Mitochondria could be a potential key mediator linking the intestinal microbiota to depression
Abstract
The intestinal microbiota has been reported to affect depression, a common mental condition with severe health-related consequences. However, what mediates the effect of the intestinal microbiota on depression has not been well elucidated. We summarize the roles of the mitochondria in eliciting beneficial effects on the gut microbiota to ameliorate symptoms of depression. It is well known that mitochondria play a key role in depression. An important pathogenic factor, namely inflammatory response, may adversely impact mitochondrial functionality to maintain cellular homeostasis. Dysfunction of mitochondria not only affects neuronal function but also reduces neuron cell numbers. We posit that the intestinal microbiota could affect neuronal mitochondrial function through short-chain fatty acids such as butyrate. Brain inflammatory processes could also be affected through the modulation of gut permeability and blood lipopolysaccharide levels. Aberrant mitochondria functionality coupled to adverse cellular homeostasis could be a key mediator for the effect of the intestinal microbiota on the progression of depression.
1 INTRODUCTION
Depression is a common mental disorder with severe health-related consequences and loss in quality of life. Although mild depression can be treated with various medicines, major depressive disorder (MDD) is difficult to treat. MDD symptoms include low mood, anhedonia, decreased energy, altered appetite, weight loss, irritability, sleep disturbances, and cognitive deficits.1 The severity of MDD is caused by physical illnesses such as cardiovascular disorders, stroke, metabolic syndromes, and psychiatric conditions such as anxiety.2-4 The first line pharmacotherapy for MDD include selective serotonin reuptake inhibitors (SSRIs) with a patient response rate of approximately 30%.5 Additional pharmacotherapy prescriptions include anti-psychiatric agents that can produce severe side-effects.6, 7 Therefore, there is an urgent requisite for additional effective therapeutic options for the treatment of MDD.
The intestinal microbiota is now recognized to be associated with depressive symptoms and manipulation of the intestinal microbiota may provide novel approaches for the effective treatment of MDD.8, 9 Certain commensal bacteria have been demonstrated to play an important role in the alleviation of depressive symptoms while intestinal dysbiosis has been reported to exacerbate MDD severity and decrease MDD treatment efficacy.10-12 For example, it has been shown that the Bacteroides genus in patients with MDD was increased, while the Lachnospiraceae genus was decreased.8, 13 Investigations with various probiotics have been developed to encourage restoring and or improving the gut microbiota towards an adysbiotic gut to ameliorate symptoms of depression. Emerging evidence with the administration of probiotics has demonstrated a decrease in neuroinflammation, a pathogenic characteristic of depression.5, 14, 15
Further consideration on the mechanisms central to the role that gut microorganisms may have on the pathogenesis of MDD is of significant importance. What is yet to be clarified is the factors that mediate the effects attributed to the intestinal microbiota in MDD. We posit that the mitochondria may be a key mediator, that is, the microbiota may affect mitochondrial functionalities through the elaboration of metabolites and gut permeability and adversely altered mitochondrial function that results in skewed shifts that increase the pathogenesis of MDD (Figure 1). Indeed, bacteria and mitochondria are very closely linked.16 Bacteria embrace the earliest form of independent life and link the metabolic activity in the intestines to the brain and mood disorders. Mitochondria, a genetically functional mosaic and proposed ancestor to an alphaproteobacterium, exhibits an operational inhibitory susceptibility to the antibiotic chloramphenicol.17 This inhibitory action is consistent and teaches that the gut microbiota displays a cross-talk adaptive interaction with the mitochondria.18
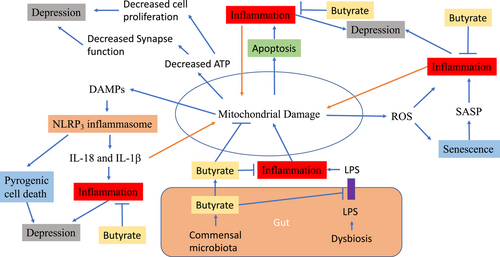
Mitochondrial damage and depression.Mitochondrial damage causes inflammation through several mechanisms. It releases ROS, which results in inflammation directly or by SASP secretion due to senescence. Mitochondrial damage also causes the release of mitochondrial DAMPs, resulting in activation of NLPR3 inflammasome and subsequently pyrogenic cell death and increased production of proinflammatory cytokines IL-18 and IL-1β. Mitochondrial damage also results in apoptosis which also causes inflammation. On the other hand, inflammation can accelerates mitochondrial damage. Butyrate produced by gut microbiota can enter to blood stream and brain to act on neural mitochondria. It can increase ATP production by mitochondria and thus, increase cell proliferation and functions. Butyrate can decrease inflammatory status through various mechanisms. Butyrate can also act on tight junction to reduce gut permeability and thus reduce blood levels of LPS, which causes inflammation. ATP, adenosine triphosphate; DAMPs, damage-associated molecular patterns; IL-18, interleukin 18; IL-1β, interleukin 1β; LPS, lipopolysaccharide; NLPR3, NACHT, LRR, and PYD domains-containing protein 3; SASP, senescence-associated secretory phenotype; ROS, reactive oxygen species
2 CRITICAL ROLE OF MITOCHONDRIA IN DEPRESSION
While the pathogenesis of depression still remains relatively obscure, many explanations have been proposed such as the role of melatonin, increased inflammatory responses, the role of sirtuins, tryptophan catabolites, oxidative, and nitrosative stress.19-21 Mitochondria have been considered to play key roles in these changes in depression.20, 22 Dysfunction of mitochondria has been well documented in various types of depression. The key roles of mitochondria are rationalized by their involvement in several important MDD mechanisms.23-25
The major role of mitochondria is in the provision of energy produced through the electron transport chain (ETC) in cristae formed within the inner mitochondrial membrane (IMM).26 The ETC coupling with ATP-synthase produces ATP through four multimeric protein complexes (I, III, IV, and V) and two membrane-permeable electron carriers via the process of oxidative phosphorylation (OXPHOS). In addition to ATP generation, mitochondria are involved in additional roles in cell physiology that include metabolism of amino acids, fatty acids, and carbohydrates. Mitochondria also elaborate and regulate pro-oxidant signaling system molecules involved in cell survival.27 Therefore, defects in mitochondria can be associated with numerous disease states.28 Mitochondria fine tune intracellular regulation to maintain normal function, which alludes to mitochondrial-cellular quality control. The quality control of mitochondria is achieved by various physiological processes that principally include unfolded protein responses (UPRmt), mitochondrial dynamics, and mitophagy.28
Through mitophagy, unfolded or misfolded proteins are cleaved by proteases and produced peptides are exported to cytoplasma for reuse.28 These peptides activate transcription of the activated transcription factor associated with stress 1 (ATFS-1) gene to produce ATFS-1 proteins that are imported into the mitochondria to stimulate UPRmt. The feed-forward regulation ensures all unfolded and misfolded mitochondrial proteins are cleared so that mitochondrial function can proceed normally.
Mitochondria are also highly dynamic, undergoing continuous fission and fusion.29 Mitochondrial fusion is the merging of two mitochondria to form one functional entity, while mitochondrial fission refers to its division into two.29 Fusion protects the organelles from degradation through inter-organelle content exchange under nutrient and aberrant redox potential changes. A three-step process that includes organelle approximation, mitochondrial outmembrane tethering/fusion, and IMM fusion under control of mitofusion 1, 2 (Mfn1 and Mfn2), and dynamin-related GTPases.30 Fission is increased under nutrient rich conditions as well as with dying cells.31 Fission is under control of dynamin-related protein-1 (Drp-1) and fission 1 protein (fis-1).32-34 Through the processes of fusion and fission, mitochondria undergo segregation or exchange of components and material compounds that facilitates mitochondrial repair following damage.
If damage is severe and an inability to repair by UPRmt ensues, mitochondria undergo autophagy (mitophagy).35 The components of mitochondria are engulfed by autophagosomes and degraded by lysosomes. This process hence avoids precipitating inflammation. Mitophagy is regulated by Pink1 and parkin.35, 36
The number of mitochondria is balanced by mitobiogenesis and mitophagy. Mitobiogenesis is intricately regulated by growth factors and signaling molecules.37 It is increased by nuclear respiratory factors (NRF1 and NRF2), estrogen-related receptors (ERR-α, ERR-β, and ERR-γ), and peroxisome proliferator-activated receptor gamma (PPAR-γ) and PPAR-γ coactivator 1-alpha (PGC-1α). Signaling molecules AMPK, mTOR, and YY1 (YingYang1) can upregulate PGC-1α to increase mitobiogenesis. Therefore, mitobiogenesis is important in maintaining sufficient numbers of mitochondria to sustain cellular homeostasis functions.
Mitochondrial quality control is damaged in depression. As such mitochondria lose the ability to maintain normal functions, leading to decreased ATP production, decreased cell proliferation, and increased cell death as well as increased inflammation. Neurons are one of the cell types that are most sensitive to mitochondrial defects as neurons have and make high energy demands with concomitant low-regenerative capacity.38 The brain's energy source is generated from mitochondrial glucose oxidation to produce ATP.39 The requisite is about 20% of total body basal oxygen.39 Therefore, the brain is highly sensitive to redox potential changes.38, 40 Hence neuronal mitochondrial damage is a key pathogenic change in MDD.
First, dysfunctional mitochondria in MDD are associated with decreased neuronal functions.41 Mitochondria are organelles involved in the generation of cellular energy, which produces ATP for the provision of cellular functions. Furthermore, mitochondria elaborate reactive oxygen species (ROS) that are involved in numerous intracellular and extracellular signaling reactions. Reports show that enteric commensal bacteria can elicit markedly different levels of ROS and that by rapidly generating ROS negotiate an acceptance by the intestinal epithelia.42, 43 Reports demonstrate that microbial redox signaling pathways involving formyl peptide receptors and intestinal epithelial NADPH oxidase 1 originate from microbial ROS productions.44 Hence, mitochondria elaborated redox signaling metabolites support a low level tolerable redox state equilibrium. Furthermore, any damage to mitochondria redox signaling can be causal for somatic cell dysfunction including those neurons in the intestinal mucosa. Dysfunction of neurons is hypothesized to lead to depression and other mood disorders.41
Mitochondria are also associated with decreased neuron cell numbers in MDD.45 Studies have revealed that hippocampal volume is decreased in patients diagnosed with MDD.46, 47 The causal factor of this decrease in volume could be due to impaired cell proliferation and increased cell death (Figure 1). The increase in cellular turnover and death of neurons is a characteristic of MDD. Mitochondrial damage can cause cell death in various forms such as autophagy, apoptosis, and pyrogenic cell death.
If the damage to mitochondria can not be repaired by UPRmt, damaged mitochondria release damage-associated molecular patterns (DAMPs), which may lead to various forms of cell death. Cell autophagy is a benign form of cell death,48 that minimizes the release of proinflammatory molecules into extracellular matrix, avoiding extracellular tissue damage. The organelles of the autophagic cells are engulfed by autophores to form autophagosomes which fuse with lysosomes and the organelles are degraded by lysozymal activity.48
Mitochondria are closely associated with apoptosis, that is, programmed cell death through the mitochondrial apoptotic pathway, which is also known as the intrinsic pathway.49 The pathway is initiated by protein p53, leading to an imbalance of proapoptotic protein Bax and antiapoptotic protein Bcl-2, which triggers cytochrome c leakage and apoptosome formation, leading to apoptosis.50 This is very much in response to an internal (intrinsic) or external (extrinsic) cell death stimulus. Thus, dysregulation of mitochondrial energy metabolism and redox potential preservation in MDD could activate mitochondrial apoptotic pathway, initiating neuronal cell death.27, 50
Pyrogenic cell death is caused by activation of the inflammasome. Mitochondrial damage can lead to an increased release of ROS, oxidized mtDNA, and cardiolipin, resulting in activation of NLRP3 inflammasome.51 Activated NLRP3 inflammasome causes pyrogenic cell death via the pore-forming protein-gasdermin D.51 The released macromolecules from pyrogenic cell death can further damage extracellular tissue.
There are cross-talk among various forms of cell death caused by mitochondrial dysfunction. Activated caspase-1 in NLRP3 inflammasome can cleave Parkin, and thus reduce autophagy (mitophagy).51 Activated caspase-1 can also cleave pro-caspase 3 and 7, and thus promotes apoptosis.51 However, cleavage of caspase-1 by caspase 3 and 7 blocks the NLRP3 inflammasome-caused pyrogenic cell death.
In addition, mitochondrial dysfunction can also cause senescence, which decreases cell proliferation and function. Dysfunctional mitochondria produce high levels of ROS that cannot be controlled, leading to macromolecular damage and cellular senescence.52 It has been reported that telomeres, which protect DNA integrity, are highly sensitive to changes in redox potential triggered injury.53 In senescent cells, it has been reported that cell cycle arrest occurs with increased gene expression of p16, ARF, p21, and p53 and decreased proliferation marker Ki67.54, 55 Particularly, these cells secret proinflammatory cytokines called senescence-associated secretory phenotype (SASP), which can accelerate the cells’ further senescence (autocrine effect) or induce other cells’ senescence (paracrine effect).56-58 Senescence has been identified as an important pathogenic characteristic of MDD.59, 60
Mitochondria can affect synapse activities and instigate abnormal transportation of neurotransmitters.61, 62 Kwon et al63 demonstrated that mitochondria regulated presynaptic calcium homeostasis and thus in turn controlled neurotransmitter release.63 Mitochondria can also affect second messenger signal transduction pathways that follows binding of monoamine (5HT and NE) to their receptors. Energy in the form of ATP is also needed for activation of downstream pathways of 5HT and NE receptors.64
Mitochondria can also promote proinflammatory processes, a characteristic of MDD. The inflammation is well demonstrated in MDD and recently, the inflammasome has been characterized in MDD.65 Mitochondria under cell stress may release their components into the cytoplasm and extracellular matrix to promote inflammation.66 Due to the origin of bacteria, mitochondrial components can be recognized as DAMPs. DAMPs including mtDNAs, ROS, ATP, and cardiolipin from the inner mitochondrial membrane can interact with NLRP3 to activate the inflammasome (Figure 1).67 Activated inflammasome increases the release of proinflammatory cytokines IL-1β and IL-18, which cause depression. van den Biggelaar et al68 found that IL-1β preceded depressive symptoms in investigating aged 267 people who did not have previous psychiatric symptoms.68 Chronic central administration of IL-1β in rats caused depressive-like symptoms.69 Intracerebral infusion of adenovirus associated virus-IL-1β induced p38 MAPK activation and neuronal apoptosis.70 In stressed rats, intracerebral infusion of RNAi against IL-1β ameliorated neuronal apoptosis and depression-like behaviors.70
ROS is well known to cause inflammation. It not only stimulates inflammation directly but also through senescence. Senescent cells release proinflammatory cytokines through SASP as described above (Figure 1). More mechanisms of mitochondrial-caused inflammation have been elucidated. A recent study revealed that exogenous ATP released from damaged mitochondria can activate purinergic receptor to promote inflammation.22
On the other hand, inflammation has been well demonstrated to cause mitochondrial dysfunction. In patients with sepsis, various indicators of mitochondrial function were decreased.71 In an animal model, lipopolysaccharides (LPS) administration caused mitochondrial dysfunction through inhibition of complex IV of ETC.72 Therefore, inflammation and mitochondrial dysfunction form a feed-forward loop, which accelerates depression pathogenesis.
Overall, mitochondria are involved in depression through both decreased neuron function due to decreased energy supply and decreased neuron cell number due to increased cell death and decreased cell proliferation. Antioxidant compounds such as coenzyme Q10, zinc, vitamin E, and glutathione are reported being decreased in MDD.73 Whereas autoimmune responses against redox modified nitrosylated proteins and oxidative specific epitopes are increased. These studies support the important role of mitochondria in depression. Therefore, improvements in mitochondrial function for ameliorating depression symptoms is a plausible posit.
3 EFFECTS OF THE INTESTINAL MICROBIOTA ON MITOCHONDRIA
The gut microbiota plays an important role in brain development and functionality. Consequently, intestinal microbiome dysbiosis has been associated with many mental disorders including depression. Evidence has accumulated from both animal studies and clinical investigations. Certain commensal bacteria have been demonstrated to play an important role in the alleviation of depressive symptoms while intestinal dysbiosis has been reported to exacerbate MDD severity and decrease MDD treatment efficacy.10 It has been shown that Bacteroides genus in patients diagnosed with MDD was increased, while the Lachnospiraceae genus was decreased.8, 13 As such the intestinal microbiota has become recognized associated with depressive symptoms and manipulation of the gut microbiota could be a novel treatment approach to improve MDD symptoms.8 In this respect, a clinical trial has shown that probiotics improve mitochondrial function along with glycemic control, body composition, gut microbiome, and hormone changes.74
Bacteria and mitochondria are closely linked as the organelles may have evolved from an ancient proteobacterial ancestor; this is evident given that bacteria and mitochondria have similar structural and functional features.16 Studies have revealed that the microbiota can affect mitochondrial functions through the elaboration of metabolites including short-chain fatty acids (SCFAs), colonic acid, pyrroloquinoline quinone, fermentation gases, and modified fatty acids.75-77 By modulating genes involved in colonic acid metabolism, production of colonic acid by bacteria can be increased, leading to improved host mitochondrial function and longevity.76
The intestinal microbiota is also known to produce SCFAs, which include acetate, butyrate, and propionate from dietary fiber.78 These SCFAs have been demonstrated to benefit mitochondrial function. Moreover, acetate and butyrate have been demonstrated to systemically circulate to the brain, increasing brain mitochondrial function.79 In an animal model, it was demonstrated that the oral administration of butyrate to pigs increased hippocampal granular cell layer volume and neurogenesis.79 Administration of butyrate to depressed mice induced by chronic stress showed antidepressant effects.80 The ability of butyrate to increase mitochondrial function has also been demonstrated in a lymphoblastoid cell line.81
The important role of butyrate in mitochondrial functionality has been demonstrated in colonocytes. It has been shown that germ-free mice showed lower NADH/NAD + ratios and decreased ATP levels than that in conventionally raised mice.82 In the transcriptome and proteome studies of these mice, the butyrate metabolism pathway was reported altered. Correspondingly, 3-hydroxybutyrate was greatly decreased in germ-free mice. This was associated with downregulation of beta-oxidation enzymes of fatty acids acyl CoA dehydrogenase which is specific for SCFAs. The decreased butyrate oxidation was further demonstrated by decreased conversion of 13C labeled butyrate to CO2. Butyrate is well known as an energy source provider to colonocytes. These changes led to decreased energy supply that then caused cellular autophagy. The addition of butyrate to cultured germ-free colonocytes reversed the changes and of cellular autophagy. The effect of butyrate was diminished by fatty-acid oxidation inhibitor etomoxir, indicating that butyrate exerts its effect through the energy pathway. An additional study reported that donkey milk and human milk increased mitochondrial activities associated with increased fecal butyrate and propionate, providing supportive evidence that butyrate can increase mitochondrial function.83 It has also been demonstrated that propionate has a similar effect as to that of butyrate.82
SCFAs can also decrease gut permeability and thus decrease blood levels of LPS. It is well known that intestinal dysbiosis increases gut permeability and thus increases the transfer of LPS to the blood circulation.84 LPS that translocates across the intestinal epithelia into the gut mucosa can trigger and maintain an inflammatory response, which is associated with many metabolic diseases including MDD.85 It has been demonstrated that infusion of LPS into the striatum of rats causes damage to neuronal mitochondria, leading to a deficit in mitochondrial respiration, damage to mitochondrial cristae, mitochondrial redox potential disruption, and nitration.86 Alternatively, decreased blood levels of LPS will decrease brain inflammatory status and facilitate mitochondrial function. In a further animal model, LPS was shown to cause mouse depression-like behaviors and this was reduced with the administration of butyrate.87 However, how butyrate affects neuronal function remains to be elucidated.
Other SCFAs such as propionate have also been shown to improve mitochondrial function in lymphoblastoid cell lines from patients with autistic disorder depending on the concentration, exposure duration, and cellular redox state.88 Administration of sodium propionate to rats with chronic stress and depression was reported to induce decreased symptoms of depression.89
4 PERSPECTIVES
It is likely that mitochondria play an important role in the interaction between the gut microbiota and MDD. Further studies are needed to elucidate the mechanisms by which the gut microbiota alters mitochondrial function in neurons and improves depressive symptoms. Based on the proposed links between the intestinal microbiome, mitochondria and MDD, it is plausible that by modulating the intestinal microbiota cohort increased beneficial concentrations of butyrate could be achieved that improve mood disorders. Increased levels of intestinal butyrate could facilitate the symptom improvement in MDD. Although it has been shown that the gut microbiota is associated with MDD, it has not been well reported as to what sort of intestinal microbiota profiles can alleviate MDD. Based on current studies, treatment regimens that are effective in modulating the intestinal microbiome could be formulated for the treatment of MDD. SCFAs could be used to treat the symptoms of MDD individually. Currently there are no studies on the direct effects of SCFAs on MDD. SCFAs may be tested for its direct effect on neuronal proliferation and cell death. Improved delivery systems of SCFAs may be tested to improve their effects on MDD in animal models. As bioavailability is a major problem for SCFAs to be used for the treatment of diseases other than gastrointestinal disorders, nano-delivery of SCFAs could be applied to increase the concentrations of circulating SCFAs. Targeted nano-delivery of active metabolites may be developed which could noticeably increase SCFAs concentrations. Furthermore, SCFAs could be coadministered together with uridine and omega-3-fatty acids as the combinations have been shown to relieve MDD.90
In summary, emergent scientific trends posit that mitochondria could be a key participant in the association of the intestinal microbiota with MDD. Requisite studies are needed to elucidate the associated mechanism(s). Manipulation of the intestinal microbiota and or the metabolites of the commensal intestinal bacteria may lead to novel treatment approaches for MDD.
CONFLICT OF INTERESTS
We declare that we have no conflict of interests.