Neuroprotective activities of bacopa, lycopene, astaxanthin, and vitamin B12 combination on oxidative stress-dependent neuronal death
Abstract
Oxidative stress is considered the common effector of the cascade of degenerative events in many neurological conditions. Thus, in this paper we tested different nutraceuticals in H2O2 in vitro model to understand if could represent an adjuvant treatment for neurological diseases. In this study, nutraceuticals bacopa, lycopene, astaxanthin, and vitamin B12 were used alone or in combination in human neuronal differentiated SH-SY5Y cells upon hydrogen peroxide-induced injury and neuroprotective, neuronal death pathways were analyzed. The nutraceuticals analyzed were able to protect H2O2 cytotoxic effects, through increasing cell viability and proteins involved in neuroprotection pathways and restoring proteins involved in cell death pathways. On this basis, it is possible to propose the use of these compounds as dietary supplement for the prevention or as adjuvant to the only symptomatic treatments so far available for neurodegenerative diseases.
1 INTRODUCTION
Oxidative stress is considered the common effector of the cascade of degenerative events in many neurological syndromes.1 The oxidative stress led to altered mitochondrial function and neuronal cell death, characteristic of age-related cognitive impairment, including Alzheimer's disease (AD) and Parkinson's disease (PD). Neurodegeneration underlying mechanisms continue to be unknown and there is still a lack of effective therapies. Recently, it has been recognized lycopene, a potent antioxidant in tomatoes, flavonoids and vitamins as beneficial compounds in the amelioration of human neurological disorders.2 Several studies reported that diet and antioxidants control the incidence of neurological disorders and cognitive impairment.3, 4
Lycopene, the lipid-soluble carotenoid present in tomatoes and red fruits,5 has various biological functions, including dampening the inflammation and suppressing carcinogenesis and tumors growth.6 Increasing studies showed that lycopene significantly counteracts brain damage. A population follow-up study reported that lycopene is able to decrease stroke risk in male.7 Furthermore, in rat models lycopene treatment prevented brain lesions caused by ischemia and reperfusion,8 and improved cognition decline induced by colchicine and rotenone.9 Lycopene counteracted neuronal death induced by neurotoxic agents, such as amyloid β, 1-methyl-4-phenylpyridinium (MPP+), 6-hydroxydopamine, trimethyltin, and methylmercury.8 Lycopene solubilized in 10% water and using 0.5 to 5 µM range concentrations, no cytotoxic effects were detected.10
Astaxanthin is a xanthophyll carotenoid which is present in different marine animals and microorganisms, including salmon, trout, algae, yeast, shrimp krill, and crayfish.11 It is a red fat-soluble pigment with stronger biological activity respect to carotenoids.11
A previous study reported that astaxanthin (10 µM) protected glioblastoma cell line U937 from H2O2-induced cytotoxicity reducing the secretion of proinflammatory cytokines.12
Bacopa monnieri is a creeping herb widely studied for its therapeutic and pharmacological activities. Its ethanol extract is composed of a mixture of triterpenoid saponins defined as bacosides A and B.13 A study showed that bacopa treatment in a MPP+ in vitro model counteracted MPP+-induced toxicity. Bacopa alone did not produce any cytotoxicity not even to the highest concentration assessed (100 μg/mL).14 In vitro studies using Bacopa monnieri have shown that it inhibits free radical formation and DNA damage in a dose-dependent way.15
Vitamin B12, also called cobalamin, is a water-soluble vitamin with a pivotal role in brain and nervous system performances, and the formation of red blood cells.16 Serum levels in the subclinical low-normal range (<250 ρmol/L) are related with vascular dementia, AD, and PD.17
Vitamin B12 is a coumarin class of compounds with different physiological and pharmacological effects, comprising antibacterial, antioxidant, anti-inflammatory.18 In a previous study the neuroprotective activity of vitamin B12 versus H2O2-induced neuronal cell injury in SH-SY5Y cells undifferentiated was shown19. This cell line was pretreated with different doses (0.2-200 μM) of vitamin B12 for 12 hours, followed by exposure to 200 μM of H2O2 for another 24 hours.
In this study the natural antioxidants, described above, were used, alone or in combination, to evaluate their ability to prevent or modulate the severity of neuronal death, following oxidative stress.
2 MATERIALS AND METHODS
As in vitro model, the human neuroblastoma cell line SH-SY5Y differentiated for 7 days in vitro with N2 supplement (Thermo Fisher Scientific) was used (differentiated towards cholinergic phenotype). It is commonly used as human neuronal model and to study various neurodegenerative diseases and aging.20, 21
Cells were plated at 1 × 104 cells/cm2 and maintained for 7 days in fetal bovine serum (FBS)-free Roswell Park Memorial Institute 1640 (RPMI 1640) medium containing the differentiating supplement N2 to induce neuronal differentiation.
The cytoprotective effects of the different antioxidants after H2O2-induced oxidative stress were evaluated. After a H2O2 concentration curve study, the condition 35 µM H2O2 for 2 hours was selected, to obtain at least 40% of cell death.
Subsequently, the differentiated SH-SY5Y cells were treated for 24 hours with the four antioxidants administered in single treatment or in different combinations utilizing the compounds at different concentrations.
The antioxidants were prepared diluting the powder in FBS-free RPMI 1640 and two concentrations for each antioxidant were selected, according the existing literature. In particular, the concentrations used for the carotenoid lycopene were 2 and 4 µM, for bacopa 3 and 6 µg/mL were selected, for astaxanthin 12.5 and 25 µM, and for vitamin B12 0.3 and 0.6 µM.
Antioxidants provided by “Menarini Industrie Farmaceutiche Riunite” were lycopene, C40H56, CAS No. 502-65-8; Bacopa monnieri extract, CAS No. 93164-89-7; astaxanthin, C40H52O4, CAS No. 472-61-7; vitamin B12 (cobalamin) C63H88CoN14O14P, CAS No. 68-19-9.
2.1 Immunofluorescence
SH-SY5Y were seeded at 10 000 cells/cm2 and differentiated using N2 supplement (Thermo Fisher Scientific) for 7 days in vitro and then assessed the expression of βIII tubulin, to evaluate the neuronal differentiation and neuronal identity. For the immunofluorescence technique was performed as previously described22 and as primary antibody rabbit βIII tubulin (Abcam, UK) 1:500 was used.
2.2 Cell viability
Control and treated cells were seeded on 96 multiwells 10 000 cells/cm2 differentiated with N2 supplement. Upon different treatments, cells were incubated with CellTiter Solution for 30 minutes. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay is a colorimetric method based on the quantity of formazan produced, as a function of viability, detected at absorbance 490 nm using an enzyme-linked immunosorbent assay plate reader. This assay was performed in triplicate.
2.3 Western blot analysis
Treated and control cells were collected and lysated in ice-cold radioimmunoprecipitation assay buffer as previously described.23 Protein lysates (30 μg) were run on 8% to 20% sodium dodecyl sulfate-page gel and blotted onto a polyvinyl difluoride membranes (Sigma-Aldrich), then were blocked with 5% nonfat milk (blocking solution; Bio-Rad Laboratories) in Tris buffered saline for 1 hour at room temperature and then incubated at 4°C overnight with the subsequent primary antibodies diluted in the same blocking solution: rabbit horseradish peroxidase-conjugated actin (1:10 000; Cell Signaling Technology, Massachusetts); rabbit brain-derived neurotrophic factor (BDNF) (1:500; Abcam, UK); rabbit p-TrkB (1:1000; Cell Signaling Technology); rabbit TrkB (1:200; Santa Cruz); mouse p-Erk1,2 (1:200; Santa Cruz); rabbit Erk1,2 (1:200; Santa Cruz); rabbit P75 (1:1000; Abcam, UK); rabbit p-ERK5 (1:1000; Cell Signaling Technology); rabbit extracellular-signal-regulated kinase 5 (ERK5) (1:1000; Cell Signaling Technology); rabbit PSD95 1:1000 (Cell Signaling Technology), rabbit p-synapsinI (Ser603) (1:500; Novus Biological), synapsinI (1:500; Invitrogen); cleav caspase 9 and 3 (1:500; Cell Signaling Technology), rabbit catalase (1:10 000; Rockland), and rabbit manganese superoxide dismutase (MnSOD) (1:500; Sigma-Aldrich).
After different washes, the membranes were incubated with secondary antibodies, peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin E (1:30 000; Thermo Fisher Scientific) were used. Immunoreactive bands were detected using luminol (Bio-Rad Laboratories) and immunoreactive bands were assessed using UVtec software and lamp and analyzed using the ImageJ software and as housekeeping actin was used. Values were reported as relative units. A representative figure for each protein assayed in the main figure is shown, while the other replicates in Supplementary Figures are provided.
2.4 Statistical analyses
For statistical analyses, samples were analyzed through Graphpad 8 software using t test. The experiments were run in triplicates (n = 3). Data were expressed as mean ± standard errors. P < .05 were considered statistically significant.
3 RESULTS
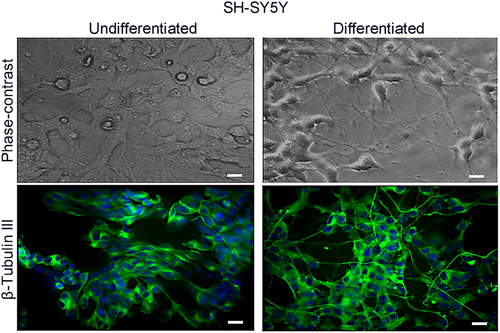
Neuroblastoma cell differentiation was evaluated by immunofluorescence for βIII tubulin, its expression correlates with the earliest phases of neuronal differentiation and as a marker of positive neuronal identity (Figure 1).
Cell viability, assessed by MTS assay, in treated and control cells in Figure 2 is reported. When compared with the control group, cells exposed for 2 hours to H2O2 at the concentrations of 35 µM showed a viability significantly decreased, to about 50% (Figure 2A). The single treatment with each compound, improved cell survival, more significantly with the higher concentration used. Particularly, lycopene 4 µM, astaxanthin 25 µM, and the vitamin B12 0.6 µM almost restored cell viability to the control values (Figure 2A).
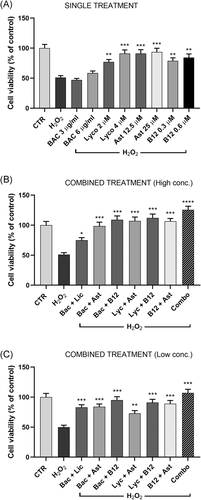
The combination of two compounds in couple or all antioxidants together enhanced cell survival, especially at the higher concentrations. In addition, the combo treatment showed viability levels higher than those of control cells (Figure 2B,C).
On the basis of these results, we analyzed BDNF/TrkB survival pathway in neuronal cells upon the combination of the four antioxidants (Figure 3). Interestingly, we noted that this combination was able to rescue the cells from H2O2-induced oxidative stress, increasing BDNF, the phosphorylated (active) form of TrkB and p-ERK5 as well.
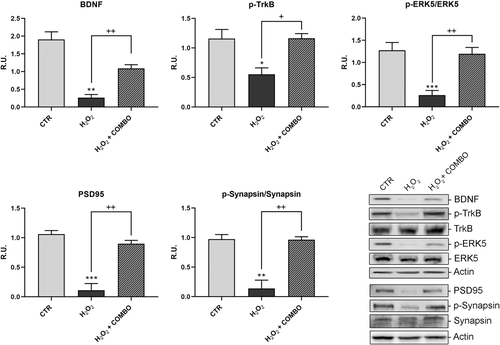
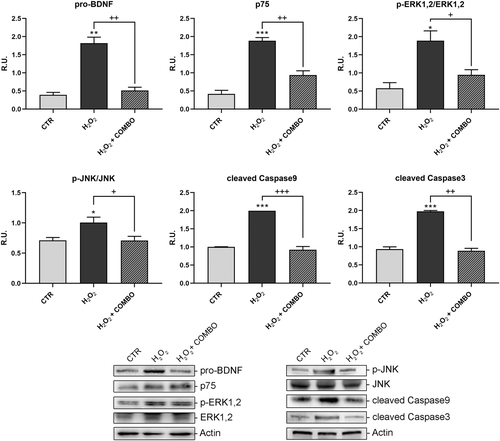
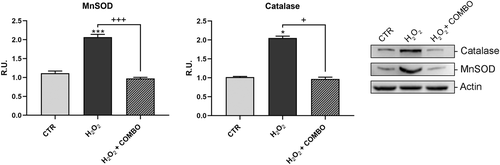
Moreover, the treatment was able to restore to control values the proteins involved in synaptic plasticity and morphology, PSD95 and synapsin (Figure 3). Afterwards, the neuronal death pathway was assessed, upon the same treatment, by analyzing pro-BDNF, P75, p-ERK1,2, p-JNK, and cleaved caspases 9 and 3 (Figure 4). As shown in Figure 4, the level of these proteins was significantly increased upon pro-oxidant treatment while the combined treatment was able to revert this effect. On this basis, the reactive oxygen species (ROS) scavenger enzymes, catalase, and MnSOD were also assayed (Figure 5). In agreement with the previous results, the oxidative challenge significantly increased the scavenger proteins, while the presence of combo treatment restored the control levels.
4 DISCUSSION AND CONCLUSION
Dementia and cognitive impairment impact on life span and life quality of the elderly. Not only neuronal dementia, which include AD, PD, and Huntington's disease, but also vascular dementia affect aging populations with progressive memory decline, motor impairment, or progressive inability.24 Oxidative stress exerts a crucial role in the pathophysiology of dementia.25 It occurs when an unbalance is developed between defensive cellular antioxidant activity and ROS production. In human neurodegenerative disease, this balance is altered and the oxidative damage increase, triggering tissue injuries.26
BDNF has a crucial role in neuronal cells survival, neural integrity and synaptic plasticity.27 Alterations in BDNF levels or activity lead to impaired development, neuronal plasticity and connectivity, leading to age-related disorders and neurodegeneration.28 Moreover, BDNF is impaired by oxidative stress and this relation is fundamental for several symptoms of neurodegenerative and neuropsychiatric diseases. BDNF is also involved in stress-related disorders and depression.29, 30
Age-related disorders gained a lot of interest due to their lack of effective treatment, irreversibility, and accompanied social and economic impacts. To improve aging and age-related disorders, the search for anti-ageing drugs has received great interest.
Recently, emerging evidence indicated that dietary antioxidants, in particular polyphenols, may exert beneficial effects on the brain, protecting neurons against oxidative stress-induced injury, reducing cardiovascular risk factor, control and suppressing neuroinflammation.22 The regular inclusion of antioxidants in the diet is really important to maintain the brain health, but it could be difficult especially in elderly. To this purpose, antioxidants dietary supplement may represent an effective strategy to counteract cognitive age-related decline.
Notably, regarding the nutraceuticals tested, it has been already demonstrated a potential involvement in ameliorating cognitive impairment and synaptic plasticity. Indeed, in diabetic rats, chronic treatment with lycopene reduced cognitive decline, ameliorating spatial version of the Morris water maze test.31 While, in a recent investigation, astaxanthin treatment was able to ameliorate synaptic plasticity, cognitive function and behavioral tests in young and aged mice,32 indicating astaxanthin as potential coadjuvant for age-related disorders.
Bacopa monnieri supplementation enhanced synaptic plasticity, in particular, animal studies reported that bacopa is able to upregulate calcium dependent kinases in the synapse and post-synaptic cell, critical for improving neuronal connections.33 Moreover, Bacopa monnieri extract administered in Wistar rats by oral gavage ameliorated the escape latency time in Morris water maze test. Further, neurons decline, and cholinergic neuron densities were also diminished.33
During aging, it has been found decreased vitamin B12 that may contribute to neurological disorders,34 thus, B-vitamins could be used in counteracting cognitive impairment and decreasing the risk of depression.35
Supporting by the literature, our in vitro study indicated that the nutraceuticals analyzed were able to protect human neuronal differentiated SH-SY5Y cells against hydrogen peroxide-induced injury, counteracting its cytotoxic effects. In fact, it has been reported that H2O2 treatment decreased the viability of SH-SY5Y cells, induced apoptosis, and decreased superoxide dismutase activity.20, 21
The effects of the combo treatment were not merely antioxidant, but their presence upregulated the expression of neurotrophic pathways while decreasing death pathways. This implies that the combined treatment restored in neuronal-like cells the correct oxidative balance, by upregulating ROS scavenger enzymes and, probably as a consequence, reducing cell death execution and improving cell survival. It is worth noting that in neurodegeneration, an impairment of BDNF occurs, paralleled by cognitive decline and decrease of synaptic plasticity and memory loss. In this context, is important to underline that the combo treatment was able to increase BDNF as well as proteins involved in synaptic plasticity and in the maintenance of synaptic morphology. On this basis, it is possible to propose the use of these compounds as adjuvant agent to the only symptomatic treatments so far available for neurodegenerative diseases.
CONFLICT OF INTERESTS
Francesco Melani is an employee of Menarini Industrie Farmaceutiche Riunite, Italy. The company has interests in the development of antioxidant combinations as dietary supplement. The other authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
GD, AC, CF, and VC conceived and designed the study. VC performed and analyzed the in vitro experiments. MdA analyzed the experiments. EB verified the analytical methods. MC contributed to in vitro sample preparation. MdA prepared all the figures of the manuscript. VC and AC wrote the manuscript. MdA, CF, GD and GD reviewed the manuscript, providing critiques of the study. AC, AG, and CF supervised the project. All authors reviewed the results and impacted on the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The datasets analyzed during the present study are available from the corresponding author on reasonable request.