NDV-D90 inhibits 17β-estradiol-mediated resistance to apoptosis by differentially modulating classic and nonclassic estrogen receptors in breast cancer cells
Abstract
Newcastle disease virus (NDV) is endowed with the oncolytic ability to kill tumor cells, while rarely causing side effects in normal cells. Both estrogen receptor α (ERα) and the G protein estrogen receptor (GPER) modulate multiple biological activities in response to estrogen, including apoptosis in breast cancer (BC) cells. Here, we investigated whether NDV-D90, a novel strain isolated from natural sources in China, promoted apoptosis by modulating the expression of ERα or the GPER in BC cells exposed to 17β-estradiol (E2). We found that NDV-D90 significantly killed the tumor cell lines MCF-7 and BT549 in a time- and dose-dependent manner. We also found that NDV-D90 exerted its effects on the two cell lines mainly by inducing apoptosis but not necrosis. NDV-D90 induced apoptosis via the intrinsic and extrinsic signaling pathways in MCF-7 cells (ER-positive cells) during E2 exposure not only by disrupting the E2/ERα axis and enhancing GPER expression but also by modulating the expression of several apoptosis-related proteins through ERα-and GPER-independent processes. NDV-D90 promoted apoptosis via the intrinsic signaling pathway in BT549 cells (ER-negative cells), possibly by impairing E2-mediated GPER expression. Furthermore, NDV-D90 exerted its antitumor effects in vivo by inducing apoptosis. Overall, these results demonstrated that NDV-D90 promotes apoptosis by differentially modulating the expression of ERα and the GPER in ER-positive and negative BC cells exposed to estrogen, respectively, and can be utilized as an effective approach to treating BC.
1 INTRODUCTION
Breast cancer (BC) is one of the most prevalent types of malignancy in women worldwide, and both the incidence rate and the mortality rate of the disease are rising.1 In addition to being managed surgically, BC is also managed via varied conventional adjuvant treatments; however, these treatments display equal limitations with respect to their ability to kill tumor cells. Therefore, novel treatments for BC are urgently needed. Estrogen receptors (ERs), which are overexpressed in 70% of BC cells, comprise two classic subtypes in BC cells, estrogen receptor α (ERα) and ERβ.2, 3 ERs are known to play major roles in BC development by binding to their ligand, estrogen.4, 5 ERα status but not ERβ status is commonly used to evaluate BC development and progression. In addition, the G protein estrogen receptor (GPER), which is a novel 17 β-estradiol (E2)–binding protein, is structurally distinct from the classic ERs. The GPER is known to act as a nonclassic receptor upstream of signaling networks to mediate a multitude of rapid biological signaling events in response to estrogen in BC cells.6
Newcastle disease virus (NDV), or avian paramyxovirus serotype 1, is a nonsegmented and negative-strand RNA virus of the family Paramyxoviridae. NDV has a natural avian host range and has been confirmed to possess the oncolytic ability to kill tumor cells, while rarely causing side effects in normal cells.7-9 The evidence from the available literature demonstrates that NDVs, including recombinant NDV strains, have the ability to promote apoptosis via multiple signaling networks in a variety of malignancies.10-13 In addition, several strains of genetically recombined NDVs derived from the wild virus have been utilized in phase I/II clinical trials investigating antitumor therapies.14-17 NDV-D90, a novel strain isolated from natural sources in China, has been confirmed to induce apoptosis in lung cancer cells and oral squamous carcinoma cells.18, 19
In contrast to what occurs in other hormone-independent malignancies, in BC cells, both ERα and GPER by binding to E2 participate in multiple signal transduction pathways and modulate downstream signaling pathways, including apoptosis-related signaling pathways.20-24 A series of studies regarding the mechanisms by which the virus exerts its effects revealed that NDV-D90 promotes apoptosis by differentially modulating the expression of ERα and the GPER in ER-positive and ER-negative BC cells exposed to E2, respectively.
2 MATERIALS AND METHODS
2.1 Cell culture
The BC cell lines MCF-7 and BT549 were obtained from the Type Culture Collection Cell Bank (Chinese Academy of Sciences Committee, Shanghai, China) and were routinely cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2. The MCF-7 and BT549 cells were infected with NDV-D90 at different concentrations (multiplicity of infection [MOI] = 1, 10−1, 10−2, and 10−3), and matched control cells were treated with 200 μL of RPMI-1640 without FBS. The cells were then cultured in phenol red-free RPMI-1640 plus 5% charcoal-stripped, steroid-depleted FBS for 2 days before the E2 (Sigma-Aldrich, St. Louis, MO) stimulation experiments. The ERα antagonist ICI 182,780 (Sigma-Aldrich) was utilized to prevent E2 from binding to ERα, and G1 (Sigma-Aldrich) was a GPER-specific agonist.
2.2 Virus
NDV-D90 was obtained from the Harbin Veterinary Medicine Research Institute of the Chinese Academy of Agricultural Sciences and was prepared as reported previously.14, 25
2.3 Antibodies and reagents
The antibodies (Abs) used in this study included primary Abs against Bcl-2, Bax, cytochrome-C, tumor necrosis factor-alpha (TNF-α), tumor necrosis factor related apoptosis-inducing ligand (TRAIL), X-linked inhibitor of apoptosis protein (XIAP), caspase-3/7/6/8/9, estrogen receptor-alpha (ERα), the GPER, and glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Santa Cruz, CA). Secondary horseradish peroxidase-conjugated rabbit antigoat, goat antimouse, and goat antirabbit Abs (Zhongshan Golden Bridge Biotechnology Co, Ltd, Beijing, China) were also used.
2.4 Caspase activity detection
Caspase-3/7/8/9 assay kits (Promega, Beijing, China) were utilized to detect caspase activity.
2.5 Cell viability detection
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc, Beijing, China) was utilized to detect cell viability. The above cells were seeded in 96-well plates at a density of 1 × 104 cells/well. At different time points, the culture medium was replaced with 100 μL of fresh medium containing 10 μL of CCK-8 solution. The cells were then incubated for 2 hours at 37°C, and the optical density at 450 nm was measured. The experiment was repeated three times.
2.6 Assessment of cell apoptosis
Cells were seeded in six-well plates and subjected to different treatments. The cells were then harvested and incubated with 5 μL of annexin V and 5 μL of propidium iodide in the dark for 15 minutes at room temperature, according to the manufacturer's instructions (BD Biosciences, San Jose, CA). Apoptosis rates were measured by flow cytometry (FCM) with a Beckman Coulter Epics Altra II Cytometer (Beckman Coulter, CA). The experiments were repeated three times.
2.7 Western blot analysis
These experiments were performed as reported previously.26, 27
2.8 Animal experimental protocols
All surgical and animal care procedures performed on the animals were conducted in accordance with institutional ethics guidelines.
2.9 Tumorigenicity study
Six-week-old female nude mice were purchased from the Beijing Vital River Laboratory Animal Technology Company (Beijing, China). Cells (5 × 106) were subcutaneously injected into one flank of each mouse. Tumor volume was estimated by the following formula: π/6 × a2 × b, where a is the short axis, and b is the long axis. The mice were randomly divided into two groups when their tumor volumes reached 100 mm3. The mice were then subjected to intratumoral injections of 1 × 107 TCID 50 of NDV D90 or phosphate buffer saline (PBS) in a total volume of 100 μL. These treatments were repeated every other day for a total of four treatments. Tumor volumes were measured every four days, and the animals were killed 28 days later.
2.10 Immunohistochemistry
These experiments were performed as reported previously.26, 27
2.11 Statistical analysis
All data are expressed as the mean ± standard deviation. Comparisons among multiple groups were made with one-way analysis of variance followed by Dunnett's t test, and comparisons of tumor volumes between the two mouse groups at different time points were conducted with repeated measurements. A P value of less than 0.05 was considered statistically significant.
3 RESULTS
3.1 NDV-D90 kills BC cells
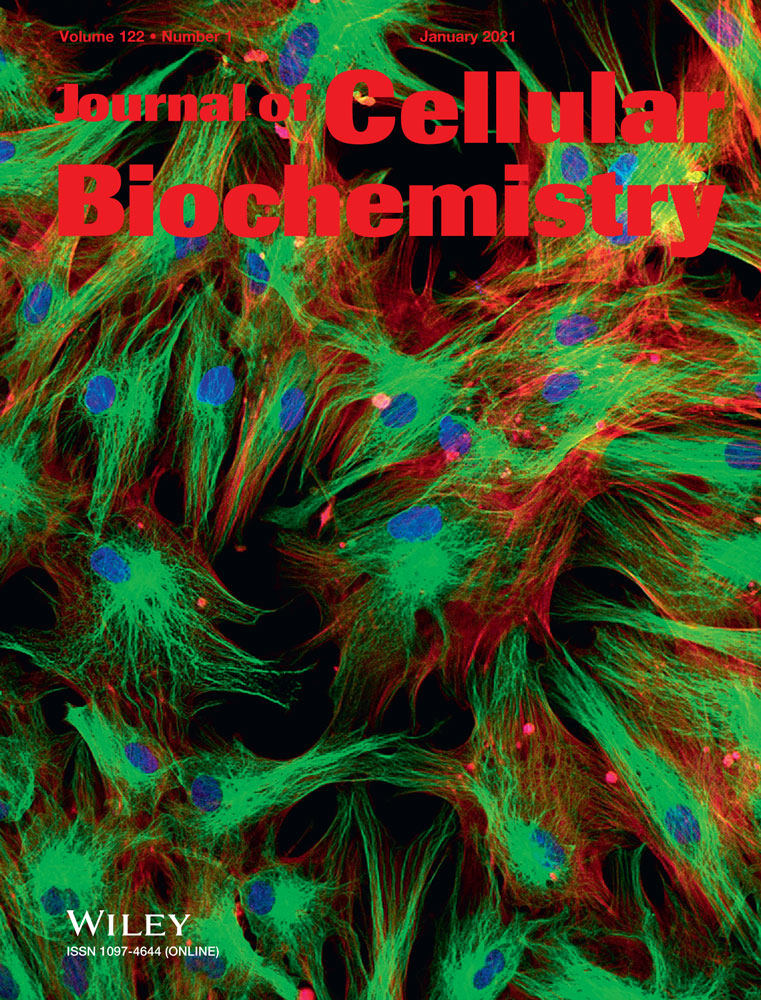
Cell counting kits were used to detect the cell-killing effects of NDV-D90 on MCF-7 and BT549 cells. As depicted in Figure 1A, NDV-D90 exerted time- and dose-dependent cytotoxic effects on the two cell lines. NDV-D90 demonstrated a slight ability to kill MCF-7 cells as early as 6 hours postinfection when administered at an MOI of 1 or 10−1 and the mortality rate was more than 10% (both P < 0.05). Extending the NDV-D90 (MOI = 1) treatment time from 6 to 9 hours or to 12 hours, the mortality rate in MCF-7 cells treated with NDV-D90 at an MOI of one was increased to almost 25% (P < 0.01) or to more than 40% (P < 0.001), respectively. The mortality rate in the NDV-D90-treated group (MOI = 1) reached its maximum level at 24 hours after infection, at which time it was almost 60% (P < 0.001) higher than that in the matched control group. NDV-D90 also decreased the survival rate by almost 20% (P < 0.05), by 25% (P < 0.01), and by more than 30% (P < 0.001) in the treated group compared with the matched control group when administered at an MOI of 10−1 for 9, 12, and 24 hours, respectively. A reduction in survival occurred relatively late in MCF-7 cells treated with NDV-D90 at an MOI 10−2 or 10−3, as survival was merely 10% at 12 hours (P < 0.05) or 20% at 24 hours (P < 0.05) lower in these cells than in their matching control cells. The cell-killing effects of NDV-D90 (MOI = 1 or 10−1) on BT549 cells were delayed until 9 hours postinfection, with a reduction of 10% in survival rate (both P < 0.05). NDV-D90 reduced cell survival by an additional 10% in treated cells when administered at an MOI of one compared with control cells at 12 hours postinfection. NDV-D90 displayed its strongest cell-killing effects when administered at its highest concentration over 24 hours and reduced cell survival by 40% in BT549 cells compared with control cells, showing an effect similar to that elicited by the virus when it was administered to MCF-7 cells over 12 hours under the same conditions. Comparably, NDV-D90 at an MOI of 10−1 in BT549 cells elicited a slow decrease of cell survival as time progressed, with the mortality rate of 15% at 12 hours (P < 0.05) and 25% at 24 hours (P < 0.01). NDV-D90 displayed a slight ability to kill BT549 cells when administered at an MOI of 10−2 or 10−3, as it reduced survival by 10% (both P < 0.05) when administered at the indicated concentrations over 24 hours. In addition, the two cell lines displayed typical morphologic changes in response to treatment with NDV-D90 at its highest concentration for 12 hours. Both cell lines displayed pyknotic nuclei and nuclear fragmentation, as demonstrated by microscopy (Figure 1B). Based on the above results, NDV-D90 was used at an MOI of 10−1 in subsequent experiments.
3.2 NDV-D90 induces apoptosis
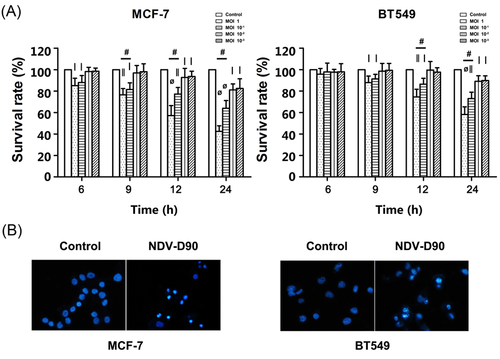
Based on the above results, we evaluated the characteristics of the cell-killing effect of NDV-D90 by FCM. A representative histogram depicting the antitumor effects of treatment with NDV-D90 at an MOI of 10−1 is shown in Figure 2. The dots in the lower- and upper-right quadrants represent apoptosis, while the dots in the upper left quadrant represent necrosis (Figure 2). The apoptosis rate was almost 20% in MCF-7 cells exposed to NDV-D90 for 12 hours and was more than 30% in MCF-7 cells exposed to NDV-D90 for 24 hours. NDV-D90 also promoted apoptosis in BT549 cells. The apoptosis rate was almost 15% and over 20% at the 12-and 24-hours time points, respectively.
3.3 NDV-D90 promotes apoptosis via the intrinsic/extrinsic signaling pathways
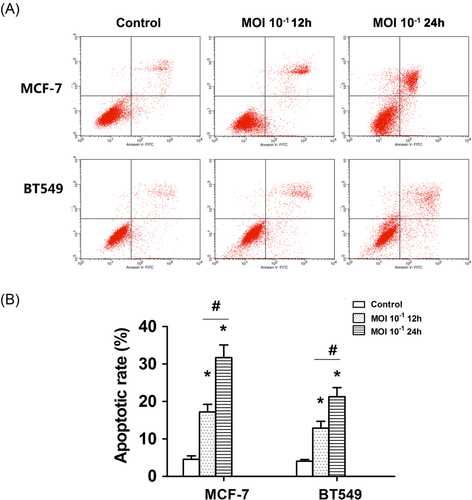
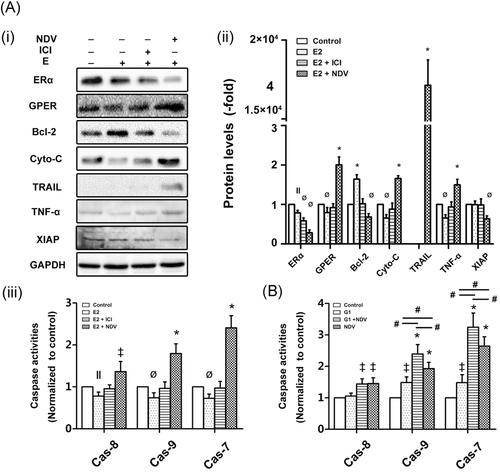
To determine the mechanism by which NDV-D90 induces apoptosis, we utilized Western blot analysis to determine the protein expression profiles of the two BC cell lines undergoing treatment with NDV-D90 at an MOI of 10−1. As shown in Figure 3A, NDV-D90 promoted mitochondrial apoptosis in a time-dependent manner in MCF-7 cells. This process was characterized by decreases in the protein expression levels of Bcl-2 and pro-caspase-9 and increases in the protein expression levels of cytochrome-C and Bax in NDV-D90-treated cells compared with control cells. Extrinsic apoptosis was not elicited by treatment with NDV-D90 until 12-hours time point. This process was characterized by an increase in the expression of TRAIL, as well as a decrease in the expression levels of pro-caspase-6 and 8. Notably, only caspase-7 levels were detected in MCF-7 cells, as caspase-3 was not detectable in this cell line. NDV-D90 treatment elicited a time-dependent decrease in pro-caspase-7 expression in MCF-7 cells (Figure 3A). Similar alterations in the expression levels of apoptosis-related proteins involved in the intrinsic signaling pathway were detected in BT549 cells (Figure 3A). However, no alterations in TRAIL expression were detected in BT549 cells. Similar to the expression levels of the apoptosis-related proteins, the activity levels of caspase-8 and 9, as well as that of the coexecutor caspase-7, were increased in a time-dependent manner in MCF-7 cells exposed to NDV-D90 (Figure 3Bi), demonstrating that NDV-D90 induces apoptosis via the intrinsic and extrinsic signaling pathways. In contrast, NDV-D90 promoted increases in caspase-9 and 3 activity but had no effect on caspase-8 activity in BT549 cells (Figure 3Bii), implying that NDV-D90 induces apoptosis via the intrinsic signaling pathway in this cell line.
3.4 NDV-D90 mediates apoptosis by differentially modulating the expression of ERs
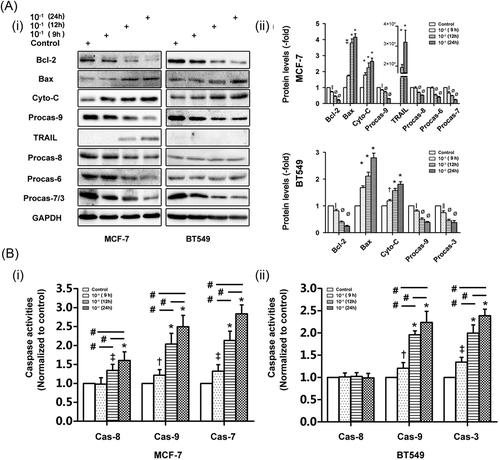
As mentioned above, ERs comprise the classic receptor ERα and the nonclassic ER GPER and function as crucial upstream modulators of multiple downstream signaling pathways in BC cells. The GPER exerts contrasting effects on carcinogenesis in ER-positive and ER-negative BC cells. As shown in Figure 4A, E2 increased MCF-7 cell resistance to apoptosis, a change accompanied by decreases in the protein expression levels of ERα, GPER, cytochrome-C, and TNF-α and increases in the protein expression levels of Bcl-2 in treated cells compared with control cells. Consistent with these findings, in the presence of E2 (Figure 4Aiii), caspase-8, 9, and 7 activity levels were decreased in MCF-7 cells compared with control cells. The ERα antagonist ICI decreased the resistance to apoptosis elicited by E2; however, ICI alone was unable to reverse the antiapoptotic effects of E2 (Figure 4Aiii). Although ICI antagonized the E2-induced decreases in the expression levels of the above-mentioned proteins, ICI did not increase ERα expression in NDV-D90-treated cells rather than decrease its expression compared with E2-treated cells (Figure 4A). The above results demonstrate that the binding of E2 to ERα promotes MCF-7 cell resistance to apoptosis and this resistance is antagonized by the disruption of the interaction between E2 and ERα. NDV-D90 completely reversed E2-mediated resistance to apoptosis and, in contrast to E2 (Figure 4A), enhanced caspase-8, 9, and 7 activity levels (Figure 4Aiii). In addition, the expression of TRAIL and XIAP, a member of the inhibitor of apoptosis proteins (IAPs), were modulated by NDV-D90 in MCF-7 cells. Neither E2 alone nor E2 and ICI in combination influenced the expressions of the two proteins (Figure 4A). The GPER-specific agonist G1 was used to determine the function of the GPER in ER-positive BC cells. As shown in Figure 4B, G1 increased caspase-9- and 7-dependent apoptosis, while NDV-D90 induced caspase-9- and -7-dependent apoptosis and enhanced caspase-8 activity in treated cells compared with control cells. The combination of NDV-D90 and G1 induced greater enhancements of caspase-9 and 7 activities than G1 or NDV-90 alone. In addition, NDV-D90 induced increases in caspase-8 activity similar to those induced by the combination of NDV-D90 and G1. Our data suggest that NDV-D90 induces apoptosis via the intrinsic/extrinsic signaling pathways in ER-positive BC cells not only by disrupting the E2/ERα axis but also by enhancing GPER expression.
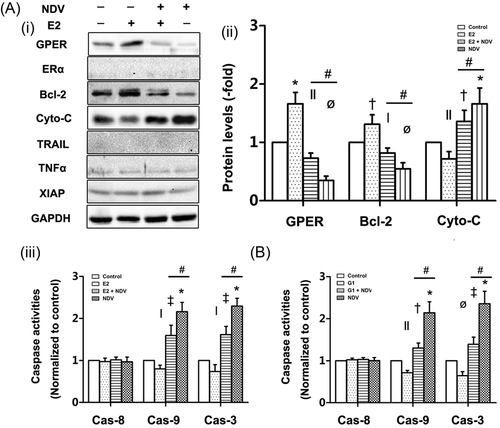
As triple-negative breast cancer (TNBC) cells lack ERα, ICI was not utilized to determine effects of ERα in BT549 cells. As shown in Figure 5Ai and ii, E2 also enhanced resistance to apoptosis in BT549 cells, a change accompanied by increases in the protein expression levels of GPER and Bcl-2 and decreases in the protein expression levels of cytochrome-C in treated cells compared with control cells. Consistent with these findings, caspase-9 and -3 activity levels were decreased in treated cells compared with control cells (Figure 5Aiii). NDV-D90 remarkably promoted apoptosis in BT549 cells despite the presence of E2, while NDV-D90 treatment alone displayed a stronger ability to enhance apoptosis in BT549 cells, accompanied by reversed alterations in the expression levels of the above proteins induced by E2 alone (Figure 5Ai and ii). Consistent with these findings, NDV-D90 enhanced caspase-9 and 3 activity levels (Figure 5Aiii). In contrast to MCF-7 cells, BT549 cells displayed no alterations in TNF-α and XIAP expression, nor did display in ERα or TRAIL expression, in response treatment with E2 alone, E2 with NDV-D90 or NDV-D90 alone (Figure 5Ai and ii). These observations suggest that NDV modulates the intrinsic apoptotic pathway in ER-negative BCs. Interestingly, the GPER-specific agonist G1 decreased caspase-9 and 3 activities, while NDV-D90 reversed these effects and promoted intrinsic apoptosis (Figure 5B). These results suggest that NDV-D90 promotes apoptosis by impairing E2-mediated GPER expression in at least some subtypes of ER-negative BCs.
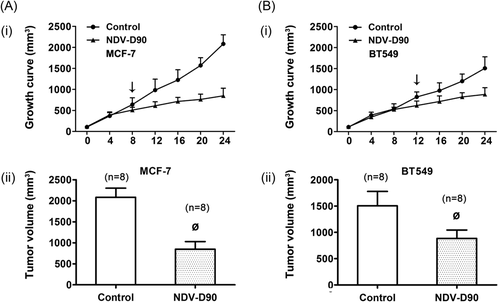
3.5 NDV-D90 exerts antitumor effects by inducing apoptosis in vivo
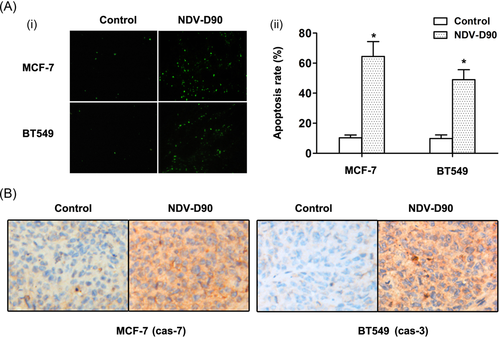
NDV-D90 significantly suppressed tumor growth in nude mice, as shown in Figure 6 (both P < 0.01). The inhibitory effect of NDV-D90 on MCF-7 cell growth in vivo was detectable as early as day 8 postinjection (P < 0.05), while the effect of NDV-D90 on BT549 cell growth was delayed and was not apparent until day 12 postinjection (P < 0.05). Furthermore, as depicted in Figure 7A, the apoptosis rate, as demonstrated by terminal deoxynucleotidyl transferase dUTP nick end labeling assay, was increased in MCF-7 cells (P < 0.01) and BT549 cells (P < 0.01) undergoing NDV-D90 treatment compared with control cells. Consistent with these findings, NDV-D90 promoted apoptosis by enhancing activities of caspase-3 or 7 (Figure 7B), functioning as coexecutor of caspase-mediated apoptosis in the two cell types.
4 DISCUSSION
NDV is well known for its ability to trigger apoptosis in infected tumor cells.7, 9, 10, 13 In the present study, NDV-D90 exerted stronger killing effects in MCF-7 cells than in BT549 cells (Figure 1), and NDV-D90 induced apoptosis at an earlier stage in MCF7 cells (6 hours) than in BT549 cells (9 hours). Furthermore, NDV-D90 exerted its cell-killing effects mainly by inducing apoptosis as opposed to necrosis (Figure 2), which occurred in less than 8% of cells (data not shown). These observations demonstrate that NDV-D90 may induce apoptosis in BC cells in a subtype-dependent manner. Further, the results of the apoptosis characterization experiments showed that NDV-D90 induced apoptosis via both the intrinsic and extrinsic apoptosis signaling pathways in MCF-7 cells and merely via the intrinsic apoptosis signaling pathway in BT549 cells (Figure 3). Therefore, these results drove us to pay more attention to how NDV-D90 differentially promoted apoptosis in the two BC cell lines.
As stated above, the classic estrogen receptor ERα and the nonclassic estrogen receptor GPER are crucial biomarkers that participate in modulating the activity of multiple downstream signaling pathways in BC cells exposed to E2, especially for the GPER that performs contrasting functions in ER-positive and ER-negative BC cells.4-6, 20-22, 28, 29 Therefore, based on the above results, we investigated whether both ERα and the GPER are involved in the effects of NDV-D90 on promoting apoptosis in MCF-7 (ER-positive cells) and BT549 (ER-negative cells) cells exposed to E2. As anticipated, E2, which served as a sustained extracellular stimulus, bound to the ERα and strengthened the ability of the E2/ERα axis to exert antiapoptotic effects in MCF-7 cells (Figure 4Ai, lane 1), consistent with the idea that ERα commonly functions as a crucial oncogenetic protein in BCs.20-22 One point that should be emphasized is that E2 partially promotes ERα degradation through a negative-feedback mechanism mediated by the ubiquitin pathway; however, this hormone-dependent feedback mechanism is generally coupled to enhanced activity of E2/ERα axis rather than its impairment.5, 28 These findings explain the enhancement of MCF-7 cell resistance to apoptosis that occurs in the presence of E2 (Figure 4A). ICI, an ER antagonist, promotes ERα degradation through a process that may be distinct from the hormone-mediating ERα proteolysis as mentioned above.5, 30 Although ICI blocked E2-mediated resistance to apoptosis by partially inhibiting ERα expression to impair the antiapoptotic effect of E2 in MCF-7 cells (Figure 4Aiii), ICI treatment alone seemed to be insufficient to initiate apoptosis (Figure 4A). In contrast, NDV-D90 disturbed the E2/ERα axis by remarkably impairing ERα expression and triggered apoptosis in MCF-7 cells (Figure 4A). Further, there are several mechanisms responsible for the apoptotic effects of NDV-D90 in MCF-7 cells exposed to E2. NDV-D90, which is endowed with oncolytic characteristics, may directly contribute to the impairment of ERα expression in MCF-7 cells. Undoubtedly, impaired ERα expression can contribute to enhancements of intrinsic/extrinsic apoptotic signaling by widely modulating the expression of multiple apoptosis-related proteins.31-34 However, the decrease in ERα expression caused by NDV-D90 was accompanied by increases in GPER protein expression. The GPER has been confirmed to be a tumor suppressor in ER-positive BCs.35, 36 GPER is negatively modulated by E2 to prevent GPER-dependent tumor suppression in ER + BCs, and GPER expression is inversely related to ERα expression,28 which may explain why GPER expression was enhanced after NDV-D90 impaired ERα expression (Figure 4Ai, lane 4). However, whether the increase in GPER expression elicited by NDV-D90 exposure had antitumor effects in MCF-7 cells required further exploration. We performed a study in which the GPER-specific agonist G1 promoted the enhancement of caspase-9 activity but failed to induce caspase-8-dependent apoptosis in MCF-7 cells (Figure 4B), suggesting that the GPER is a tumor suppressor and exhibits an ability to induce apoptosis mostly via the intrinsic caspase-9-dependent pathway in ER + BCs. Therefore, the modulatory effects of NDV-D90 on ERα and the GPER may partially contribute to increases in the intrinsic/extrinsic apoptosis pathway activities. In the present study, XIAP and TRAIL seemed to fail to respond to E2 alone or E2 combined with ICI in MCF-7 cells; however, alterations in the expression levels of the two proteins were noted after NDV-D90 treatment (Figure 4Ai). Although TRAIL specifically induces apoptosis, either resistance to TRAIL or difficulties in its induction limits the effective application of TRAIL in BC cells.37, 38 XIAP is the only IAP that negatively modulates apoptotic pathways by directly binding to several caspases.39, 40 However, XIAP may not be adequately sensitive to E2 stimulation in MCF-7 cells,41 which may explain why XIAP expression was not altered under E2 stimulation (Figure 4Ai). However, NDV-D90 succeeded in inducing TRAIL expression and decreasing XIAP expression, which may partially contribute to the intrinsic/extrinsic apoptotic pathway activity, suggesting that NDV-D90 overcomes E2-induced resistance to apoptosis by modulating the expression of anti- and proapoptotic proteins that are not readily modulated by conventional treatments in ER-positive BC cells. Interestingly, a previous study demonstrates that the enhancements of extrinsic pathway activity mediated by NDV-induced increases in expressions of TNF-α and TRAIL may be dispensable for the execution of apoptosis, which may be indirectly modulated by the intrinsic signaling.42 However, in the present study, NDV-D90 promoted the caspase-8-dependent extrinsic apoptosis in the MCF-7 cells, accompanied by upregulation of TNF-α and TRAIL, while similar events were not detectable in the BT549 cells under the same condition (discussed below), possibly suggesting that NDV-D90-mediated inductions of TNF-α and TRAIL expressions seems to be undeniably related to the extrinsic apoptosis. Thus, we surmised that the direct involvement of NDV-D90 in mediating the extrinsic apoptosis may be cellular- or viral-subtype dependent. Overall, the above results suggest that NDV-D90 is endowed with a strong ability to sensitize ER-positive BC cells to programmed cell death via the intrinsic and extrinsic apoptotic signaling pathways despite the presence of E2 not only by modulating the expression of ERα and the GPER but also by distinctly modulating the expression of several apoptosis-related proteins through ERα- and GPER-independent manners.
Almost 20% to 30% of breast cancers are classified as TNBCs, which lack ERα, the progesterone receptor, and EGFR2 (Her-2). Thus, therapies targeting certain signaling pathways are not applied for or are less effective against TNBCs. In the present study, E2 also strengthened the resistance of BT549 cells to apoptosis; however, this effect seemed to be unrelated to ERα because ERα expression was not successfully induced, while GPER expression was unexpectedly enhanced in BT549 cells (Figure 5A). This finding may be explained by the idea that the GPER is likely modulated by E2 in a positive-feedback manner in TNBCs and that this modulation may be distinct from the manner in which it is modulated in ER-positive BCs.43, 44 The fact that contrasting changes in GPER expression occur in response to NDV-D90 treatment in ER-positive and negative BCs raised the question of whether the enhancement of GPER expression is proportional to the E2-mediated enhancement of BT549 cell resistance to apoptosis, similar to roles of ERα in the MCF-7 cells. G1 was used to validate the function of the GPER in ER-negative BCs. Interestingly, instead of promoting apoptosis, G1 enhanced BT549 cell resistance to apoptosis and thus fulfilled a role that is entirely different from its role in MCF-7 cells (Figure 5B). However, NDV-D90 thoroughly reversed the resistance induced by G1 and also significantly increased caspase-9 activity, suggesting that the GPER, in at least certain subtypes of TNBC, is characterized as an oncogenic protein and NDV-D90 is able to enhance apoptosis by impairing GPER expression in these ER-negative tumor cells. The results of our study support the idea that GPER-1 functions as an oncogenic protein and contributes to the malignant behavior of TNBCs.23, 45, 46 Therefore, the GPER plays a role that is functionally opposite of its role in MCF-7 cells (ER-positive cells). In addition, GPER participates in the resistance of TNBCs to apoptosis by modulating the expression of Bcl-2 family members.24 NDV-D90 was unable to modulate the expression of TNF-α, TRAIL, and XIAP, which may explain why the virus did not induce caspase-8-dependent apoptosis in BT549 cells. These results suggest that NDV-D90 contributes to the induction of the mitochondrial apoptosis pathway in BT549 cells at least in part by impairing E2-mediated GPER expression. Furthermore, the in vivo studies involving the xenograft tumor model showed that NDV exerts antitumor effects on the two BC cell lines (Figures 6, 7). NDV-D90 displayed a decreased ability to induce programmed cell death in BT549 cells than in MCF-7 cells in vitro and in vivo, possibly because of deficiencies in the expression of certain upstream biological targets involved in modulating apoptosis in TNBC. Currently, a recombinant NDV by means of inserting foreign genes promotes the TRAIL-induced apoptosis, indicating that the antitumor potency of NDVs could be effectively modified by selectively interfering with tumor cell resistance to extrinsic apoptosis.10
The induction of programmed cell death is known as a crucial hallmark of NDV-mediated oncolytic properties in infected tumor cells. Although controversy exists regarding the involvement of NDVs in the modulation of the extrinsic apoptosis,42 it is commonly accepted that NDVs are more likely to impact intrinsic apoptosis. This may be because mitochondria are sensitive to a variety of stress-inducing stimuli and NDVs may directly modulate the expressions of Bcl-2 family members in available NDV-infected cells.47-49 However, it should be noted that NDV-mediated intrinsic apoptosis may be at least partially attributable to the modulatory effects of NDV on ERs, including ERα and the GPER, in hormone-dependent BC cells. Therefore, it can be speculated that antitumor effects of NDVs on hormone-dependent tumors may be partially dependent on their ability to modulate hormone-related receptors, which is separate from their oncolytic functions that may directly participate in modulating other tumor-associated proteins in many other malignancies.
Taken together, the findings of this study show that NDV-D90 initiates apoptosis at least in part by differentially modulating the expression of the classic estrogen receptor ERα and the nonclassic estrogen receptor GPER and by specifically modulating the expression of certain apoptosis-related factors in ER-positive and negative BC cells. Future research should focus on recombined NDV-D90 strains generated by selective genetic modification to compensate for deficiencies in the expression of crucial biomarkers capable of exerting antitumor effects in BC cells. NDV-D90, also identified as avian paramyxovirus serotype-1, is a novel virus that displays an oncolytic ability to induce apoptosis and thus warrants further investigation, as it may be a promising treatment for a variety of cancers.
ACKNOWLEDGMENTS
The authors would like to thank Professor Xi Li (State Key Laboratory of Veterinary Biotechnology, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences) for providing NDV-D90. This study was funded by grant from the National Natural Science Foundation of China (81400350).