microRNA-320a prevent Müller cells from hypoxia injury by targeting aquaporin-4
[Figure 2 was updated September 23, 2020, after online publication of this article]
Abstract
Müller cells are closely related to diabetic retinopathy (DR). Aquaporin-4 (AQP4) can effectively promote the diffusion of water across cellular membranes. However, the dynamic balance of water plays key role in many diseases, such as cerebral edema. Meanwhile, the unusual expression and distribution of AQP4 in the retina are the significant causes of ocular hypertension and reperfusion injury. To explore the functional significance between microRNA-320a (miR-320a) and AQP4 in pathological hypoxia-induced DR related retinal edema, we hypothesized that miR-320a regulates AQP4 expression and internalization to relieve the edema of Müller cells under the pathological retinal hypoxia stress by targeting AQP4, thereby attenuate the damage of Müller cells. Results demonstrated that miR-320a mimics inhibited the expressions of AQP4 in Müller cells. Furthermore, overexpression miR-320a protected Müller cells by suppressing superoxide anion. In addition, overexpression miR-320a markedly attenuated hypoxia-induced injury, significantly increased the cell viability, and promoted the internalization of AQP4. Furthermore, miR-320a can also regulate the stable anchoring of AQP4 on the cell membrane. Our study indicated that miR-320a may be a potential modulator which can mediate AQP4 expression and attenuate the hypoxia damage of Müller cells. In conclusion, miR-320a may be a potential target for DR therapy by targeting AQP4.
1 INTRODUCTION
Diabetic retinopathy (DR) is the most common complication of patients with diabetic in the eye and the greatest threat to visual health. With the increase in the incidence of diabetes and the prolongation of the survival of patients with diabetic, the incidence of DR and other eye diseases secondary to DR is also increasing.1 DR is the first blinding disease in the working-age population around the world.2 The typical fundus lesions of DR are retinal microangioma, “flaky exudation,” neovascularization, vitreous hemorrhage, and even retinal detachment. It can be divided into nonproliferative and proliferative DR. In nonproliferative DR, small capillaries rupture and leakage in the retina. Proliferative DR is the development of nonproliferative DR, which has new blood vessels, can easily cause the detachment of proliferative membranes and even retina. Moreover, proliferative DR is more harmful to vision, which can lead to serious vision loss or even complete blindness.1
As a glial cell that plays a leading role in the retina, Müller cells play a vital role in creating and maintaining neural retinal structures, supporting retinal neuron survival and information processing. Meanwhile, Müller cells can differentiate into retinal neurons by proliferation to promote retinal repair when the retina is damaged.3, 4 Loss of retinal function and death of neurons in diabetes can be attributed to the reactivity changes in Müller.5 Moreover, Müller cells are also involved in many eye diseases. Seriously, Müller cells are sensitive to hypoxia, and persistent hypoxia can lead to swelling and death.6 Aquaporin (AQP) is a family of transmembrane proteins composed of six transmembrane monopeptide chains, which regulating water transport in vivo.7 AQP4 is the main AQP in the brain and retina, located in the membrane of glia cells, including astrocytes and granulocytes, promoting the water along the gradients of hydrostatic and osmotic pressures from high to low.8 AQP4 maintains the balance of water, electrolytes, and osmotic pressure in the normal retina and regulates the intraocular pressure, and the misplacement of which is associated with the retinal edema caused by various diseases and glial swelling.9 Studies have shown that hypoxia, high intraocular pressure, and reperfusion injury can induce the abnormal expression of AQP4 in Müller cells, resulting in the swelling of Müller cells.10 Furthermore, the location of AQP4 in the retina can be changed by high intraocular pressure or strong light irradiation. In addition, AQP4 can be internalized and eventually degraded by lysosomes.11 Studies have shown that Agrin facilitated the aggregation of AQP4 to form orthogonal aggregate particles (OAPs), thereby enhancing the water transfer capacity ability.12, 13 Laminin, Dp71, syntrophin, and dyntrophin are involved in the clustering of the AQP4 water channel on the astrocyte glial membranes.14
micro-RNAs (miRNAs) have the function of inhibiting the transcription and translation of the target messenger RNAs (mRNAs) or splicing the target mRNAs to promote its degradation, which plays important roles in the regulating developmental processes.15 The literature indicated that miRNAs were involved in the regulation of retinopathy.16 There is increasing evidence that miRNAs play important roles in the regulation of diabetes and its complications, which regulate DR through a variety of closely related biological pathways.17 Therefore, miRNAs have potential application prospects in the prognosis, diagnosis, and treatment of retinopathy. However, the functional significance of between miR-320a and AQP4 in pathological hypoxia-induced DR related retinal edema has not been elucidated.
In the present study, we hypothesized that miR-320a could regulate AQP4 expression and internalization to relieve the edema of Müller cells under the pathological retinal hypoxia stress by targeting AQP4, thereby attenuate the damage of Müller cells. We first explored the expression of miR-320a and AQP4 in Müller cells under hypoxia as well as normal ones, was significantly reduced, while the expression of AQP4 was enhanced in hypoxia-induced Müller cells. Moreover, we investigated the effects of miR-320a overexpression on intracellular superoxide anion and cell proliferation under hypoxia. Meanwhile, we found that miR-320a could regulate AQP4 expression in hypoxia conditions. Furthermore, we sought to determine the effects of miR-320a overexpression or inhibition, and AQP4 knockdown and AQP4 internalization and cell physiological status under hypoxia. Moreover, miR-320a mimics increased the protein expressions of Dp71, α-dystroglycan (DG), β-DG and laminin, while decreased the expressions of α-syntrophin, β1-syntrophin, and Agrin compared to the ones under hypoxia. Meanwhile, the effects of miR-320a mimics on those proteins were suppressed by its inhibitor, but promoted by AQP4 small interfering RNA (siRNA). The results demonstrated that miR-320a may be a potential therapeutic target for DR by targeting AQP4.
2 MATERIALS AND METHODS
2.1 Primary culture of müller cells
According to the method described in the previous study,18 Isolated and cultured Müller cells from Sprague-Dawley (SD) rats. SD rats pups at postnatal 4 to 7 days, and their eyes were removed. Then, the retinas were isolated with care to avoid contamination from the RPE and the ciliary epithelium. They were cut into pieces of about 1 mm2 and cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Thermo Fisher Scientific, CN) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific) in a humidified environment of 5% CO2 at 37°C for 3 to 4 days. The retinal aggregates were washed by severe rinsing, and the cells were maintained by 90% DMEM/F12 and 10% FBS. The squamous cell population attached to the bottom of the culture dish was the purified Müller cells. After the second passage, the cultured cells were identified using the antibodies against the glial fibrillary acidic protein (GFAP) and glutamine synthetase (GS). The third passaged cells were used for the subsequent experiments.
2.2 Prediction of miRNA candidate targets
Databases including PicTar (http://pictar.mdcberlin.de/), miRNATarget2, and miRNAanda (microRNA.org) were used to predict the target genes of miR-320a.
2.3 Luciferase assay
The AQP4 3′-untranslated region (3′-UTR) wild type (WT) and the mutant (Mut) plasmid were constructed using AQP4 plasmid as a vector. miR-320a mimics, inhibitor, and negative control (NC) were obtained from RiboBio Co Ltd (Shanghai, China). The cells were divided into miR-320a NC + AQP4 3′-UTR (WT) group, miR-320a mimics + AQP4 3′-UTR (WT) group, miR-320a inhibitor + AQP4 3′-UTR (WT) group, miR-320a NC + AQP4 3′-UTR (Mut) group, miR-320a mimics + AQP4 3′-UTR (Mut) group, and miR-320a inhibitor + AQP4 3′-UTR (Mut) group. 293T cells which transfected with miR-320a NC, mimics, and inhibitor were seeded into 24-well plates (5 × 104 cell/well) for 24 hours, respectively. After that, the luciferase plasmid containing 50 nM of AQP4 3′-UTR WT or Mut were cotransfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The fluorescence intensity was measured by a fluorescence detector after 48 hour.
2.4 Cell transfection and RNA interference
Müller cells were inoculated with six-well plates (1 × 106 cells/well) for 24 hours. Thirty nanometers of miR-320a inhibitor, mimics, NC, AQP4-siRNA (GenePharma, Shanghai, China) were transfected into Müller cells according to the manufacturer's protocol, respectively. The miR-320a mimics sequence was 5′-AAAAGCUGGGUUGAG AGGGCGA-3′. The miR-320a inhibitor sequence was 5′-CCUCUCAACCCAGCUUUU-3′. The sequence for negative control was 5′-CAGUACUUUUGUGUAGUACAA-3′. The sequence for AQP4-siRNA was 5′-GCAGUUAUCAUGGGAAACUTT-3′. The synthetics were transfected using Lipofectamine, 2000 (Invitrogen). To determine the efficiency of miRNA mimic/inhibitor/AQP4-siRNA, the expressions of miR-320a and AQP4 were assessed by qRT-PCR or Western blot.
2.5 Induction of hypoxia and detection of intracellular superoxide anion
Müller cells (1 × 104 cells per well) were seeded into 24-well cell culture plates. The plates were inoculated in a hypoxic incubator (1% O2, 5% CO2, 94% N2) with a humidified environment of 5% at 37°C for 1, 2, 4, 6, 12 hours, respectively. Dihydrogenin can freely permeate the cells, which can be oxidized to ethidium bromide and inserted into the DNA in the presence of superoxide anions. The superoxide anion was characterized in situ with an oxidative fluorescent dye, dihydroethylene bromide. Added 5 μM of dihydroethidium to Müller cells and incubated for 30 minutes. Detected the fluorescence intensity by Fluorescein Light microscopy (IX71; Olympus Inc, Japan).
2.6 Cell Counting Kit-8 assay
To determine the effects of miR-320a and AQP4 on the proliferation of Müller cells in hypoxia conditions, Cell Counting Kit-8 (CCK-8) assay was performed. miR-320a mimics, miR-320a inhibitor, and AQP4 siRNAs were transfected into cells for 12 hours. Next, cells were challenged with hypoxia for 6 hours. Ten microlitres of CCK-8 reagent was added to each well, and the absorbance was measured at 450 nm. Calculated the cell proliferation rate according to OD values.
2.7 Immunofluorescence labeling
Removed the medium, washed three times with 0.01 mol/L phosphate buffer saline, and fixed in phosphate buffer for 10 minutes with 4% formaldehyde, after Müller cells (5 × 104 cells per well) were seeded into 24-well plate and incubated for 24 hours. It was infiltrated with 0.3% Triton X-100 for 30 minutes and blocked by 5% FBS for 30 minutes at 37°C. The cells were incubated with a mixture of primary antibodies and allowed to stand overnight at 4°C. The antibodies were as follows: AQP4 (1:200; Santa), early endosome antigen1 (EEA1, a early endocytic marker; 1:100; Abcam), mannose-6-phosphate receptor (M6PR, a late endocytic marker; 1:100; Sigma) and lysosome-associated membrane glycoprotein 1 (LAMP1, a lysosomal marker; 1:250; Abcam) at 4°C overnight. Immunofluorescence double-labeling was performed by Dylight488-coupled (1:1000 green), Cy3-coupled (1:1000 red), and Cy5-coupled (1:1000 cyan). Laser confocal scanning to detect its distribution and expression.
2.8 RNA extraction and quantitative real-time polymerase chain reaction
Collected cells and extrated total RNA by TRIzol reagent (Ambion). Total RNA (2 μg) was reverse-transcribed into complementary DNA (cDNA; 20 μL) using the TaqMan Reverse Transcription Reagents Kit (Thermo Fisher Scientific). 0.5 μL of cDNA product was used as a quantitative polymerase chain reaction (PCR) amplification template. β-actin was used as an internal reference. Reaction conditions: pre-denaturation 50°C for 2 minutes, 95°C for 10 minutes; 95°C denaturation for 15 seconds, 60°C annealing and extension for 30 seconds, 40 to 45 cycles. The measurement results were analyzed by 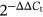 method. The primers used in the qRT-PCR were showed in Table 1.
method. The primers used in the qRT-PCR were showed in Table 1.
| Name | Forward primer(5′-3′) | Reverse primer(5′-3′) |
|---|---|---|
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
| miR-320a | AAAAGCGGGGAGAGGGCG | GCGAGCACAGAATTAATACGACTCAC |
| AQP4 | GGAAGGCATGAGTGACGGA | CAGACGCCTTTGAAAGCCAC |
| Agrin | CATGAACCTGGAGGGCTGG | TATCGAACCAGCATGGAGGC |
| Dp71 | AATGCCCCTGGAAAGCCAAT | TCTGCCCAAATCATCTGCCA |
| α-Dystroglycan | ATCTCCAGCCATTGCACCTC | AATCTGGCCAGGAACTGTGG |
| β-Dystroglycan | CCTCCTCCTACCGGTTCTCAA | CGGGGTGGAGCAACCTAAAA |
- Abbreviations: AQP4, aquaporin-4; miR, microRNA; qRT-PCR, quantitative real-time polymerase chain reaction.
2.9 Western blot analysis
Total protein was extracted and protein concentration was determined by bicinchoninic acidprotein assay kit (Beyotime, Shanghai, China). Protein separation was carried out using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10%, 200 V, 300 mA) for 50 minutes, followed by semi-dry transfection for 45 minutes. Membranes were blocked with skim milk power at room temperature and incubated with the primary antibodies overnight. The primary antibodies used in this study included monoclonal anti-AQP4 (1:500), α-syntrophin (1:1000), α-DG (1:500), β-DG (1:500), Laminin (1:1000), Agrin (1:1000), and hypoxia-inducible factor-1α (HIF-1α; 1:500; Cell Signaling Technology, Beverly, MA), anti-Dp71 (1:500; Proteintech, Chicago, IL), and anti-β-actin (1:2000; Santa Cruz, CA). After washing, the secondary antibody (anti-rabbit or anti-mouse antibody; Cell Signaling Technique, Beverly, MA) was incubated with horseradish peroxidase for 2 hours at room temperature. The membranes were exposed in darkroom, using a Bio-Rad imaging system (Hercules, CA) to capture images and analyzed the gray values.
2.10 Statistical analysis
GraphPad Prism 5.0 and SPSS 13.0 were used for the Student t test and one-way analysis of variance statistical analyses. Data were represented as mean ± SD from at least three separate experiments. At P < .05, the difference was considered significant.
3 RESULTS
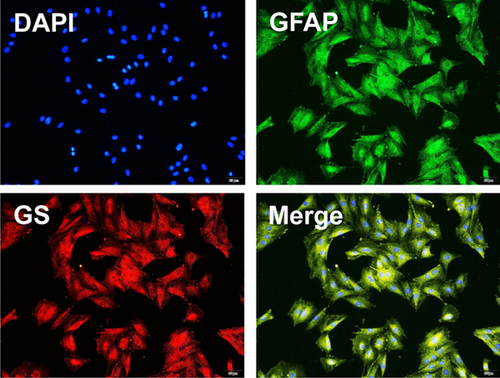
3.1 Identification of müller cells by immunofluorescence
To determine whether the primary cells are Müller cells, we first isolated and cultured primary Müller cells from rat and identified the cells by using the antibodies against GFAP and GS, a specific marker of the Müller cells in the retina. The immunostaining results revealed that most of the primary cells were GFAP- and GS-positive Müller cells (>90%; Figure 1).
3.2 Hypoxia increases the expression of AQP4 and decreases the expression of miR-320a in müller cells
Hypoxia markedly induced the damage of Müller cells in a time-dependent manner (Figure 2A,B). Compared with normal cells, AQP4 expression was gradually increased with the prolongation of hypoxia induction time (Figure 2C). Furthermore, the expression of miR-320a was reduced by hypoixa treatment and the expression pattern of which was contrary to that of AQP4 (Figure 2D). Furthermore, we detected the expressions of HIF-1α mRNA and protein (as shown in Figure 2E-L). The results showed that the expressions of HIF-1α protein and mRNA were increased with the prolongation of hypoxia, indicating that the changes of AQP4 and miR-320a expressions were due to hypoxia. Meanwhile, the results showed that hypoxia for 6 and 12 hours had similar effects on Müller cells. Therefore, Müller cells cultured for 6 hours were selected for further studies.
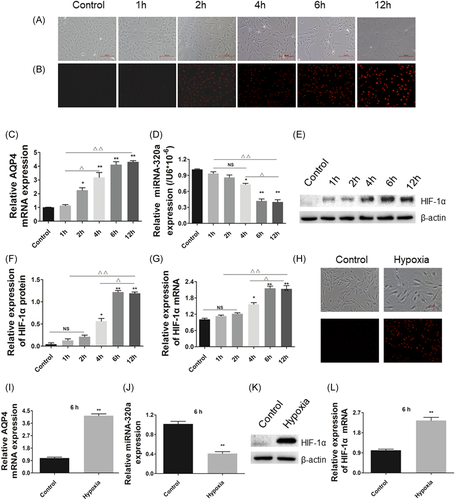
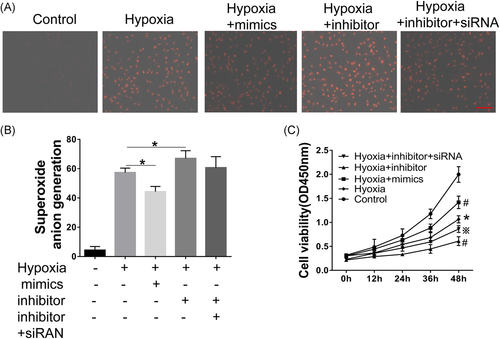
3.3 Overexpression miR-320a and knockdown AQP4 reduces hypoxia-induced superoxide anion and increases the cell viability in müller cells
Superoxide anion probe detection was performed according to the procedure of the Kit's instructions after hypoxia treatment. The results showed that hypoxia increased the production of superoxide anion in a time-dependent manner (Figure 2B). Moreover, miR-320a mimics markedly inhibited the production of superoxide anion induced by hypoxia compared with the control group. Conversely, miR-320a inhibitor significantly enhanced the effect of hypoxia on superoxide anion, while the effects of miR-320a inhibitor can be attenuated by AQP4-siRNA (Figure 3A,B). Moreover, hypoxia caused a significant decrease in cell viability, and as the increasing of induction time, the cell viability gradually decreased (Figure 2A). Cell viability reached a lowest level after hypoxia for 6 hours. In addition, compared with the hypoxia group, the cell viability was significantly increased by miR-320a mimics, but reduced by miR-320a inhibitor, while the effect of miR-320a inhibitor on cell viability can be improved by AQP4-siRNA (Figure 3C). These results indicated that miR-320a can protect the Müller cells against hypoxia, and AQP4 was involved in this process.
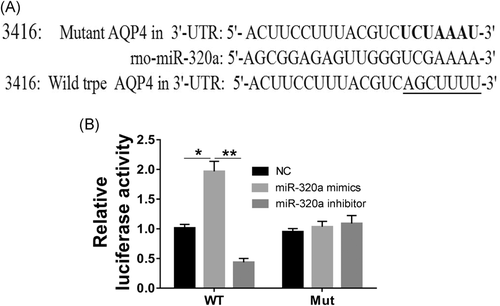
3.4 AQP4 is a target of miR-320a
To further explore the underlying effects and mechanism of miR-320a and AQP4 in Müller cells under the pathological retinal hypoxia stress, target genes of miR-320a were predicted according to miRNA target prediction program PicTar and miRNA. Org. It was found that the 3′-UTR of ADAM10, RAB14, AQP4, vascular endothelial growth factor (VEGF) as well as SND1, and the 5′ end of miR-miR-320a had better binding sites. As shown in Figure 4A, there was a putative miR-320a binding site in the 3′-UTR of the AQP4 gene. Furthermore, compared to the control group, luciferase reporter gene activity was significantly mimicked by miR-320a mimics in the cells which transfected with AQP4 3′-UTR (WT), but was inhibited by miR-320a inhibitor (Figure 4B). Moreover, in cells transfected with AQP4 3′-UTR (Mut), miR-320a mimics and inhibitors had no significant effect on luciferase reporter gene activity. The results indicating that AQP4 is indeed a target gene of miR-320a.
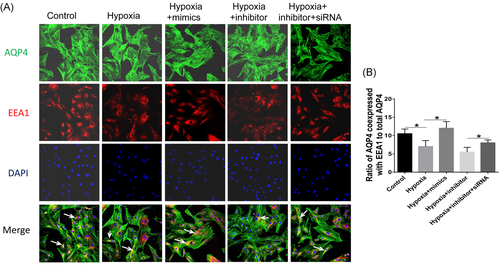
3.5 miR-320a regulates the internalization of AQP4
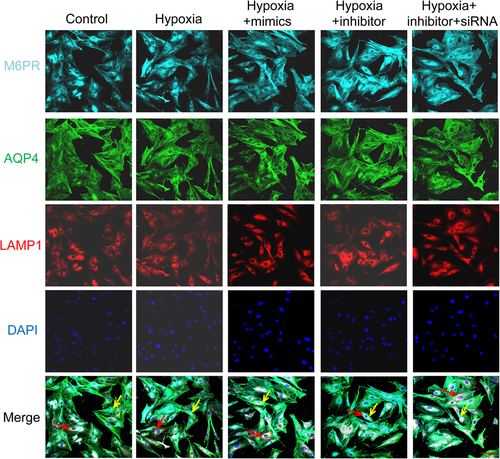
Immunofluorescence results showed that the coexpression of AQP4 and EEA1 markedly decreased by hypoxia and miR-320a inhibitor, but increased by miR-320a mimics. Moreover, the effect of AQP4-siRNA on the coexpression of AQP4 and EEA1 was opposite to that of miR-320a inhibitor (Figure 5A,B). In addition, there was no significant difference in the coexpression of AQP4, M6PR, and LAMP1 between different treatment groups (Figure 6). These results revealed that inhibition of the expression of miR-320a aggravated the internalization of AQP4 and lysosomal degradation induced by hypoxia. Conversely, hypoxia-induced AQP4 internalization and lysosomal degradation can be improved by miR-320a overexpression and AQP4 knockdown.
3.6 Effects of miR-320a on the hypoxia injury
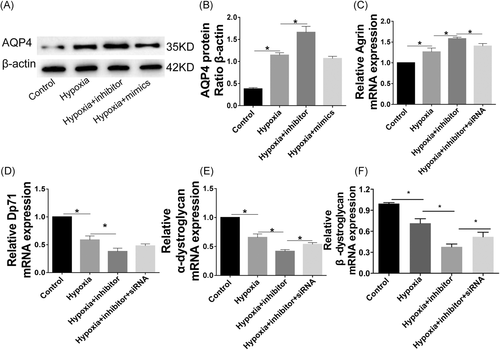
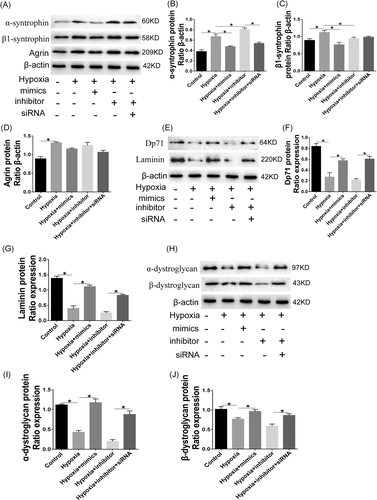
As shown in Figure 7, the expressions of AQP4 protein and Agrin were increased, while the mRNA expressions of Dp71, α-DG, and β-DG were decreased under hypoxia condition. Meanwhile, the effects of hypoxia on those factors were significantly exacerbated by miR-320a inhibitor. The knockdown of AQP4 reversed the effects of miR-320a inhibitor on cells. Furthermore, hypoxia markedly increased the protein expressions of α-syntrophin, β1-syntrophin, and Agrin, while significantly reduced the protein expressions of Dp71, α-DG, β-DG and laminin, which can be attenuated by miR-320a mimics. Meanwhile, the effects of hypoxia on cells were aggravated by miR-320a inhibitor but were promoted by AQP4-siRNA (Figure 8).
4 DISCUSSION
DR is a common and serious diabetic microvascular complication. Statistics have shown that the incidence of DR in patients with diabetes was as high as 70%.19 Müller cells perform multiple functions in the retina, including energy and nutrient metabolism, as well as visual information processing. Moreover, Müller cells are also involved in the metabolism of retinal neurotransmitters, maintaining the stability of water, ionic environment, and pH in the retina.20, 21 Nevertheless, the blood supply to retina comes from double circulation. The inner retinal tissue receives blood supply from the central retinal artery, which is sensitive to hypoxia. In the present study, hypoxic treatment markedly inhibited the viability of Müller cells in a time-dependent manner.
The occurrence of retinal edema is not only related to the breakdown of the blood-retinal barrier, leading to the choroidal tissue fluid and intravascular fluid or leakage into the extracellular space of the retina, but also to the clearance of intraretinal water.22 In the development of some retinal diseases, because of the ischemia and hypoxia of retinal tissue, the disorder of cell energy metabolism, the ion pump failure to maintain the ion gradient inside and outside the cell membrane, and large quantities of water entering the cells, leading to the increase of intracellular osmotic pressure and edema. However, the Müller cells play an important role in this process. AQP4 is a bidirectional channel protein located on the membrane of Müller cells, which can affect water transport through transmembrane transport.23 Thus, we conclude that the abnormal expression of AQP4 is related to the development of retinal tissue lesions. There is growing evidence that numerous miRNAs were responsible for the lesion of Müller cells.24 Particularly, miR-320a as a negative factor also related to many pathological processes. For instance, miR-320a serves as a novel endogenous regulator of AQP1 and AQP4, contributes to the treatment of cerebral ischemia.15 In this study, we confirmed that AQP4 was a direct target of miR-320a. And the increasing expression of AQP4 induced by hypoxia was negatively regulated by miR-320a. The results were consistent with the previous study.15 In the DR, capillary basement membrane thickening, cell damage, local hypoxia, and platelet accumulation occur. These changes can stimulate the synthesis and secretion of HIF-1α, and further aggravate the damage of the retina, forming a vicious circle. Hypoxia and ischemia can inhibit the degradation of prolylhydroxylase, resulting in the accumulation of HIF-1α.25 In this study, results showed that the expressions of HIF-1α protein and mRNA were increased with the prolongation of hypoxia, indicating that the damage of Müller cells, the changes of AQP4 and miR-320a expressions were caused by hypoxia.
Hypoxia refers to the pathological process that causes abnormal changes in tissue metabolism, function, and morphological structure due to insufficient oxygen supply or oxygen barriers in tissues, which is an important inducer in retinal lesions.26 The main pathological mechanism of DR is retinal ischemia and hypoxia.27 Excessive peroxy anion induced by hypoxia can affect the normal function of cells by damaging the proteins and nucleic acid.28 In our study, we found that miR-320a mimics markedly reduced the production of peroxygen anions induced by hypoxia. It has been reported that hypoxia can increase the endothelial permeability through upregulating the level of VEGF, which leads to retinal tissue edema and other pathological changes.29 In addition, AQP4 expression was significantly increased, while the internalization and lysosomal degradation of which were remarkably reduced in retinal edema tissue compared to the normal ones.30 The membrane-bound proteins including AQP4 can be regulated by internalization which alters subcellular distribution. However, little is known about the redistribution of AQP4 in the Müller cells under hypoxia.
AQP4 internalization means that AQP4 enters the early endosome and metastasizes to the late endosome. Compared to the controls, miR-320a mimics significantly improved the coexpression of the early endosome markers EEA1 with AQP4 caused by hypoxia. The late endosomal M6PR and lysosomal marker LAMP1 also were colocalized with AQP4. The studies suggested that reducing the expression of AQP4, and enhancing the internalization and lysosomal degradation of which may be related to the inhibition effect of miR-320a on retinal edema. Furthermore, AQP4 is membrane protein in cells that can stably anchor to the cell membrane, which is the result of interaction with many other proteins. AQP4 is anchored by syntrophin, an adapter protein in the dystrophin-associated complex.31 Abnormal expression of syntrophin, for instance, α-syntrophin and β1-syntrophin, can result in the mislocalization of AQP4 and lead to a reduction in the AQP4 expression.31 Our results demonstrated that overexpression miR-320a or knockdown AQP4 can promote AQP4 stably anchor in the Müller cells membrane under hypoxia. Studies have shown that Agrin facilitated the aggregation of AQP4 to form OAPs, thereby enhancing the water transfer capacity ability.12, 13 The dystrophin-glycoprotein complex proteins, which are comprised of DG, dystrophin, Dp71, α-dystrobrevin, and α-syntrophin, provide a link between laminin and the cytoskeleton and are critical for the localization of AQP4.32 Moreover, the levels of Dp71, α-DG, β-DG, and Agrin are the physiological markers of normal cells. In the present study, overexpression miR-320a and knockdown AQP4 significantly decreased the expressions of AQP4, α-syntrophin, β1-syntrophin, and Agrin, while markedly promoted the expressions of β-DG, α-DG, laminin, and Dp71. The results suggested that the miR-320a may play an important role in regulating the hypoxia-induced Müller cells injury via targeting AQP4.
5 CONCLUSION
In conclusion, the present study demonstrated the function of miR-320a as a suppressive miRNA in Müller cells edema, at least partially through the downregulation of AQP4, which in turn regulates the hypoxia-induced Müller cells edema.
ACKNOWLEDGMENTS
This study was supported by the Joint project of Yunnan Science and Technology Department- Kunming Medical University Applied Fundamental Research [2017FE468 (-119)], the Applied Basic Research Key Project of Yunnan Provincial Fundus Disease Research Center (2016NS239), Science and Technology Talents and Platform Project (Academician Workstation of Zhengqin Yin, 2017IC021), National Natural Science Foundation of China (81560496). Furthermore, we are especially grateful to Dr. SZ for his reviewing comments and technical support in statistical analysis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.