | 911 | 0.09 (0.08, 0.11) | 0.26 (0.24, 0.29) | 0.46 (0.44, 0.48) | 0.62 (0.61, 0.64) | 0.80 (0.78, 0.82) |
| 50–59 years | 98 | 0.14 (0.09, 0.19) | 0.30 (0.25, 0.37) | 0.49 (0.43, 0.54) | 0.61 (0.57, 0.67) | 0.78 (0.71, 0.85) |
| 60–69 years | 476 | 0.10 (0.09, 0.13) | 0.27 (0.25, 0.31) | 0.46 (0.43, 0.49) | 0.62 (0.60, 0.65) | 0.78 (0.77, 0.82) |
| 70–79 years | 312 | 0.07 (0.06, 0.10) | 0.23 (0.20, 0.28) | 0.44 (0.40, 0.49) | 0.64 (0.59, 0.67) | 0.81 (0.79, 0.84) |
| ≥80 years | 25 | 0.07 (0.03, 0.15) | 0.22 (0.12, 0.48) | 0.51 (0.27, 0.64) | 0.69 (0.58, 0.85) | 0.91 (0.77, 0.94) |
| KDSQ-C score <6 | ||||||
| Total | 1.423 | 0.10 (0.10, 0.12) | 0.27 (0.25, 0.30) | 0.47 (0.44, 0.48) | 0.62 (0.61, 0.64) | 0.81 (0.80, 0.83) |
| 50–59 years | 125 | 0.13 (0.09, 0.18) | 0.30 (0.25, 0.37) | 0.49 (0.44, 0.53) | 0.61 (0.57, 0.65) | 0.78 (0.71, 0.90) |
| 60–69 years | 718 | 0.10 (0.09, 0.12) | 0.27 (0.25, 0.30) | 0.46 (0.43, 0.49) | 0.62 (0.60, 0.65) | 0.80 (0.78, 0.83) |
| 70–79 years | 526 | 0.09 (0.08, 0.11) | 0.26 (0.23, 0.31) | 0.46 (0.42, 0.48) | 0.63 (0.61, 0.67) | 0.82 (0.80, 0.85) |
| ≥80 years | 54 | 0.07 (0.03, 0.13) | 0.26 (0.13, 0.46) | 0.55 (0.43, 0.62) | 0.69 (0.62, 0.77) | 0.91 (0.77, 0.94) |
| MMSE-DS score >26 | ||||||
| Total | 1,003 | 0.10 (0.10, 0.12) | 0.25 (0.23, 0.28) | 0.45 (0.43, 0.47) | 0.62 (0.61, 0.64) | 0.79 (0.78, 0.82) |
| 50–59 years | 113 | 0.10 (0.09, 0.15) | 0.29 (0.23, 0.35) | 0.47 (0.41, 0.53) | 0.59 (0.55, 0.63) | 0.78 (0.71, 0.85) |
| 60–69 years | 524 | 0.10 (0.09, 0.13) | 0.27 (0.25, 0.31) | 0.45 (0.42, 0.48) | 0.62 (0.60, 0.65) | 0.78 (0.77, 0.83) |
| 70–79 years | 340 | 0.08 (0.07, 0.11) | 0.22 (0.19, 0.26) | 0.44 (0.39, 0.48) | 0.63 (0.59, 0.67) | 0.81 (0.78, 0.83) |
| ≥80 years | 26 | 0.07 (0.03, 0.15) | 0.22 (0.12, 0.48) | 0.54 (0.27, 0.62) | 0.69 (0.61, 0.85) | 0.91 (0.77, 0.94) |
- Abbreviations: KDSQ-C, Korean Dementia Screening Questionnaire-Cognition; MMSE-DS, Mini-Mental State Examination-Dementia Screening.
4 DISCUSSION
This study has demonstrated that plasma MDS-OAβ values reflect cognitive function as assessed using the KDSQ-C and MMSE-DS. RIs of the plasma MDS-OAβ were established in reference subjects with normal general cognition according to common cognitive screening tools.
The performance of MDS-OAβ test in differentiating AD patients from subject with normal cognition has been studied in clinical settings,18-20 but far less so in community-based population with apparently normal cognition. Wang et al.20 found that plasma MDS-OAβ values were higher in patients with AD than in normal individuals. In addition, they confirmed that plasma MDS-OAβ values were correlated with other conventional amyloid biomarkers such as CSF Aβ42, CSF pTau, CSF tTau, and 11C-Pittsburgh compound B standardized uptake value ratio. They suggested that MDS-OAβ could be used in routine clinical practice for detecting OAβ related to AD pathology. Although the purpose of screening in clinical practice is to correctly distinguish patients with AD from subjects with normal cognition, many people in the community cannot be dichotomized in this way. In primary clinical settings in the community, cognitive assessments are needed for the screening of risk groups of AD for further neuropsychological testing rather than for distinguishing AD from normal cognition. Therefore, MDS-OAβ evaluation is needed in primary clinical settings for assessing cognitive function.
The present study found that MDS-OAβ values reflected the cognitive function in primary clinical settings in the community. The median MDS-OAβ value differed significantly not only between subjects with KDSQ-C scores ≥8 and those with KDSQ-C scores of 6–7, but also between subjects with MMSE-DS scores of 21–26 and those with MMSE-DS scores ≥27. Simultaneous multi-category classifications of cognitive dysfunction have been proposed in Korea.21, 22 The MMSE is the most widely used cognitive test and exhibits moderate accuracy for ruling out a diagnosis of cognitive dysfunction. Choi et al.21 evaluated the K-MMSE with cutoff score analysis. They used the cutoff score from the medication guideline of the Health Insurance Review and Assessment Service, Korea (http://www.hira.or.kr). This suggested ≥27 for normal cognitive control, 21–26 for mild cognitive impairment (MCI), and ≤20 for dementia. In addition, Lee et al.22 showed that the optimal cutoff points of KDSQ-C were 2 and 8 for normal cognitive control, MCI, and dementia. The Korean government include screening for cognitive dysfunction in the national general health screening program, in which the KDSQ-C has been used as a screening tool. The KDSQ-C assesses not only memory but also other cognitive functions, is not influenced by age or education period, and has high validity and reliability for distinguishing between subjects with and without dementia.14
Atrophic changes in the AD brain have been detected using voxel-based morphometry.19, 23, 24 Youn et al.19 demonstrated that plasma MDS-OAβ values were negatively correlated with changes in the brain volume in AD, with reductions reflecting typical areas of degeneration in AD. They suggested that the oligomerization of Aβ in the blood can cause a pattern of AD in the brain. The frequency of brain atrophy found using NeuroQuant did not differ significantly among the three groups of MDS-OAβ values in the present study. In addition, all those with suspected atrophic changes in the brain based on NeuroQuant analyses had borderline-risk MDS-OAβ values. This result suggests that most of our subjects who were analyzed using NeuroQuant in healthcare centers were either normal or only in the very early stage of cognitive dysfunction. Some studies have shown that the brain volume decreases as AD progresses, with a sigmoid relationship.25, 26 In addition, while plasma MDS-OAβ measures the dynamics of Aβ oligomerization, amyloid PET only reveals the fibrillar form of Aβ in the brain.27 Changes in plasma MDS-OAβ may occur in the early stage of cognitive dysfunction before the brain volume changes.
We determined the RIs of MDS-OAβ values using a large reference population with normal general cognition according to cognitive screening tools. This large group of reference subjects could represent the general population since most of them were recruited from the national health screening program across the country. Although a value of 0.783 has been proposed for the 95% RI of MDS-OAβ values in normal controls,13 that was based on a small reference population. Moreover, no correlation was found between the MDS-OAβ value and age in normal control subjects, which resulted in speculation that age does not significantly influence MDS-OAβ values. Meanwhile, Lee et al.28 reported that plasma MDS-OAβ values were strongly correlated with aging in their population. In our study, although the 95th percentiles of MDS-OAβ appeared to increase with the age-group, the median MDS-OAβ values did not differ significantly among age-groups.
The present study has several strengths. It involves a large sample and had a multicenter design involving 16 health-promotion centers in 13 cities across the country. Participants were mostly recruited from the national health screen program as a community-based cohort, rather than from tertiary university hospitals or dementia centers. This suggests that our participants were representative of the Korean general population. However, there are also some limitations in the present study. Most of all, the standards of the cognitive function in this study are common cognitive screening tools without clinical follow-up and/or independent measures of brain amyloid deposition. In primary clinical settings, cognitive assessments are needed for the screening of risk groups of AD for further neuropsychological testing rather than for distinguishing AD from normal cognition. In fact, cognitive assessments in primary clinical settings have been underwent using cognitive questionnaires such as KDSQ and MMSE in the real world. Although KDSQ and MMSE have demonstrated high validity and reliability for screening for early dementia, cognitive assessment with cognitive questionnaires has limitations such as being subjective and yielding different outcomes when completed by participants and their informants. Authors tried to evaluate whether plasma MDS-OAβ values reflect cognitive function through cognitive questionnaires for considering an adjuvant or alternative to cognitive screening questionnaires for screening cognitive dysfunction in primary healthcare settings. Second, although the participants were recruited from the national health screening program, NeuroQuant with MRI was additionally performed in those subjects who chose to undergo an MRI examination voluntarily during health checkups. It is possible that these individuals were more concerned about their cognitive condition. In addition, the number of cases that underwent NeuroQuant with MRI was not enough to determine the association between MDS-OAβ values and structural changes of the brain in this study. Third, the authors could not analyze the association of subdomains of neurocognitive batteries with the MDS-OAβ values.
In conclusion, the RI of plasma MDS-OAβ obtained from a large community-based sample has been revealed in this study. The plasma MDS-OAβ value reflects cognitive function in the general Korean population. This suggests that the plasma MDS-OAβ test might be considered an adjuvant or alternative to cognitive screening questionnaires for screening cognitive dysfunction in primary healthcare settings. However, this study does not have clinical follow-up and independent measures of brain amyloid deposition. Authors understand the necessity of future studies using clinical follow-up and larger samples with measures of brain amyloid deposition such as brain volumetry and, ultimately, a longitudinal design to establish the mechanisms for cognitive impairment.
ACKNOWLEDGMENTS
This study was supported by PeopleBio, Co., Ltd. through Investigator Initiated Study, which provided reagents for testing plasma MDS-OAβ. The authors would like to thank all participants who participated in this study and all staffs of health-promotion centers and MediCheck Lab, Korea Association of Health Promotion, Seoul, Korea.
CONFLICTS OF INTEREST
No potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
All the authors participated in designing this study. SC, HP, and IH performed data collection. SC undertook the statistical analyses. EN, SC, DN, and HC analyzed and interpreted the data. EN wrote the first draft of the manuscript, which was reviewed by all of the other authors, who also provided further contributions and suggestions.
Open Research
DATA AVAILABILITY STATEMENT
Data are available on request due to privacy/ethical restrictions.




