Histone deacetylases 1 and 2 inhibition suppresses cytokine production and osteoclast bone resorption in vitro
Abstract
The regulation of epigenetic factors is an emerging therapeutic target of immune function in a variety of osteolytic pathologies. Histone deacetylases (HDAC) modify core histone proteins and transcriptional processes, in addition to nonhistone protein activity. The activated immune response in rheumatoid arthritis, periodontitis, and prosthetic implant particle release stimulates the catabolic activity of osteoclasts. In this study, we investigated the effects of novel therapeutic agents targeting HDAC isozymes (HDAC 1, 2, and 5), previously shown to be upregulated in inflammatory bone disorders, in cytokine-stimulated human monocytes and osteoclasts in vitro. Inhibiting HDAC 1 and 2 significantly reduced gene expression of IL-1β, TNF, MCP-1, and MIP-1α in TNF-stimulated monocytes, while suppressing secretions of IL-1β, IL-10, INF-γ, and MCP-1 (P < .05). Osteoclast formation and bone resorption were also significantly diminished with HDAC 1 and 2 inhibition, through reduced NFATc1 expression and osteoclast specific target genes, TRAF6, CTR, TRAP, and Cathepsin K (P < .05). Similar trends were observed when inhibiting HDAC 1 and to a lesser extent, HDAC 2, in isolation. However, their combined inhibition had the greatest anti-inflammatory and antiosteoclastic effects. Targeting HDAC 5 had minimal effects on these processes investigated in this study, whereas a broad acting HDACi, 1179.4b, had widespread suppressive outcomes. This study demonstrates that targeting HDACs is a potent and effective way of regulating the inflammatory and catabolic processes in human monocytes and osteoclasts. It also demonstrates the importance of targeting individual HDACs with an overall aim to improve efficiency and reduce any potential off target effects.
1 INTRODUCTION
Over the past decade, the important interrelationship between skeletal maintenance and the immune system has identified essential processes leading to pathological break down of bone in inflammatory conditions. Specifically, rheumatoid arthritis (RA), periodontitis (PD), and during prosthetic implant wear-particle release into adjacent tissue, the activation of the immune system can lead to enhanced osteoclast driven bone resorption causing osteolysis.1 An important therapeutic goal for the preservation of the arthritic or prosthetic joints, or maintaining periodontal health and tooth support in PD, is by targeting both the inflammation and resulting bone destruction.
During the inflammatory reaction, activated immune cells have the capacity to stimulate bone resorbing osteoclasts through the production of cytokines such as tumor necrosis factor (TNF) and receptor activator of nuclear factor-κB ligand (RANKL) that possess pro-osteoclastic properties.2, 3 Investigations assessing inflammation related bone pathologies have utilized the pro-catabolic features of cytokines such as TNF, to establish in vitro techniques that represent this stimulated environment and the formation of inflammatory osteoclasts.2, 4 Furthermore, the production of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1α (MIP-1α) by immune cells has profound effects on exacerbating this response, while directly influencing the catabolic processes of bone metabolism.5-7 A number of studies have demonstrated increased expression and production of these molecules in inflamed periodontal gingival tissues and crevicular fluids in PD,8, 9 and synovial samples from human or mouse models of RA,10-12 supporting a need to reduce both the immune response and the activity of inflammatory osteoclasts.
Given the increased understanding of the relationship between the immune and skeletal system,7 novel therapeutic targets continue to be identified in a range of bone loss diseases. Recently, epigenetic regulation of cells has been established as a potent method for regulating cell phenotype and activity. A number of studies have revealed the anti-inflammatory and antiosteoclastic activity of histone deacetylase (HDAC) inhibitors (HDACi).13-15 HDACs are a group of 18 human enzymes that are involved in the epigenetic processes of gene regulation and posttranscriptional protein modification.16 These include class I HDACs (1, 2, 3, and 8), class IIa (HDAC 4, 5, 7, and 9), class IIb (HDAC 6 and 10) and class IV (HDAC 11). The remaining seven atypical enzymes are classified as Sirtuins (class III) due to their differing structure and cofactor activity. Physiologically, the catalytic domain of HDACs effects the removal of acetyl groups from histone lysine side chains to condense chromatin and repress gene expression; counteracting the acetylation capacity of histone acetyl transferase (HAT) enzymes.16 However, dysregulation of their expression or activity can lead to altered epigenetic modifications, potentially explaining the functional changes in cell cycle and immunoregulatory processes, perpetuating the development of disease. Recent evidence reveals discrepancies in HDAC expression or activity in inflammatory bone loss conditions.17-19 Specifically, we identified significantly upregulated HDAC 1, 5, 8, and 9 expression in human PD gingival tissue, with HDAC 1 shown to colocalise with TNF and TRAP (tartrate resistant acid phosphatase) positive osteoclastic cells.19
To date, investigations into bone metabolism and inflammation with the use of HDACi have primarily focussed on broad acting compounds (commonly referred to as pan inhibitors) that suppress a number of enzymes in each of the classes. These include 1179.4b, suberanilohydroxamic acid (SAHA), trichostatin A (TSA), romidepsin (FR901228), and givinostat (ITF2357), as reviewed by Cantley et al.15 Despite reported antiosteoclastic and anti-inflammatory activities in several in vitro and in vivo models, only Givinostat has progressed to phase 2 clinical trials for the treatment of juvenile arthritis with positive outcomes.20 As the enzyme suppression profiles of these HDACi are broad, with variations in HDAC expression and activity between cell types in addition to redundancies between HDAC gene and protein targets,21 further progression of these compounds to clinical use may be challenging. Subsequently, more recent studies using HDACi have focused on characterizing class specific inhibitors with high affinity for individual isoforms. We and others have recently shown that class I specific inhibitors (such as MS-275 and NW-21; with high affinity for HDAC 1 and 2) have presented favorable antiosteoclastic and anti-inflammatory properties,22-24 specifically through regulation RANKL and NFATc1 signaling. Conversely, the HDAC 5 isoform from class II has the ability to shuttle between the cell nucleus and cytoplasm. As such, it has been reported to deacetylate NFATc1 leading to down regulation of its transcriptional activity.25 Overexpression of HDAC 5 in a recent study using bone marrow macrophages (BMMs) identified impairments in macrophagic and osteoclastic activity, indicating suppression of its activity necessary for osteoclast differentiation and activity.15, 25 However, no research to date has investigated inhibitors that target this enzyme in osteoclastic cell types. HDAC 7, a class II enzyme may also have an important role during the macrophagic/osteoclastic maturation, as a study investigating BMM fusion and osteoclast differentiation recorded HDAC 7 as a negative regulator of this process.26 As such, its overexpression significantly inhibited BMM fusion, resulting in decreased size, and number osteoclast like cells in vitro. Further research into these pro- or anticatabolic processes is required to elucidate the protective or destructive properties of HDAC enzymes in osteolytic conditions.
As we now have access to a range of compounds designed to inhibit individual or combinations of HDAC isoforms, the current study aimed to investigate the effects of suppressing class I (HDACs 1 and 2) and class IIa (HDAC 5 and 7) on (a) cytokine/chemokine production by TNF-activated human monocytes and (b) human osteoclast formation and activity during TNF stimulation in vitro.
2 MATERIALS AND METHODS
2.1 Histone deacetylase inhibitors (HDACi)
Selective HDACi for HDAC 1 (BRD0302) and HDAC 2 (BRD6688)27 were designed and synthesized, along with Merck60 (HDAC 1 and 2 inhibitor) by the Stanley Centre for Psychiatric Research at the Broad Institute of MIT and Harvard (Cambridge).27 Compound 39 (targets HDAC 5 and 7 [Class IIa HDACs]) and 1179.4b (Class I and II inhibitor), supplied by the University of Queensland's Institute of Molecular Bioscience, were also investigated in this study.28, 29 HDACi (Table 1) used for in vitro treatments were suspended in a dimethyl sulfoxide (DMSO) vehicle at 0.01%.
| Compound | Target | HDAC 1 | HDAC 2 affinity | HDAC 3 affinity | HDAC 5 affinity | HDAC 7 affinity | HDAC 8 affinity | Other class II HDACs affinity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| affinity | |||||||||
| BRD0302 | HDAC 1 | 0.113 μM ± 0.015 | 1.29 μM ± 0.55 | 9.22 μM ± 2.06 | Wagner et al27 | ||||
| BRD6688 | HDAC 2 | 0.021 μM ± 0.013 | 0.100 μM ± 0.048. (preferential binding kinetics with extended half-life for HDAC2 compared to HDAC1) | 11.48 μM ± 2.54 | Wagner et al27 | ||||
| Merck 60 | HDAC 1 and 2 | 0.04 μM | 0.1 μM | >20 μM | >20 μM | >20 μM | >20 μM | HDAC 4, 6 > 20 μM | Compound 20 in Wagner et al30 |
| Compound 39 | Class IIa | HDAC 1 > 10 μM | HDAC 2 6.6 μM | 0.14 μM | 0.39 μM | 66 μM | HDAC 4 0.75 μM, HDAC 6 > 10 μM, | Tessier et al31 | |
| 1179.4b | Class I and II (pan inhibitor) | 48 nM | >3000 nM | HDAC 6 107 nM | Cantley et al24; Compound 5228 |
2.2 In vitro human monocyte/macrophage and osteoclast assay
Isolation of peripheral blood mononuclear cells (PBMCs) from healthy human blood donors (Australian Red Cross, AUS) using a Ficoll-Paque media (Pharmacia Biotech, SWE) and differential centrifugation was conducted as previously described.24 PBMCs were then suspended in α-minimal essential media (α-MEM; Invitrogen, AUS) with 10% fetal bovine serum, 1% penicillin/streptomycin, 1% L-glutamine, 100 nM dexamethasone (Invitrogen, AUS), and 25 ng/mL recombinant human (rh)-macrophage colony stimulating factor (M-CSF; Millipore) before being seeded into Falcon assay plates at 2 × 106 cells/mL. Nonadherent cells were removed after 24 hours at 37°C, 5% CO2, leaving the remaining monocyte/osteoclast progenitors to culture for the remainder of experimental assays.
To assess the anti-inflammatory activity of HDAC suppression, isolated PBMCs from four donors were stimulated with the proinflammatory cytokine rh-TNF (TNF; 10 ng/mL; R&D Systems) for 24 hours to induce an activated inflammatory state in the presence or absence of HDACi: BRD0302, BRD6688, Merck60, Compound 39, and 1179.4b. PBMCs (osteoclast progenitors) from eight donors were left untreated (±24 hour stimulation of TNF; 10 ng/mL) for 7 days until induction of osteoclastic differentiation with rh-RANKL (RANKL; 10 ng/mL; Millipore). Treatments of HDACi targeting HDAC 1, HDAC 2, HDAC 1 and 2, and class IIa HDACs were then analyzed for their antiosteoclast/antibone resorbing efficacy (BRD0302, BRD6688, Merck60, and Compound 39) over the remaining 10 days of the 17-day assay period, as previously established.24 Effects of HDACi on cell viability/proliferation was evaluated by colorimetric WST-1 based (Roche, DEU) assay as per manufacturer's instructions. All HDACi treatments were compared with 0.01% DMSO vehicle treated cells.
2.3 Assessment of HDACi activity on cytokine/chemokine production
TNF (10 ng/mL) activated monocyte/macrophages were treated with HDACi (10 nM and 100 nM) for 24 hours as previously described. Cytokine and chemokine levels (interleukin [IL]-1β, IL-10, regulated on activation, normal T cell expressed and secreted [RANTES], TNF, interferon [IFN]-γ, MCP-1, and MIP-1α) in cell culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA) or by multiplex immunoassay. Assays were performed in accordance with manufacturer's directions.
2.4 HDACi effect on osteoclast formation and bone resorbing activity
RANKL (10 ng/mL) induced differentiation of TNF (10 ng/mL) stimulated preosteoclastic cells were treated with HDACi (10-1000 nM) for 10 days as outlined above. After 1 week of treatment, osteoclastic cells cultured on 16-well Falcon chamber slides were fixed for 10 minutes in 4% glutaraldehyde solution, followed by serial washes with distilled water. Cells were then stained for TRAP using an established method,24 and imaged by light microscopy (Nikon Microphoto FXA Photomicroscope). Large TRAP-positive staining cells with more than three nuclei, characteristic of mature osteoclasts, were quantified for comparative analysis between treatments.24
Remaining TNF (10 ng/mL) stimulated osteoclastic cells were cultured on whale dentine disks in the presence of HDACi (10 nM). After a total of 17 days in culture, dentine was trypsinized and washed vigorously to remove adherent cells. Disks were then carbon coated for imaging under a Philips XL30 Field Emission Scanning Electron Microscope (FE-SEM) at x200 magnification (Adelaide Microscopy, University of Adelaide, AUS). Analysis of osteoclast activity was conducted by the measurement of osteoclastic resorption pits present on the dentine surface using Adobe Photoshop Elements (7.0 v, Adobe Systems Software Ireland Ltd) and Image J software (1.47 v, National Inst of Health). The total area of resorption present within three representative images per dentine slice was used to quantify the average overall resorption per donor, and compared between treatment groups.24 Osteoclast activity was determined by calculating the percent area of dentine resorbed per number of TRAP-positive multinucleated cells forming, and expressed as a percentage of resorption per osteoclast.
2.5 Analysis of inflammatory and osteoclastic gene expression with HDACi treatment
Total RNA was extracted from TNF (10 ng/mL) stimulated PBMCs using a TRIzol (Thermo Fisher Scientific; AUS) based on methods as per manufacturers’ guidelines after 24-hour incubation with HDACi, or on day 10 and 14 following osteoclast differentiation with RANKL (10 ng/mL) commencing on day 7 of the 17-day osteoclast assay. Complimentary DNA (cDNA) was generated using Rotor-Gene Q (Qiagen, AUS) reverse transcription with 250 ng of random hexamer and 200 U of superscript III reverse transcriptase (Geneworks, AUS), followed by amplification of samples in triplicate using a Platinum SYBR Green qPCR Supermix-UDG (Life Technologies, Pty, Ltd, AUS) as previously described.24 Gene expression was quantified using the 2−ΔCT method32 and expressed relative to an endogenous reference gene, human acidic ribosomal protein (hARP).33 Inflammatory cytokine/chemokine molecules investigated in this study included TNF, IL-1β, 1L-10, IFN-γ, MCP-1, MIP-1α,23 and RANTES.34 Osteoclast-related genes analyzed were nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1), TRAP, cathepsin K (Cath K), calcitonin receptor (CTR),35 TNF associated receptor activator factor-6 (TRAF6),24 dendritic cell-specific transmembrane protein (DC-STAMP), and β3-integerin, in addition to HDACs 1, 2, and 5.24
2.6 Statistical analyses
Statistical analysis of osteoclast formation and activity, along with gene and protein expression studies used one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparisons tests to identify significant variations between experimental groups and control cells with statistical significance being accepted at P < .05. GraphPad Prism version 7 was used in this study.
3 RESULTS
3.1 HDAC expression in monocytes and osteoclasts
As TNF is a key molecule involved in a variety of cellular functions, including orchestrating the cytokine cascade during an immune response, it was used to induce and mimic inflammatory events in vitro in all experiments. Stimulation of monocytes with TNF (10 ng/mL) for 24 hours resulted in increased messenger RNA (mRNA) expression of class I HDACs 1 (P < .01) and 2 (P < .05). Although a positive trend in the class II HDACs (5 and 7) was observed following TNF stimulation, this did not achieve statistical significance (P > .05) (Figure 1).
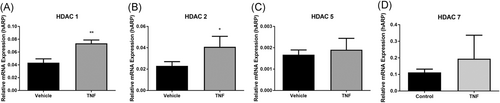
TNF regulated HDAC expression in human monocytes relative mRNA gene expression of A, HDAC 1; B, HDAC 2; C, HDAC 5; D, HDAC 7 ± TNF (10 ng/mL) as outlined in methods. Values expressed relative to housekeeping gene, human acidic ribosomal protein (hARP). *P < .05. HDAC, histone deacetylases; mRNA, messenger RNA; TNF, tumor necrosis factor
3.2 HDACi alter the cytokine profile of TNF-stimulated monocytes
Stimulating human PBMCs with TNF (10 ng/mL) for 24 hours significantly enhanced the production of proinflammatory cytokines and chemokines IL-1β, TNF, IFN-γ MCP-1, and MIP-1α both at the mRNA (Figure 2 and Figure 3) and protein level (Table 1) (P < .05). Expression of RANTES and the anti-inflammatory cytokine, IL-10, was not however significantly altered (P > .05) in activated monocytes.
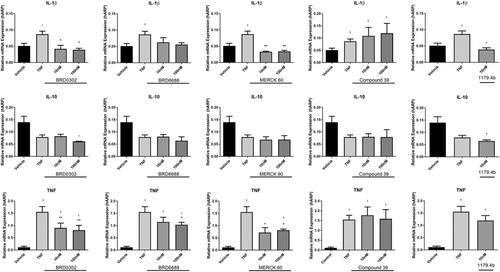
Effects of HDACi (10-100 nM) targeting HDAC 1 (BRD0302), HDAC 2 (BRD6688), HDAC 1 and 2 (MERCK 60), HDAC 5 (compound 39), and broad suppression of class I and II HDACs (1179.4b) on mRNA expression of cytokines in TNF (10 ng/mL) stimulated monocytes. Row (1) IL-1β; row (2) IL-10; and row (3) TNF. Values expressed relative to housekeeping gene, human acidic ribosomal protein (hARP). *(P < .05) compared with TNF-stimulated cells; †(P < .05) compared with vehicle control. HDAC, histone deacetylases; mRNA, messenger RNA; TNF, tumor necrosis factor
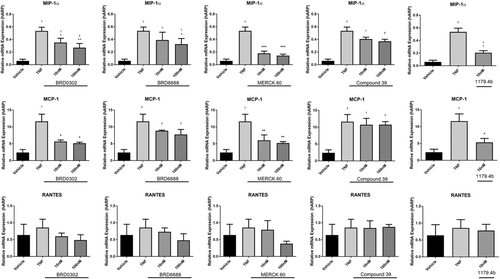
Effects of HDACi (10-100 nM) targeting HDAC 1 (BRD0302), HDAC 2 (BRD6688), HDAC 1 and 2 (MERCK 60), HDAC 5 (compound 39), and broad suppression of class I and II HDACs (1179.4b) on mRNA expression of chemokines in TNF (10 ng/mL)-stimulated monocytes. Row (1) MIP-1α; row (2) MCP-1; and row (3) RANTES. Values expressed relative to housekeeping gene, human acidic ribosomal protein (hARP). *(P < .05) compared with TNF-stimulated cells; †(P < .05) compared with vehicle control. HDAC, histone deacetylases; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; mRNA, messenger RNA; RANTES, regulated on activation, normal T cell expressed and secreted; TNF, tumor necrosis factor
The HDAC 1 inhibitor (BRD0302) and HDAC 2 inhibitor (BRD6688) both reduced the expression of cytokines IL-1β and TNF (Figure 2) and chemokines MIP-1α and MCP-1 (Figure 3) at the mRNA level in a dose-dependent mechanism (10-1000 nM). However, only protein levels of IL-1β and MCP-1 were significantly reduced in cell supernatant with inhibition of HDAC 1 or HDAC 2 at doses investigated (10-100 nM) (P < .05) (Figure 4). Secreted TNF, MIP-1α, RANTES, IFN-γ, and IL-10 levels were not affected by selective suppression of HDAC 1 or 2.
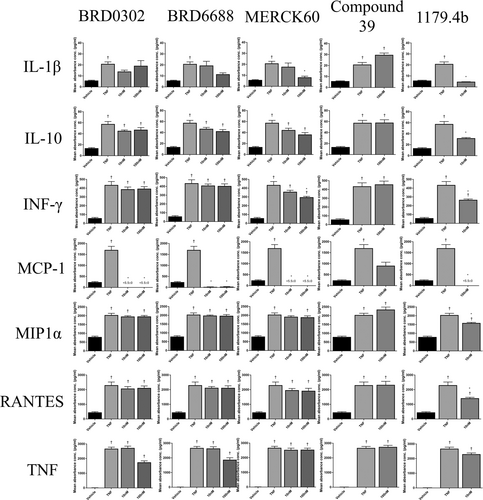
Effects of HDACi (10-100 nM) targeting HDAC 1 (BRD0302), HDAC 2 (BRD6688), HDAC 1 and 2 (MERCK 60), HDAC 5 (compound 39), and broad suppression of class I and II HDACs (1179.4b) on protein expression from cell supernatant of chemokines in TNF (10 ng/mL)-stimulated monocytes. *(P < .05) compared with TNF-stimulated cells; †(P < .05) compared with vehicle control. HDAC, histone deacetylases; IL, interleukin; INF-γ, interferon gamma; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; RANTES, regulated on activation, normal T cell expressed and secreted; TNF, tumor necrosis factor
Targeting HDAC 1 and 2 in combination using HDACi Merck 60, significantly reduced both mRNA expression and protein secretions of IL-1β, MCP-1, and IFN-γ (P < .05) (Figures 2-4). Gene expression of TNF and MIP-1α was also suppressed, although notable reductions in protein secretions were observed, these did not achieve statistical significance. Interestingly, IL-10 levels in cell supernatants were reduced at the highest dose (100 nM) of the HDACi, Merck 60. Levels of RANTES were unchanged with Merck 60.
Compound 39 (class IIa HDACi – targets HDAC 5 and 7) resulted in no significant change in the cytokine profile of activated monocyte/macrophages in these assays (Figures 2-4).
Broad suppression of class I and II HDACs with low doses (10 nM) of HDACi 1179.4b had a similar anti-inflammatory profile as the class 1 inhibitor Merck 60, with majority of proinflammatory factors evaluated in this study being significantly reduced both at the mRNA and protein level. These included IL-1β, IFN-γ, MCP-1, and MIP-1α gene expression and protein secretions (P < .05) (Figures 2-4).
3.3 HDACi suppresses osteoclast formation and bone resorbing activity
Induction of inflammatory osteoclast formation and activity in vitro was achieved by treating osteoclastic progenitors with TNF (10 ng/mL) as outlined above. Fewer numbers of TRAP-positive cells formed with TNF stimulation (Figure 5). Interestingly, these cells appeared larger in size with greater numbers of nuclei present, and had significantly (P < .05) greater resorptive capacity than unstimulated vehicle control cells. This finding is consistent with previous investigations stimulating osteoclasts with TNF.2
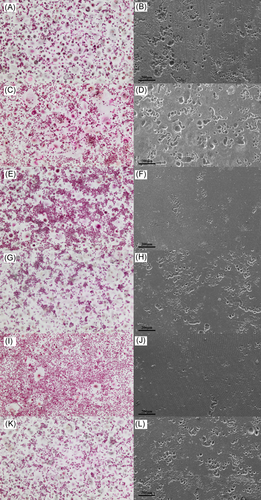
Representative images of TRAP-positive multinucleated osteoclastic cell formation (left) and resorption pits on dentine slices (right) in response to HDACi treatments (100 nM). A, B, vehicle control; C, D, TNF control; E, F, TNF + BRD0302; G, H, TNF + BRD6688; I, J, TNF + MERK60; K, L, TNF + Compound 39. HDAC, histone deacetylases; TNF, tumor necrosis factor; TRAP, tartrate-resistant acid phosphatase
HDAC 1 inhibition with BRD0302 or HDAC 2 with BRD6688 had very little effect on osteoclastic formation in the absence of TNF, with numbers of TRAP-positive multinucleated cells being similar to untreated vehicle control (data not shown). However, TNF- (10 ng/mL) stimulated cells were sensitive to HDAC 1 suppression, with high doses of BRD0302 (1000 nM) reducing the number and size of osteoclastic cells forming in vitro (Figure 5 and Figure 6). Interestingly, the osteoclast activity was diminished in the presence of HDACi targeting either HDAC 1 or 2 with significant reduction in surface resorption being observed at doses 100 to 1000 nM for BRD0302 (P < .01) and 1000 nM for BRD6688 (P < .05; Figure 5 and Figure 6).
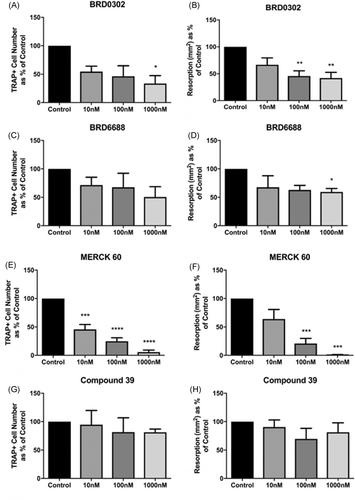
HDACi regulate human osteoclastic cell formation and bone resorptive activity in vitro. Fold change in the number of TRAP-positive multinucleated osteoclastic cell formation (left) and area (mm2) of topographical resorption in dentine slices (right). A, B, BRD0302 (HDACi 1); C, D, BRD6688 (HDACi 2); E, F, MERCK 60 (HDACi 1 & 2); G, H, compound 39 (class IIa HDACi). *(P < .05) Compared with TNF-stimulated control cells. HDAC, histone deacetylases; TNF, tumor necrosis factor; TRAP, tartrate-resistant acid phosphatase
Suppressing both HDAC 1 and 2 with Merck60 reduced TRAP-positive multinucleation at all HDACi concentrations investigated (10-1000 nM), which translated to significant (P < .05) declines in bone resorptive capabilities at concentration-dependent rates (Figure 5 and Figure 6). Conversely, Compound 39 that targets class IIA HDAC 5 and 7 failed to have any substantial influence on osteoclastic formation or resorptive activity in vitro, with no statistical variations from the untreated control (Figure 5 and Figure 6). Furthermore, combining BRD0302, BRD6688, or Merck60 with Compound 39 resulted in no alterations to targeting the class I HDACs alone, indicating no observable osteoclastic effect of the class IIa inhibitor (Compound 39).
3.4 Suppression of human osteoclasts through decreased osteoclast signaling, activity, and fusion factor expression with HDACi targeting HDAC 1 and HDAC 2
Gene expression via quantitative real-time polymerase chain reaction (qRT-PCR) of essential osteoclast factors was analyzed after TNF stimulation and HDACi treatments, targeting HDAC 1 (BRD0302), HDAC 2 (BRD6688), or HDAC 1 and 2 (Merck60) at day 10 and 14 of the osteoclastic culture. Both HDAC 1 and 2 inhibitors BRD0302 and BRD6688 (10 nM) reduced the mRNA expression of the signaling factor TRAF-6, activity markers TRAP and Cath K, and the fusion factor DC-Stamp at day 14 (Figure 7).
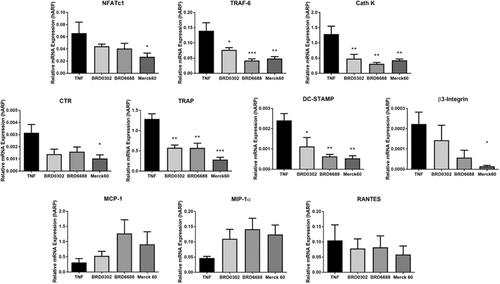
Regulation of osteoclast-related gene expression by HDACi. Relative mRNA gene expression of osteoclast-related genes in TNF-stimulated osteoclastic cells. A, NFATc1; B, TRAF6; C, Cath K; D, CTR; E, TRAP; F, DC-STAMP; G, β3-integrin; H, MCP-1; I, MIP-1α; and J, RANTES *(P < .05) Compared with TNF stimulated control cells. Cath K, cathepsin K; CTR, calcitonin receptor; HDAC, histone deacetylases; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; mRNA, messenger RNA; NFATc1, nuclear factor of activated T-cells, cytoplasmic 1; RANTES, regulated on activation, normal T cell expressed and secreted; TNF, tumor necrosis factor; TRAF6, TNF associated receptor activator factor-6
Conversely, suppressing both HDAC 1 and 2 with Merck60 (10 nM) was more suppressive of osteoclastic factors, with significant (P < .05) reductions in the mRNA expression of NFATc1, TRAF-6, TRAP, Cath K, CTR, β3-integrin, and DC-STAMP at day 14 (Figure 7).
Interestingly, inhibition of HDACs 1 and/or 2 had no significant effect on the chemokines investigated (MCP-1, MIP-1α, and RANTES; Figure 7).
4 DISCUSSION
The proinflammatory effects of TNF have been extensively researched over the past decade, with a wide range of systemic and cellular outcomes being reported.2 In chronic inflammatory conditions, such as PD, RA, and in prosthetic implant loosening, where levels of TNF are substantially upregulated,19, 36 its destructive influence appears to be through the enhancement of cytokine/chemokine production leading to increased cellular infiltration and activity.7 These inflammatory cells and mediators then have both direct and indirect effects in stimulating osteoclastic bone resorption.2 The current study utilized the stimulatory effects of TNF to promote the inflammatory activity of human monocytes/macrophages and osteoclastic cells in vitro for the assessment of novel therapeutic compounds designed to suppress the actions of individual HDAC enzymes. The results revealed that suppressing HDAC 1 and 2 in combination suppresses production of a range of key inflammatory cytokines and chemokines including IL-1β, TNF, MCP-1, and MIP-1α. Inhibiting this combination of HDACs was subsequently shown, in a concentration-dependent manner, to suppress the formation and activity of human osteoclasts in vitro.
As expected, TNF induced the synthesis of a range of inflammatory mediators by human monocytes (IL-1β, IL-10, RANTES, TNF, IFN-γ MCP-1, and MIP-1α). Broad suppression of HDAC activity by 1179.4b (pan-inhibitor) reduced the stimulatory effect of TNF, as concentrations of these inflammatory molecules (IL-1β, TNF, IFN-γ MCP-1, and MIP-1α) in cell supernatants were significantly reduced. This anti-inflammatory effect observed with broad HDAC inhibition is consistent with the effects observed in a variety of inflammatory animal models, as recently reviewed.15 Interestingly, treatment with 1179.4b at 1 mg/kg/day was not able to suppress inflammation in a mouse model of periodontitis,23 despite markedly reducing alveolar bone loss. However, inflammation was assessed via histological assessment of cellular infiltrate in gingival tissue and not levels of cytokines or chemokines in the tissues. This dose was also low (1 mg/kg/day), while still significantly reducing alveolar bone loss. In the current study, 1179.4b (10 nM) treatment of stimulated human monocytes resulted in decreased levels of the anti-inflammatory cytokine IL-10 at both the mRNA and protein level. IL-10 is produced by stimulated monocytes and reported to modulate and suppress the proinflammatory cascade. This may explain different effects observed with broad acting inhibitors in relation to their anti-inflammatory abilities. Broad inhibition of HDACs has the ability to suppress both anti- and proinflammatory molecules as recently reviewed,37 supporting the need to characterize the actions of individual HDAC isoforms, and targeting those involved in promoting particular pathological conditions.
The expression of class I HDAC 1 and 2 was both significantly increased in TNF-stimulated monocytes. We subsequently demonstrated that targeting HDAC 1 and 2 with novel inhibitors (BRD0302 [HDAC 1] and BRD6688 [HDAC 2]) regulates the expression of individual molecules involved in the proinflammatory cascade. Inflammatory cytokine IL-1β and chemokine MCP-1 levels were both significantly reduced with HDAC 1 and/or 2 inhibition both at the mRNA and protein level by TNF-activated monocytes. IL-1β, like TNF, is integral to the immune response, mediating cellular and cytokine networks. High levels of IL-1β have been observed in the serum of patients with chronic PD38 and are substantially increased when patients suffer from additional systemic conditions, such as cardiovascular disease.39 Furthermore, MCP-1 is an essential chemotactic factor that is involved in promoting the migration and recruitment of mononuclear inflammatory cells.40 Recent research suggests that levels of IL-1β, combined with the actions of MCP-1, could be a causal link to the development of chronic PD in addition to its contributing exacerbation of systemic conditions such as coronary heart disease.39 The suppression of key inflammatory mediators by targeting HDAC 1 highlights this isoform as being an important epigenetic factor involved in stimulating inflammatory processes. Our previous studies utilizing a novel HDACi, NW-21 that was designed to target HDAC 1 and 2 (HDAC1, 0.021 μM; HDAC2, 0.042 μM), was also shown to suppress mRNA expression of MCP-1 and to a lesser extent IL-1 β in monocytes.23 NW-21 and MS-275, also a HDAC 1 inhibitor (inhibition of HDAC activity IC50 values: HDAC1, 0.181 μM; HDAC2, 1.16 μM), were both shown to reduce inflammation with significant decreases in arthritis paw scoring and swelling which was also confirmed histologically in a collagen antibody induced arthritis mouse model.23 Interestingly, treatment with the broad acting HDACi 1179.4b did not reduce inflammation in this mouse model. Studies by others have also shown that MS-275 has greater antiarthritic activity when compared with the broad acting HDACi suberanilohydroxamic acid (SAHA).41 The suggested association of HDAC 1 with inflammation has also been shown in human tissues from inflammatory diseases, with HDAC 1 levels correlating with TNF levels in both RA18 and PD.19 Inhibitors targeting HDAC1, including Merck 60, NW-21, and MS-275, may have potential to treat inflammatory diseases such as PD and RA.
In addition to an anti-inflammatory effect, this study also demonstrated an antiosteoclastic effect of HDAC 1 and 2 inhibitors in the presence of TNF. Interestingly, BRD0302 and BRD6688 resulted in small reductions in the numbers of TRAP-positive cells and their subsequent activity in unstimulated control cells. In the presence of TNF, however, these inhibitors were more efficient at suppressing both the formation and activity of the cells. TNF may activate the cells making them more responsive to the inhibitors and this could be valuable for their use as treatments in inflammatory diseases. When HDAC 1 or 2 were suppressed (BRD0302 or BRD6688) reductions in both osteoclast formation and activity were evident. However, when both HDAC 1 and 2 were targeted with HDACi Merck 60 (HDAC 1 and 2), the effect on bone resorption was even more pronounced. For HDAC 1 (BRD0302) and HDAC 2 (BRD6688) inhibition alone, there was approximately 50% reduction in the area of dentine resorption, whereas with Merck 60 (HDAC 1 and 2) there was almost a complete suppression at the highest dose of 1000 nM. These results suggest that not only do redundancies in the actions of HDAC 1 and 2 exist (in respect to osteoclast activity), but compensatory mechanisms of these HDACs allow for fundamental cellular processes to continue in the absence of individual select HDAC isotypes. Previous studies utilizing NW-21 (targets HDAC 1 and 2) revealed significant suppression of human osteoclast formation and activity in vitro confirming the importance of targeting these two HDACs.23 HDAC 1 and 2 are also known to function in a protein complex suggesting that redundancies could exist.42 The possibility of redundancy in HDACs in regard to the osteoclast activity has also been demonstrated in past studies where the combination of MS-275 (HDAC 1 inhibitor) and 2664.12 (HDAC 6 inhibitor) significantly suppressed the formation and activity of human osteoclasts at similar levels to the broad acting HDACi 1179.4b.24 MS-275 or 2664.12 was less effective when utilized individually in this study.
An interesting finding of this study was the negative results observed with class IIa HDAC suppression, specifically HDAC 5 and 7 (Compound 39). HDAC 5 has been shown to be overexpressed in chronic PD tissue19 and upregulated during RANKL induced osteoclastogenesis in vitro,24 despite its activity being reported to enhance RANKL signaling, mediated through NFATc1 stabilization,25 ultimately diminishing the process of osteoclast differentiation. Furthermore, a recent study utilizing selective short hairpin RNA (shRNA)-mediated suppression of HDAC 5 reported increased the osteoclastogenic activity in isolated primary bone macrophages.43 These studies may indicate an anticatabolic role for HDAC 5, which acts to protect against and regulate osteoclastic bone resorption. Further analyses of the actions of HDAC 5 during inflammation and bone metabolism are required to elucidate its role during the destructive processes of PD. Select studies have focussed on a possible role for HDAC 7 in osteoclast differentiation. Overexpression of HDAC 7 is shown to inhibit the fusion of osteoclast precursors26 and HDAC 7 knockdown in a mouse model enhanced osteoclast differentiation.44 The lack of catabolic effects by compound 39 observed in this study, or the absence of HDAC 5 and 7 suppression by TNF could be related to the combination of HDACs targeted (class IIa predominately HDAC 5 and 7), the fact that suppressing HDAC 7 has been shown to increase osteoclast differentiation or the compound potency itself, or an absence of direct innovation by the inflammatory cytokine utilized in this study. Further studies utilizing more specific acting inhibitors would help to elucidate the roles of individual class IIa HDAC isozymes.
The HDAC 1 and 2 inhibitors (BRD0302, BRD6688, and Merck 60) appeared to inhibit osteoclastogenesis via suppression of key intercellular signaling factors TRAF-6 and NFATc1. TRAF-6 is activated early following RANKL/RANK interaction, leading to the translocation of the key transcription factor NFATc1 to the nucleus initiating the production of osteoclast genes, including CTR, Cat K, TRAP, and β3-integrin. Consistent with this, HDACi treatment resulted in significant reductions in the mRNA expression of these factors. The HDACi also resulted in a reduction in DC-STAMP, essential for cell to cell fusion during multinucleated osteoclast formation. Our previous studies have also demonstrated a suppression in TRAF-6 and NFATc1 with HDACi 1179.4b and NW-21. Although with 1179.4b, decreases in TRAF-6 were noted at day 10 and NFATc1 suppression at day 17.24 With NW-21 treatment TRAF-6 and NFATc1 suppression was noted at 10 day.23 In the current, study the key osteoclast mediators were suppressed at day 14, (Cat K, TRAP, and β3-integrin). Differences in time points to detect changes in the mRNA expression of intercellular molecules could be related to the presence of TNF-α in the culture or could be due to different donor cells used. Treatment with the HDAC 1 and 2 inhibitors in this assay did not suppress the expression of chemokines MCP-1, MIP-1α, or RANTES. This was unexpected given previous studies utilizing NW-21 in human osteoclasts resulted in a significant suppression in mRNA expression of MCP-1 at day 10. Further to this, the studies with NW-21 were conducted in unstimulated cells and hence the presence of TNF-α may alter the way in which the HDACi function in their suppression of osteoclasts.
The results of this study identified an important role for HDAC 1 and 2 during inflammatory and osteoclastogenesis in vitro, with reduced proinflammatory cytokine/chemokine production by activated monocytes and the suppression of pathological bone resorption by TNF-stimulated osteoclasts. These findings further support the importance of continuing research into HDACi as potential therapeutic options for the treatment of chronic inflammatory bone loss diseases.
ACKNOWLEDGMENTS
This study was supported by the National Health and Medical Research Council (NHMRC) (1057835). Dr Cantley was supported by a NHMRC Early Career Fellowship (1070880)
CONFLICT OF INTERESTS
All authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
KA, DRH, and MC conceived of the present idea. KA carried out experiments and wrote manuscript with technical and intellectual support by TRF, OR and PMB, under supervision of DRH and MC. FFW, EBH, RCR, and DPF designed, manufactured, and provided compounds for analysis. All authors contributed to interpretation of results and approval of final version of manuscript for submission.