Immunological characteristics and effect of cyclosporin in patients with immune thrombocytopenia
Abstract
Objective
Immune thrombocytopenia (ITP) is well-known as an antibody-mediated autoimmune disease, and it is easy to get response but often turns to relapse or refractory. Cyclosporin is a traditional immunosuppressant and had a good effect on ITP patients. In this paper, we retrospectively analyze the immunological characteristics and therapeutic effect of cyclosporin in 220 patients with ITP.
Methods
All newly diagnosed ITP patients in the Department of Hematology, Tianjin Medical University General Hospital from June 2018 to December 2020 were enrolled and divided into four groups according to the expression of autoantibodies and the occurrence of prodromal infection. The basic data and immune indexes of ITP patients in each group were collected. The clinical immunological characteristics of patients in each group and the therapeutic effect of cyclosporin in each group were analyzed.
Results
Multi-autoantibody ITP patients were more likely to have low serum albumin and high gamma globulin, and the ratio of albumin to globulin decreased. In addition, the level of IgA and IgG increased and the level of complement C3 and C4 decreased more frequently than those in other groups. The number of CD3+T lymphocytes, especially CD3+CD4+T lymphocytes, decreased in ANA+ITP patients. The number of CD16+CD56+NK cells, pDC/DC ratio, and pDC/mDC ratio were higher than those in other groups. The expression of IL-6 and the proportion of CD19+B lymphocytes increased in two groups of ITP patients with abnormal autoantibodies. The patients of pro-infected group were more likely to suffer from coagulation disorder. After treatment with cyclosporin, the response rate increased and the 3-month relapse rate decreased in all ITP patients, and the therapeutic effect of patients with high megakaryocyte number was significantly higher than that of patients with low megakaryocyte number. The impact factors that influence the effect of glucocorticoid and(or) IVIG were the number of CD3+CD8+T lymphocytes, CD4/CD8 cell ratio, and the number of CD19+B lymphocytes. The independent impact factor of cyclosporin therapeutic response rate was the number of CD3+T lymphocytes.
Conclusions
ITP is a heterogeneous disease, recurrence may occur during or rapidly after treatment. Cyclosporine included treatment can improve the effective rate of ITP and reduce the relapse rate within 3 months. The number of CD3+T lymphocytes was the only impact factor that influence the therapeutic effect of cyclosporin in ITP patients.
1 INTRODUCTION
Immune thrombocytopenia(ITP) is an autoimmune hemorrhagic disease characterized by platelet destruction and(or) insufficient platelet production leading to skin and(or) mucous membrane, nose and(or) gum hemorrhage, even vital organs hemorrhage. The specific etiology of ITP is not very clear, among which genetic, drug, infection, oxidative stress, and abnormal immune regulation play an important role in the pathogenesis.1 In humoral immunity of ITP patients, platelet-specific glycoprotein antibody not only mediates platelet destruction through mononuclear macrophage system, but also inhibits the proliferation and maturation of megakaryocytes. Th2 cells in CD4+T lymphocytes can activate B lymphocytes and promote the production of antibodies. In cellular immunity of ITP patients, CD8+T cells can directly dissolve platelets and inhibit the production of platelet of megakaryocytes. Th17 cells play an equally important role in immune regulation as Th1 cells in CD4+T lymphocytes by activating MAP kinase and NF-κB pathway.2 DCs can directly promote the proliferation and differentiation of B lymphocytes and play an important role in antigen presentation; moreover, DC in ITP patients can regulate the activation of T lymphocytes through cytokines IL-6 and IL-12. In addition, NK cells can also modulate cellular immunity in ITP patients.3, 4
In terms of drug therapy, glucocorticoid and intravenous immunoglobulin (IVIG) have been used as the first-line treatment for ITP patients for long time. Thrombopoietin receptor agonists (TPO-RA) and Rituximab are used as second-line treatment, especially Rituximab can inhibit the function of B lymphocytes secreting antibodies. Cyclosporin is a traditional but potent immunosuppressant, which can act on both humoral and cellular immunity.5 Studies found that cyclosporin mainly inhibits T helper (Th) cells proliferation and differentiation, thus indirectly regulates the activity of B lymphocytes. In addition, cyclosporin can also inhibit the production and release of IL-2 by Th1 cells, reduce the reactivity of IL-2, and further affect the differentiation of B lymphocytes.6
The clinical manifestations of ITP patients were heterogeneous. Some ITP patients have one or more kinds of autoantibodies, which are not enough to definitely diagnose any autoimmune disease. Meanwhile, other ITP patients are with detectable or undetectable bacterial or viral prodromal infections. However, the differences in clinical characteristics and immune indicators of patients with various types of ITP are currently not clear. In terms of therapeutic effect, our clinical work found that some patients responded well to glucocorticoid and(or) IVIG, others had good response to cyclosporin, and recurrence rate is low. Therefore, in this study, 220 ITP patients were classified into four groups according to autoantibodies detection and prodromal infection. The immunological characteristics, therapeutic effects in each group of ITP patients, were further compared in our research.
2 MATERIALS AND METHODS
2.1 General information of patients
All ITP patients hospitalized at the Department of Hematology, Tianjin Medical University General Hospital, China, from June 2018 to December 2020 were enrolled. The criteria for the specific inclusion were as follows: ①Adult patients with newly diagnosed ITP in our hospital, and the age of the patients was more than 18 years. ②In line with the 2019 ITP international consensus diagnostic criteria7: platelet count<100 × 109/L at least two times, and there were different degrees of hemorrhage. ③There was no obvious abnormality in liver and kidney function. ④The research program was approved by the Ethics Committee of Tianjin Medical University General Hospital. All patients were informed and signed informed consent. The criteria for the exclusion were as follows: ①adolescent ITP patients with age<18 years; ②patients diagnosed with Myelodysplastic syndrome, Evans syndrome, and other similar diseases; ③retreated ITP patients who had been diagnosed before treatment in our hospital. The age, gender, and other general information of all ITP patients were statistically analyzed.
2.2 Grouping for ITP patients
All eligible ITP patients were divided into four groups according to whether their autoantibodies were positive and whether they were complicated with prodromal infection: General ITP patients (ITP), ITP patients with positive antinuclear antibody (ANA+ITP), ITP patients with multiple autoantibody abnormalities (Multi-autoantibody ITP), and ITP patients with prodromal infection (Pro-infected ITP). ①Patients with general ITP were defined as follows: 13 kinds of autoantibodies detected by the clinical laboratory in our hospital were all negative, and there were no patients complicated with bacterial or viral prodromal infection. ②The ANA+ITP patients were as follows: among the 13 kinds of autoantibodies detected by the clinical laboratory in our hospital, only antinuclear antibody was positive, while the other 12 antibodies were all negative, and there were no ITP patients complicated with bacterial or viral prodromal infection. ③The definition of Multi-autoantibody ITP was that among the 13 kinds of autoantibodies detected by the clinical laboratory in our hospital, there were one or more kinds of positive autoantibodies besides antinuclear antibody, and there were no patients complicated with bacterial or viral prodromal infection. ④The definition for Pro-infected ITP patients was those ITP patients who were negative for 13 autoantibodies detected by the clinical laboratory, but complicated with bacterial or viral prodromal infection.
2.3 Collection of basic laboratory indexes and immune-related indexes
①The clinical data included detailed medical history, clinical manifestations, and drug therapy. ②Blood routine and coagulation indexes were detected by the clinical laboratory. ③The number of bone marrow megakaryocytes was detected by morphology Laboratory of Hematology Department in our hospital. ④13 kinds of autoantibodies were detected in the clinical laboratory in our hospital, including: antinuclear antibody, anti-dsDNA antibody, anti-nRNP antibody, anti-Sm antibody, anti-SSA antibody, anti-SSB antibody, anti-Ro-52 antibody, anti-Jo-1 antibody, anti-sc1-70 antibody, anti-centromere B antibody, anti-nucleosome antibody, anti-histone antibody, and anti-ribosomal P protein antibody. ⑤The immune indexes detected by the clinical laboratory were IgA, IgE, IgG, IgM, C3, and C4 etc. ⑥The indexes of immune cells were detected by flow cytometry in Department of Hematology in our hospital: peripheral blood T lymphocyte subsets, peripheral blood B lymphocyte subsets, peripheral blood NK cell, and DC subsets. ⑦The indexes of lymphokines were detected by flow cytometry in Department of Hematology in our hospital: peripheral blood Th1, Th2, and Th17 cytokines.
2.4 Treatment and follow-up
All ITP patients were treated according to the international consensus of ITP in 2019: Glucocorticoids included conventional dose of Prednisone (1 mg/kg for 2 weeks, to a maximum of 3 weeks) or high-dose Dexamethasone (40 mg/day for 4 days, repeated up to three times) and IVIG (0.4 g/kg/day × 5 day or 1.0 g/kg/day × 1–2 days) were given as the first-line treatment. TPO-RA, CD20 antibody, cyclosporin (3–5 mg/kg/day), and cyclophosphamide were used in relapse or refractory patients. All patients were followed up for at least 6 months to observe their therapeutic effect and recurrence. The efficacy criteria are as follows: ①Complete response (CR), PLT ≥100 × 109/L after treatment, without hemorrhage. ②Partial response (PR), PLT ≥30×109/L after treatment, and platelet count increased two times compared with basic value, without hemorrhage. ③No response (NR): PLT<30 × 109/L after treatment, or platelet count increased less than two times of the basic value after treatment, or hemorrhage occurred. ④Recurrence: After effective treatment, PLT decreased to <30 × 109/L or PLT decreased to less than half of the basic value, or hemorrhage occurred.
2.5 Statistical analysis
SPSS 24.0 statistical software was used for data analysis. The measurement data were expressed in (X ± s). Two independent samples t test was used to compare the two groups when normally distributed. Multiple groups of data were compared by one-way ANOVA, and LSD t test was used for pairwise comparison if there was statistical significance in multiple groups of data. The enumeration data were expressed in case number and rate, and chi-square test was used for comparison between groups. Multivariate analysis was performed by Logistic regression analysis. All p values were two-sided, and those p<0.05 were considered statistically significant.
3 RESULTS
3.1 Basic clinical data of ITP patients
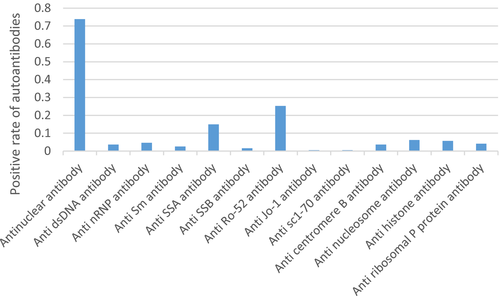
A retrospective analysis of 220 ITP patients was performed. Among them, there were 64 cases in general ITP group, 50 cases in ANA+ITP group, 87 cases in Multi-autoantibody ITP group, and 19 cases in Pro-infected ITP group. The proportion of females in all subgroups was higher than that of males; in particular, the proportion of female patients in Multi-autoantibody ITP patients was the highest, significantly higher than the proportion of female patients in general ITP patients (p = 0.004). The mean platelet count in four groups was all lower than the basic value, but no significant difference among each group. There was no significant difference in drug therapy among the groups. The specific basic information, autoantibodies, and drug therapy are shown in Tables 1-3. And the autoantibody's ratios are shown in Figure 1.
| Number of people (proportion of all) | Sex ratio (male/female) | Age (years) | Platelet count (100–300) | |
|---|---|---|---|---|
| Total ITP patients | 220 | 1:1.89 (76/144) | 55.6 (18–88) | 43.20 ± 28.68 |
| ITP | 64 (29.09%) | 1:1.21 (29/35) | 54.3 (18–88) | 46.14 ± 30.54 |
| ANA+ITP | 50 (22.73%) | 1:1.63 (19/31) | 57.9 (23–87) | 41.98 ± 26.85 |
| Multi-autoantibody ITP | 87 (39.55%) | 1:3.35 (20/67)* | 56.3 (18–86) | 42.83 ± 29.54 |
| Pro-infected ITP | 19 (8.64%) | 1:1.38 (8/11) | 51 (18–76) | 38.21 ± 20.80 |
| Antinuclear antibody | 73.85% | Anti-dsDNA antibody | 3.61% |
| Anti-nRNP antibody | 4.64% | Anti-Sm antibody | 2.58% |
| Anti-SSA antibody | 14.95% | Anti-SSB antibody | 1.55% |
| Anti-Ro-52 antibody | 25.26% | Anti-Jo-1 antibody | 0.52% |
| Anti-sc1-70 antibody | 0.52% | Anti-centromere B antibody | 3.61% |
| Anti-nucleosome antibody | 6.19% | Anti-histone antibody | 5.67% |
| Anti-ribosomal P protein antibody | 4.12% |
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| Glucocorticoid | 70.31% | 64.00% | 81.61% | 78.95% |
| IVIG | 28.13% | 18.00% | 44.83% | 52.63% |
| Platelet transfusion | 18.75% | 24.00% | 18.39% | 31.58% |
| TPO | 4.69% | 6.25% | 9.20% | 15.79% |
| Eltrombopag | 25.56% | 14.00% | 25.29% | 36.84% |
| Cyclosporin | 15.63% | 22.00% | 24.14% | 21.05% |
| Cyclophosphamide | 0 | 0 | 2.30% | 0 |
| Rituximab | 1.56% | 2.00% | 5.75% | 0 |
| IL-11 | 3.13% | 0 | 1.15% | 10.53% |
3.2 Analysis of coagulation factors in ITP patients
Compared with the general ITP group, the number of Prothrombin time (p = 0.005), International normalized ratio of prothrombin time (p = 0.004), and Thrombin time (p = 0.047) in Pro-infected ITP group were higher, and the level of Fibrinogen (p = 0.038) was lower. See Table 4 for details.
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| Prothrombin time | 11.32 ± 1.29 | 11.45 ± 1.44 | 11.35 ± 1.07 | 12.85 ± 3.49* |
| International standardized ratio of prothrombin time | 1.03 ± 0.11 | 1.05 ± 0.13 | 1.04 ± 0.10 | 1.17 ± 0.31* |
| Activated partial thromboplastin time | 29.96 ± 6.88 | 31.23 ± 7.04 | 31.51 ± 10.10 | 31.65 ± 4.85 |
| Fibrinogen | 3.01 ± 0.73 | 2.88 ± 0.53 | 2.96 ± 0.66 | 2.59 ± 0.80* |
| Thrombin time | 19.90 ± 2.61 | 20.76 ± 2.85 | 20.81 ± 2.29 | 21.39 ± 3.35* |
| Plasma D-dimer | 836.22 ± 922.79 | 634.6 ± 714.87 | 860.40 ± 848.81 | 449.95 ± 556.37 |
- * p < 0.05.
3.3 Comparison of immune-related indexes in ITP patients
Level of albumin(ALB) (p = 0.000), the ratio of Albumin/globulin (p = 0.000), level of Complement C3 (p = 0.007), and Complement C4 (p = 0.003) were decreased in Multi-autoantibody ITP group when comparing with general ITP group. The quantity of Gamma globulin (p = 0.000), Immunoglobulin A (p = 0.017), and Immunoglobulin G (p = 0.000) were significantly high in Multi-autoantibody ITP group. As shown in Table 5.
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| ALB | 59.04 ± 3.45 | 58.02 ± 3.74 | 53.38 ± 5.79* | 58.09 ± 6.39 |
| Gamma globulin | 16.84 ± 2.88 | 18.58 ± 3.46 | 22.43 ± 6.84* | 19.34 ± 7.27 |
| Albumin/globulin | 1.46 ± 0.21 | 1.4 ± 0.2 | 1.18 ± 0.25* | 1.44 ± 0.38 |
| Immunoglobulin A | 221.71 ± 105.12 | 261.96 ± 111.57 | 277.00 ± 127.21* | 236.68 ± 133.08 |
| Immunoglobulin G | 1216.88 ± 288.41 | 1333.48 ± 331.41 | 1667.59 ± 625.84* | 1356.89 ± 534.78 |
| Immunoglobulin M | 114.20 ± 53.73 | 115.45 ± 55.09 | 127.61 ± 69.03 | 183.34 ± 117.72* |
| Complement C3 | 88.44 ± 16.60 | 85.70 ± 16.66 | 78.06 ± 21.42* | 76.52 ± 21.16* |
| Complement C4 | 22.92 ± 6.08 | 20.07 ± 6.65 | 17.56 ± 10.84* | 14.83 ± 7.06* |
- * p < 0.05.
3.4 T and B lymphocyte subsets detected by flow cytometry in ITP patients
In terms of T lymphocyte subsets, the proportion of CD3+T lymphocytes (p = 0.000) and CD3+CD4+T lymphocytes (p = 0.001) was extremely decreased in ANA+ITP patients than in general ITP patients. In terms of B lymphocyte subsets, the proportion of CD19+B lymphocytes in ANA+ITP patients (p = 0.047) and Multi-autoantibody ITP patients (p = 0.008) was super higher than in general ITP group. See Table 6 for details. However, there was no significant difference in CD8+T lymphocytes and CD5+CD19+B lymphocytes among all these four groups.
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| CD3+ | 73.58 ± 7.34 | 63.96 ± 15.23* | 70.53 ± 10.66 | 71.34 ± 10.29 |
| CD3+CD8+ | 27.4 ± 10.63 | 27.91 ± 11.49 | 26.84 ± 10.43 | 26.24 ± 10.59 |
| CD3+CD4+ | 44.33 ± 9.91 | 37.18 ± 10.32* | 42.17 ± 11.06 | 42.11 ± 10.05 |
| CD3+CD4+CD8+ | 0.83 ± 2.08 | 0.48 ± 0.57 | 0.49 ± 0.53 | 0.44 ± 0.47 |
| CD4/CD8 | 2.01 ± 1.21 | 1.64 ± 0.9 | 2.00 ± 1.56 | 1.95 ± 0.94 |
| CD19+ | 13.07 ± 5.34 | 16.04 ± 8.21* | 17.13 ± 9.30* | 15.79 ± 8.29 |
| CD5+CD19+/CD19+ | 22.06 ± 20.43 | 23.08 ± 16.95 | 17.43 ± 15.03 | 17.67 ± 11.04 |
- * p < 0.05.
3.5 Lymphokine indexes of Th1/Th2/Th17 detected by flow cytometry in ITP patients
IL-2, TNF-α, and IFN-γ are mainly secreted by Th1 cells; IL-4, IL-6, and IL-10 are mainly secreted by Th2 cells; and IL-17 is mainly secreted by Th17 cells.
Our data in Table 7 show that the level of IL-6 in ANA+ITP patients (p = 0.037) and the Multi-autoantibody ITP patients (p = 0.046) were increased compared with the general ITP group, and there was no significant difference in other lymphokine indexes.
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| IL-2 | 3.50 ± 3.82 | 4.51 ± 2.01 | 5.47 ± 10.44 | 3.38 ± 1.09 |
| IL-4 | 3.87 ± 3.42 | 5.51 ± 2.53 | 5.59 ± 6.14 | 5.18 ± 2.4 |
| IL-6 | 4.57 ± 4.09 | 9.03 ± 10.28* | 7.50 ± 7.37* | 5.82 ± 3.25 |
| IL-10 | 5.08 ± 5.09 | 6.36 ± 10.19 | 4.41 ± 2.91 | 18.88 ± 45.37 |
| TNF-α | 3.10 ± 2.28 | 4.01 ± 2.43 | 3.92 ± 4.64 | 3.60 ± 1.47 |
| IFN-γ | 4.88 ± 5.80 | 4.51 ± 2.60 | 5.85 ± 9.26 | 7.38 ± 7.44 |
| IL-17 | 8.27 ± 12.59 | 6.08 ± 7.67 | 1.64 ± 1.95 | 2.48 ± 2.47 |
- * p < 0.05.
3.6 NK cells and DC subsets detected by flow cytometry in ITP patients
NK cells were labeled with CD16+CD56+, and we used CD16+CD56+ double positive to detect NK cells. Compared with the general ITP group, the proportion of CD16+CD56+NK cells in ANA+ITP patients was increased (p = 0.019). DCs can be divided into pDC and mDC according to its source and function. Moreover, the mDC in DCs can be further divided into CD11c-mDC and CD16-mDC, and CD11c-mDC is composed of mDC1 and mDC2. Compared with the general ITP group, the proportions of pDC, no matter pDC/DC (p = 0.012), or pDC/mDC (p = 0.025) in ANA+ITP group were both significantly increased. As shown in Table 8.
| ITP (n = 64) | ANA+ITP (n = 50) | Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |
|---|---|---|---|---|
| CD16+CD56+ | 13.04 ± 5.58 | 17.39 ± 10.82* | 12.13 ± 8.47 | 12.59 ± 5.19 |
| DC/PBMMNC | 0.97 ± 1.07 | 0.61 ± 0.43 | 0.52 ± 0.42 | 0.8 ± 0.70 |
| pDC/DC | 8.34 ± 5.69 | 22.97 ± 18.18* | 12.96 ± 18.71 | 8.64 ± 3.37 |
| mDC/DC | 65.34 ± 19.41 | 63.29 ± 18.60 | 61.19 ± 27.42 | 53.18 ± 27.54 |
| pDC/mDC | 0.14 ± 0.09 | 0.44 ± 0.43* | 0.61 ± 1.83 | 0.19 ± 0.14 |
| CD16+mDC/mDC | 37.21 ± 34.66 | 30.88 ± 27.78 | 33.86 ± 32.72 | 33.51 ± 28.33 |
| mDC1/mDC | 50.98 ± 32.60 | 62.26 ± 27.80 | 51.24 ± 37.29 | 58.1 ± 28.33 |
| mDC2/mDC | 1.9 ± 1.68 | 3.39 ± 2.30 | 3.71 ± 6.29 | 2.2 ± 1.78 |
- * p < 0.05.
3.7 Therapeutic effect of cyclosporin in ITP patients
The therapeutic effect and recurrence of total ITP patients and ITP patients in each group after treatment with cyclosporin are shown in Tables 9 and 10. Compared with ITP patients treated with glucocorticoid and(or) IVIG only, the therapeutic response rate of all ITP patients after treatment with cyclosporin was higher (p = 0.004), and the recurrence rate within 3 months was lower (p = 0.048). There was a statistically significant increase in therapeutic response rate between the ANA+ITP group (p = 0.017) and the Pro-infected ITP group (p = 0.004) after treatment with cyclosporin compared with the ITP patients treated with glucocorticoid and(or) IVIG only.
| CR | PR | CR+PR (ORR) | Recurrence rate within 3 months | Recurrence rate within 6 months | Incidence of infection during treatment | |
|---|---|---|---|---|---|---|
| Cyclosporin (n = 46) | 54.35% | 23.91%* | 78.26%* | 4% | 16% | 6.52% |
| Glucocorticoid or IVIg only (n = 124) | 49.19% | 4.84% | 54.03% | 21.31%* | 31.15% | 13.71% |
| p Value | 0.550 | 0.000 | 0.004 | 0.048 | 0.150 | 0.196 |
- * p < 0.05.
| Cyclosporin | Glucocorticoid or IVIg only | p Value | Cyclosporin | Glucocorticoid or IVIg only | p Value | |
|---|---|---|---|---|---|---|
| ITP (n = 64) | ANA+ITP (n = 50) | |||||
| CR | 50.00% | 43.59% | 0.716 | 54.55% | 43.33% | 0.524 |
| PR | 20.00%* | 2.56% | 0.040 | 36.36%* | 6.67% | 0.017 |
| CR+PR (ORR) | 70.00% | 46.15% | 0.178 | 90.91%* | 50.00% | 0.017 |
| Multi-autoantibody ITP (n = 87) | Pro-infected ITP (n = 19) | |||||
| CR | 16.67% | 63.83% | 0.373 | 75.00%* | 12.50% | 0.030 |
| PR | 50% | 6.38% | 0.111 | 25.00% | 0% | 0.140 |
| CR+PR (ORR) | 66.67% | 70.21% | 0.919 | 100%* | 12.50% | 0.004 |
- * p < 0.05.
3.8 Therapeutic effect of cyclosporin in patients with different numbers of megakaryocytes
In this study, according to the number of megakaryocytes in bone marrow, ITP patients were divided into three groups: 0–50, 50–200, and more than 200 megakaryocytes. There was no significant difference in response rate by glucocorticoid and(or) IVIG among these three groups. In patients treated with cyclosporin, the therapeutic effect of patients with more than 200 megakaryocytes was relatively higher (p = 0.005), when compared with the patients having <50 megakaryocytes (p = 0.025). The results are shown in Tables 11 and 12.
| Megakaryocytes≥200 (n = 42) | 200>Megakaryocytes ≥50 (n = 29) | Megakaryocytes<50 (n = 37) | |
|---|---|---|---|
| CR | 54.76% | 41.38% | 45.95% |
| PR | 7.14% | 3.45% | 2.70% |
| CR+PR (ORR) | 61.90% | 44.83% | 48.65% |
3.9 Independent and final impact factors in treatment of ITP patients
As shown in Table 13, CD3+CD8+T cells, CD3+CD4+T cells, CD16+CD56+NK cells, CD4/CD8 ratio, and CD19+B cells were the independent impact factors that influence the recurrence of patients who were treated with glucocorticoid and (or) IVIG by single-factor analysis. The impact factors that influence the effect of glucocorticoid and(or) IVIG were the number of CD3+CD8+T lymphocytes, CD4/CD8 cell ratio, and the number of CD19+B lymphocytes. As shown in Table 14, the number of megakaryocytes and CD3+T cells will influence cyclosporin effect by single-factor analysis. The independent impact factor of cyclosporin therapeutic response rate was the number of CD3+T lymphocytes.
| Single-factor analysis | Multiple-factor analysis | |||||
|---|---|---|---|---|---|---|
| p Value (Sig.) | Exp (B) | 95% CI | p Value(Sig.) | Exp (B) | 95% CI | |
| CD3+CD8+ | 0.046 | 0.939 | 0.883–0.999 | 0.049* | 1.158 | 1.001–1.340 |
| CD3+CD4+ | 0.031 | 1.060 | 1.005–1.118 | |||
| CD16+CD56+ | 0.015 | 0.874 | 0.784–0.974 | |||
| CD4/CD8 | 0.003 | 2.172 | 1.296–3.640 | 0.036* | 4.601 | 1.105–19.163 |
| CD19+ | 0.023 | 1.058 | 1.008–1.110 | 0.024* | 1.141 | 1.018–1.279 |
- * p < 0.05.
| Single-factor analysis | Multiple-factor analysis | |||||
|---|---|---|---|---|---|---|
| p Value (Sig.) | Exp (B) | 95% CI | p Value (Sig.) | Exp (B) | 95% CI | |
| Number of megakaryocytes | 0.014 | 1.013 | 1.003–1.024 | |||
| CD3+ | 0.039 | 0.904 | 0.821–0.995 | 0.040* | 0.895 | 0.805–0.995 |
- * p < 0.05.
4 DISCUSSION
ITP is a common hemorrhagic disease characterized by low platelets in peripheral blood. Bone marrow pathology shows that normal or increased number of bone marrow megakaryocytes are accompanied by bone marrow megakaryocyte development and maturation disorders. ITP is clinically based on exclusion diagnosis, and the common causes of ITP are increased platelet destruction or inadequate platelet production mediated by humoral and cellular immunity.8 In terms of aberrant cellular immune regulation, they specifically include the imbalance of Th1/Th2 cells, the abnormal differentiation of Th17 and Treg cells, and the secretion disorder of related cytokines. Moreover, DCs can accelerate the activation of B lymphocytes to produce antiplatelet antibodies, and even further activate CD8+T lymphocytes and NK cells.9 Therefore, the current treatment for ITP patients is mainly immune regulation and inhibition of the autoantibodies formation. In this paper, clinical multi-factor indicators were used to predict the efficacy of different drugs, especially the immunosuppressive agent cyclosporin in ITP patients.
This study included 220 patients. All the patients were divided into four groups according to the expression of autoantibodies and prodromal infection: general ITP group, ANA+ITP group, Multi-autoantibody ITP group, and Pro-infected ITP group. Some studies have found that ITP patients can be accompanied by some autoantibodies such as antinuclear antibodies. But all these patients with autoantibodies could not be diagnosis by a definite autoimmune disease. Especially, ITP patients with single antinuclear antibody positive should be followed up to guard against the final progress of rheumatoid arthritis, systemic lupus erythematosus, thyroid diseases, or autoimmune liver diseases etc.10 In all ITP patients included in this study, the positive rate of total antinuclear antibodies was the highest, exceeding 70%. Anti-RO-52 antibody and anti-SSA antibody are typical autoantibodies in the occurrence of Sjogren's syndrome. This study found that the positive rates of anti-RO-52 antibody and anti-SSA antibody ranked after the positive rates of antinuclear antibodies, and the expressions of anti-RO-52 antibody and anti-SSA antibody were relatively high compared with other autoantibodies.11 In our study, patients in ANA+ITP group and Multi-autoantibody ITP group have not reached the diagnostic level of other autoimmune diseases, so patients in ANA+ITP group and Multi-autoantibody ITP group have certain particularities compared with the general ITP group.
In this study, we found that compared with the general ITP group, many coagulation indexes of pro-infected ITP patients were abnormal, including prothrombin time, International standardized ratio of prothrombin time and thrombin time was higher, and the number of Fibrinogen was lower. Therefore, it is speculated that ITP patients complicated with prodromal infection are relatively few blood clots and are more likely to bleed. Further analysis of some immune indexes showed that compared with the general ITP group, the patients with Multi-autoantibody ITP had lower ALB, higher gamma globulin, and lower albumin/globulin ratio. And the number of immunoglobulin A and immunoglobulin G increased. Previous studies have found that most ITP patients have platelet-specific immunoglobulin G antibodies, which can bind to platelet surface glycoproteins GPⅡB/ⅢA and GPⅠB/Ⅸ, so it can be considered that IgG autoantibodies are the main mediators driving the autoimmune system.12 When the capacity of complement synthesis decreases or the complement consumption increases, the level of complement will decrease. Studies have shown that the decrease of complement synthesis capacity is mainly seen in liver dysfunction, and the increase of complement consumption is mainly seen in systemic lupus erythematosus or other autoimmune diseases.13 In this study, we found that the level of complement C3 and C4 decreased in patients with Multi-autoantibody ITP. Therefore, it is speculated that complement C3 and C4 will be consumed when there were various autoantibody abnormalities.
The aberrant number of lymphocytes is an important immune factor in ITP patients, especially the balance of CD4+ and CD8+ T lymphocyte subsets. During the pathogenesis of ITP, platelet-binding antibodies are produced by B lymphocytes in peripheral blood, spleen, bone marrow, and other sites. CD4+T lymphocytes can mediate the differentiation of subsequent B lymphocytes and the secretion of autoantibodies. Even the depletion of B lymphocytes can have an effect on T lymphocyte subsets.14, 15 In this study, we try to collect T and B lymphocyte subsets, NK cell, and DC subsets of peripheral blood in different groups of ITP patients. We found the proportion of total CD19+B lymphocytes in ANA+ITP patients and Multi-autoantibody ITP patients increased significantly than in general ITP group. Complement hypercatabolism and(or) production disorder may also occur in ITP like in SLE patients.
Th1/Th2/Th17 cytokines secreted by T lymphocytes form a regulatory network in the immune microenvironment of ITP patients.16 Previous studies have found that a variety of cytokines or chemokines, such as IL-1, IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α, TGF-β, and IFN-γ, are all related to the pathogenesis of ITP patients. All these cytokines could indirectly reflect the function of immune response and inflammation status in autoimmune diseases.17 Our data showed the expression of IL-6, which always highly expresses in patients with autoimmune diseases. In ANA+ITP patients and Multi-autoantibody ITP patients, the level of IL-6 was significantly higher than that in general ITP patients. Indicating the ITP patients in ANA+ITP and Multi-autoantibody groups was in high inflammation status.
Some other immune cells such as NK cells and DCs may play a regulatory role in ITP. Studies have found that NK cells are activated in peripheral blood of ITP patients; in addition, granular enzyme B, perforin, and FasL secreted by NK cells are highly expressed in ITP patients. DCs can present and provide platelet antigens to CD4+T lymphocytes, so as to help promote the differentiation of B lymphocytes.18, 19 mDC cells further promote the maturation and differentiation of Th1 cells, while pDC cells mainly promote the transformation of Th0 cells to Th2 cells. Studies have found that pDC has a weaker ability to capture antigens than mDC, but can induce innate immune responses. Moreover, pDC could induce the differentiation and activation of Treg, thus directly or indirectly inhibit a variety of helper T cells.20 CD16-mDC in mDC can secrete TNF-α factor, and the combination of TNF-α and TLR2 ligand can differentiate CD16-mDC into mature DCs with high antigen-presenting ability.21 The flow cytometry of NK cell and DCs subsets in our ITP patients was further analyzed. It was found that CD16+CD56+ cells, pDC/DC ratio, and pDC/mDC ratio were increased in ANA+ITP patients, suggesting that pDC and NK cells also participate in the process of ITP patients especially in ANA+ITP patients. We also test subgroups of mDCs, but did not find differences among four ITP groups.
Cyclosporin is a widely used immunosuppressant, which can directly inhibit the formation of antigen-antibody complex, increase the number of peripheral blood platelet, and reduce the destruction of platelets. It can also inhibit the activities of Th cells and CD8+T lymphocytes.22, 23 Our results showed that the therapeutic response rate was increased and the recurrence rate was decreased within 3 months after being treated with cyclosporin in all ITP patients. Especially in ANA+ITP group and the Pro-infected ITP group, the therapeutic response rate by cyclosporin is much higher than those treated only with glucocorticoid and(or) IVIG only. Moreover, the therapeutic effect of patients with high megakaryocyte number was significantly higher than that of patients with low megakaryocyte number. CD3+ T lymphocytes indicate that cyclosporine has a good therapeutic effect. Therefore, cyclosporin therapy can be considered for patients in ANA+ITP group and the Pro-infected ITP group, especially for patients with increased CD3+T lymphocytes.
In conclusion, ITP is a heterogeneous disease, recurrence may occur during or rapidly after treatment. Cyclosporine included treatment can improve the effective rate of ITP and reduce the relapse rate within 3 months. The number of CD3+T lymphocytes was the only impact factor that influences the therapeutic effect of cyclosporin in ITP patients.
ACKNOWLEDGEMENT
This work was funded by the National Natural Science Foundation of China (81600093, 81770118, 81770110, 81870101, 81800120, 81800119, 81970116, 81970115), and the Natural Science Foundation of Tianjin (18ZXDBSY00140, 18JCYBJC91700, 17JCQNJC11500).
CONFLICT OF INTEREST
The manuscript has been submitted solely to this journal and is not published, in the press, or submitted elsewhere and confirmed that all the research meets the ethical guidelines, including adherence to the legal requirements of the study country. This study is a clinical retrospective study, and no ethical approval is required. There is no conflict of interest among the eight authors of the article entitled “Immunological characteristics and effect of cyclosporine in patients with immune thrombocytopenia”: Ting Wang, Xin He, Ningyuan Ran, Chunyan Liu, Limin Xing, Huaquan Wang, Rong Fu, Zonghong Shao.
Open Research
Data Availability Statement
The data used to support the findings of this study are included within the article. And the raw data used to support the findings of this study are available from the first author upon request.




