Does the Harvesting Technique Affect the Properties of Adipose-Derived Stem Cells?—The Comparative Biological Characterization
ABSTRACT
The objective of this study was to evaluate complex biological properties of human stem cells isolated from adipose tissue (ASCs) harvested utilizing different methods: surgical resection (R), power-assisted liposuction (PAL), and laser-assisted liposuction (LAL). ASCs were isolated from healthy donors, due to surgical resection, power-, and laser-assisted liposuction. Isolated cells were characterized by their clonogenicity, proliferation rate, doubling time, multilineage differentiation, and senescence potential. The average number of ASCs from 1g/1 ml of solid adipose tissue/lipoaspirate was 2.9 × 105 ± 2.4 × 105, 1.1 × 105 ± 0.8 × 105, and 1.2 × 105 ± 0.7 × 105, respectively, for ASCsR, ASCsPAL, and ASCsLAL. However, number of colonies formed by ASCsR and ASCsPAL was significantly higher compared to the average number of colonies formed by ASCsLAL. Also, in comparison to other analyzed cell groups, ASCsPAL obtained the highest proliferative activity. All analyzed cells were characterized by stable expression of CD90 and CD44 markers during prolonged culture. Expression of CD34 and CD45 markers was decreasing in subsequent passages. Presented study shows that different ASCs collection method affects some basic characteristics of these cells, such as number of isolated cells, clonogeneity, or doubling time. J. Cell. Biochem. 118: 1097–1107, 2017. © 2016 Wiley Periodicals, Inc.
First applications of tissue engineering were based on the use of differentiated cells originating from adult organism [Rustad et al., 2010]. However, it was associated with high invasiveness [Howard et al., 2008]. In recent years, the range of available cell populations for tissue engineering has significantly widened. From several decades stem cells were the focus of research, especially mesenchymal stem cells (MSCs), due to their unique biological properties [Joachimiak et al., 2012]. Adipose tissue was considered only as a passive energy storage, until the mid-80s of the last century, when its participation in the metabolism of sex hormones was confirmed, and it started to be considered an important endocrine organ that controls metabolism, immunity, and satiety. The most significant breakthrough had place in 2001 when adipose tissue was described as a new source of adult stem cells, called adipose-derived stem cells (ASCs) [Zuk et al., 2001]. After several years of research, adipose tissue appears to be a great source of stem cells due to isolation in large quantities, during relatively safe and quick liposuction procedures [Zuk et al., 2002]. High content of ASCs in adipose tissue excludes a need for long-term in vitro culture, which reduces the risk of chromosomal abnormalities. Furthermore, ASCs are characterized by clonogenicity, high proliferation rate, and potential to multilineage differentiation [Guilak et al., 2004, 2006; Helder et al., 2007; Gimble et al., 2010]. Such properties make ASCs an attractive and possibly efficient tool for therapeutic purposes [Nakagami et al., 2006].
Since adipose tissue stem cells are starting to reach the clinical use stage, there is an urgent need to investigate the differences between stem cell populations isolated by different methods and identify the one generating the most clinically utilizable ones with the highest efficiency possible.
Therefore, the objective of this study was to evaluate complex biological properties of human stem cells isolated from adipose tissue (ASCs) harvested utilizing different methods: surgical resection (R), power-assisted liposuction (PAL), and laser-assisted liposuction (LAL). The comparison is aimed at describing ASCs behavior in cell culture, which could be useful for their future use in potential medical procedures.
MATERIALS AND METHODS
ORIGIN OF ADIPOSE TISSUE
- solid adipose tissue obtained during surgical procedures (n = 27),
- lipoaspirate obtained during PAL (n = 21),
- lipoaspirate obtained during LAL (n = 21).
To minimize the differences between patients, the samples were selected in terms of sex (females), age (≥40), BMI (≥25), and anatomical locations (the abdominal region).
All patients signed informed consent and were informed about the procedure according to the study protocol, which was approved by the local University Ethics Committee (KB 287/2011).
ISOLATION AND CULTURE OF ASCs
Procedure of ASCs isolation was initiated by washing adipose tissue with sterile PBS (phosphate-buffered saline, Sigma–Aldrich, Germany) containing 5 μg/ml amphotericin B, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma–Aldrich) to remove blood cells. Subsequently, vessels and fibrous tissue were gently removed and the adipose tissue was minced into small fragments (1–2 mm).
Liposuction aspirate was washed with sterile PBS containing antibiotics (as above) to eliminate blood cells, saline, and anesthetics used during tumescent liposuction.
Further isolation steps were the same for both resected fat and lipoaspirate. The washed adipose tissue fragments underwent enzymatic digestion, with type I collagenase at a final concentration of 0.075% (Sigma–Aldrich) at 37°C for 30 min. The digestion was interrupted with the addition of an equal volume of complete culture medium DMEM/Ham's F12 (Dulbecco's modified essential medium, Sigma–Aldrich) supplemented with 10% FBS, 5 μg/ml amphotericin B, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Sigma–Aldrich). Afterwards, samples were centrifuged twice at 170g for 5 min at room temperature, and SVF (Stromal Vascular Fraction) pellet was resuspended in complete DMEM/Ham's F12 medium. Suspended cells were then passed through 100 µm cell strainer (BD Bioscience, US AP) to separate the undigested tissue fragments and once again centrifuged. SVF pellet was suspended in complete culture medium and isolated cells were plated at an equivalent to ∼15 g ml of fat tissue/liposuction aspirate per T25 flask. The cells were cultured at 37°C, under 5% CO2. The medium was changed every second day until the cells reached 80–90% confluence.
COLONY FORMING EFFICIENCY ASSAY (CFE)
ASCs isolated from resected adipose tissue (ASCsR) and from lipoaspirate, harvested during PAL (ASCsPAL) and LAL (ASCsLAL), after second passage were seeded in 6-well culture plates (BD Biosciences, US AP) in the number of 1 × 103, 5 × 103, and 10 × 103/well. After 14 days of incubation, cell colonies were visualized with 2% Rhodamine B staining (Sigma–Aldrich) and counted during macroscopic observation.
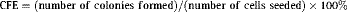
PROLIFERATION RATE AND POPULATION DOUBLING TIME
To assess the proliferation rate of ASCs, cells from second passage were seeded in 24-well culture plates (BD Bioscience, US) at a density of 1 × 104/well and cultured in standard conditions. Cells were detached from the culture flask and counted, the procedure was repeated daily for 14 days. Based on the graph showing dependency of cell number over culture time, population doubling time (PDT) was calculated according to the formula: PDT = CT/(logN/No) × 3.31, where CT—culture time, No—initiating cell number, N—harvesting cell number.
ANALYSIS OF ASCs GROWTH USING THE xCELLigence SYSTEM
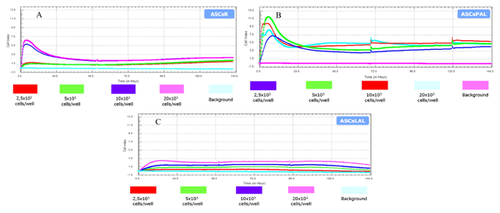
The xCELLigence system (Roche, US) illustrates cell proliferation and viability without any stain or label. After background impedance measurement, ASCsR, ASCsPAL, and ASCsLAL were seeded in 16-well E-plates at a density of 2.5 × 103; 5 × 103; 10 × 103; and 20 × 103/well. Proliferation, morphological changes, and attachment of cells to the wells surface were monitored every 15 min for 120 h. Relative change in electrical impedance that represents cells status was measured by the real-time cell analyzer (RTCA)-DP belonging to xCELLigence system, and expressed as a unitless parameter termed cell index (CI). The growth curve was recorded automatically using the integrated software system (RTCA Software 1.2.1).
SENESCENCE ANALYSIS
The senescence was evaluated by assessment of total cellular β-galactosidase activity in cell lysates. For the preparation of cell lysates, a mean number of 4 × 105 ASCsR, ASCsPAL, and ASCsLAL was collected during five following passages. Cell suspension was centrifuged at 170g for 5 min, obtained cell pellet was then resuspended in 300 µl of 0.05 M Tris/HCl (pH = 7.6) (Sigma–Aldrich), transferred to −20°C for 3 min and then placed in a water bath at 37°. Cell lysate was centrifuged for 10 min at 350g. Collected supernatant was stored at −80°C until use, but no longer than 2 months. The assay was initiated by the addition of 100 µl of the lysate to 200 µl of the reaction buffer containing 0.25 M sodium phosphate, pH 6.0 (POCH, Poland); 0.01 mM MgCl2 (Sigma–Aldrich); 0.45 M β-mercaptoethanol (ACROS, Belgium); and 4 mg/ml ONPG in distilled water. After 30 min of incubation, enzymatic reaction was inhibited by addition of 0.1 M NaOH. Absorbance was measured spectrophotometrically at 405 nm.
CD MARKER AND PLURIPOTENT MARKER ANALYSIS
Phenotype of ASCsR, ASCsPAL, and ASCsLAL was analyzed with the use of anti-CD34-PE, anti-CD44-FITC, anti-CD45-PE-Cy7, and anti-CD90-APC antibodies (BD Bioscience, US). Analysis was repeated after the first, third, and fifth passages in order to evaluate the phenotypic stability of ASCs during prolonged culture. ASCs obtained from a commercially available human hSVF fraction (Irisbiosiences, Portugal) were used as positive control.
To analyze the markers of pluripotency (hNanog, Oct3/4, Sox2), BD Human Pluripotent Stem Cell Factor Analysis Transcription Kit (BD Biosciences, US AP) was used. All of the analyses were performed according to the manufacturer's protocol. Evaluation of markers expression was performed using flow cytometry (FACSCanto II, BD Biosciences, US AP).
CELL DIFFERENTIATION ASSAY
ASCsR, ASCsPAL, and ASCsLAL were analyzed in terms of capacity to differentiate into the adipogenic, osteogenic, and chondrogenic lineages. It was the qualitative analysis, we do not measure how many cells were differentiated. Differentiation into adipocytes and osteocytes was performed in a monolayer. Cells were plated at a density of 4 × 104/well and 3 × 104/well, respectively, and cultured at 37°C for 2 weeks in a differentiation medium. Adipogenic differentiation was induced by DMEM/Ham's F12 (3:1) supplemented with 10% FBS, 10 μm insulin (Sigma–Aldrich), 1 µM dexamethasone (Sigma–Aldrich), 5 µg/ml amphotericin B, and 100 µg/ml penicillin/streptomycin [Karaoz et al., 2009; Khatri et al., 2009]. Differentiation process was visualized by staining with Oil Red O. Osteogenic differentiation was induced by DMEM/Ham's F12 (3:1) supplemented with 15% FBS, 50 μM ascorbic acid (Sigma, Germany), 0.1 μM dexamethasone (Sigma–Aldrich), 5 µg/ml amphotericin B, and 100 µg/ml penicillin/streptomycin [Payushina et al., 2006]. Differentiated ASCs were evaluated by calcified extracellular matrix (ECM) deposition using von Kossa assay (Bio-Optica, Italy).
Chondrogenic differentiation was performed in three-dimensional (3D) pellet culture. ASCs were suspended at a density of 1 × 106 in 0.5 ml of 1% alginate (Sigma–Aldrich) and the alginate beads were prepared by the ionotropic gelation method using calcium chloride as a crosslinking agent. The chondrogenic medium consisted of DMEM/Ham's F12 (3:1) supplemented with 1% ITS (Sigma–Aldrich), 10 ng/ml TGFβ3 (Sigma–Aldrich), 100 nM dexamethasone (Sigma–Aldrich), 5 µg/ml amphotericin B, and 100 µg/ml penicillin/streptomycin [Lindroos et al., 2011; Mardani et al., 2013]. Cells were incubated for 2 weeks and chondrogenesis was confirmed by the expression of collagen II. All of the differentiation procedures for each method was repeated at least three times.
STATISTICAL ANALYSIS
Each experiment presented in this manuscript was performed at least 3 times. All the data were presented as mean ± SD. Shapiro–Wilk test was used to test data normality. To assess statistical significance parametric test ANOVA and non-parametric Kruskal–Wallis test were used. Differences were considered significant when the P value was less than 0.05. Statistical analysis was performed using STATISTICA v.10 and Excel 2007.
RESULTS
ISOLATION AND CULTURE OF ASCs
ASCs isolated from fat tissue/lipoaspirate were seeded in T25 culture flasks. After 48 h of culture, non-adherent, floating cells were removed and the adhered cell population was washed with PBS. Although during the initial culture period some morphological heterogeneity of the adherent fraction could be observed, after the first 48 h of culture, most of the cells displayed fibroblastic-like morphology. The population of cultured cells became morphologically homogenous around the 4th day of culture (Fig. 1).

CELL CULTURE SUCCESS AND NUMBER OF ISOLATED ASCs
The success rates for establishing cell cultures from ASCsR, ASCsPAL, and ASCsLAL was 100%. ASCsR reached the state of 70–80% confluence within 8 ± 3 days of culture, ASCsPAL within 7 ± 3 and ASCsLAL within 7 ± 2 days. Statistical analysis showed no significant difference in the average number of days between establishing the culture and the first passage between the studied ASCs (P = 0.22). The average number of ASCs isolated from 1 g of solid adipose tissue or 1 ml of lipoaspirate was 2.9 × 105 ± 2.4 × 105, 1.1 × 105 ± 0.8 × 105, and 1.2 × 105 ± 0.7 × 105, respectively, for ASCsR, ASCsPAL, and ASCsLAL. The average number of isolated ASCsR was significantly higher than ASCsPAL and ASCsLAL (P = 0.01).
GROWTH CHARACTERISTICS OF ASCs
The clonogenicity of ASCs was evaluated after 14 days with rhodamine B staining. ASCs seeded in number of 1 × 103/well formed on average 24 ± 4 colonies, 23 ± 7 colonies, and 9 ± 3 colonies for ASCsR, ASCsPAL, and ASCsLAL, respectively (Fig. 2A). The average number of colonies formed by ASCsR and ASCsPAL was significantly higher compared to the average number of colonies formed by ASCsLAL (P = 0.00024, P = 0.00034). Clonogenicity correlates with the number of stem cells, and amounted to 2.4%, 2.3%, and 0.9%, respectively, for ASCsR, ASCsPAL, and ASCsLAL (Fig. 2B).
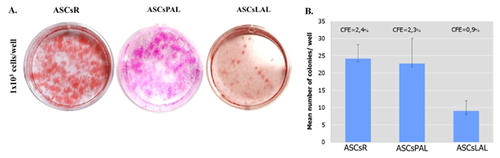
Analyzing the growth curves of isolated cells, the longest phase of adaptation to culture conditions was observed for ASCsLAL. ASCsPAL reached the plateau phase at the 8th day of the culture, while this phase was not observed for the other cell populations. The decrease in the proliferation rate for all tested cells occurred between 7th and 10th day of culture. In addition, the population doubling time was calculated, which was significantly longer for ASCsR (5.8 ± 1.4 days) in comparison with ASCsPAL (2.5 ± 0.7 days) and ASCsLAL (3.5 ± 0.3 days) (Fig. 3).
Analysis of ASCs growth kinetics at various cell seeding densities was performed with the use of xCELLigence system. Cell viability was expressed by the CI parameter, which values express the change in electrical impedance resulting from an increase in the number of cells undergoing adhesion to the gold electrode array on the surface of companion plates. Seeding density had a significant impact on proliferation of ASCsR and ASCsLAL. In comparison to other analyzed cell groups, ASCsPAL obtained the highest proliferative activity, even at the lowest plating density (Fig. 4).
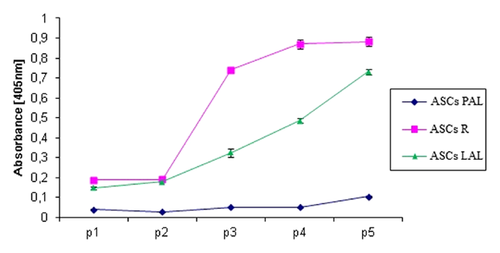
SENESCENCE OF ASCs DURING PROLONGED CELL CULTURE
The total β-galactosidase activity was determined in cell lysates prepared during the first five passages with the use of biochemical assay. The lowest activity of β-galactosidase was observed in ASCsPAL. Furthermore, the enzyme activity remained low during five following passages. β-Galactosidase activity in ASCsLAL increased gradually with each passage. ASCsR showed similar levels of β-galactosidase activity to ASCsLAL during the first two passages. However, the enzyme activity rapidly increased with the beginning of passage 3 (Fig. 5). These results were confirmed by a colorimetric assay of β-galactosidase activity (data not shown).
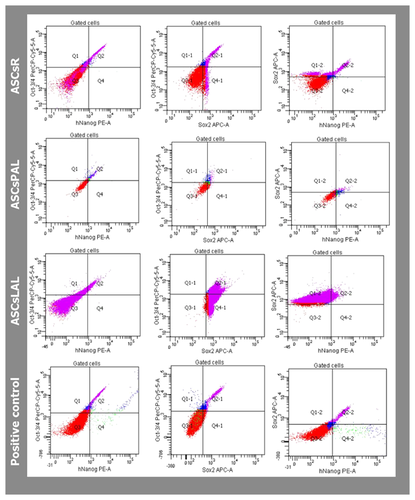
IMMUNOPHENOTYPING OF DIFFERENT ASCs POPULATIONS
To analyze the stem cell phenotype of isolated ASCs, the expression of CD34, CD44, CD45, and CD90 surface markers was analyzed after first, third, and fifth passages. All three studied cell populations showed high expression of CD44 and CD90 markers (Table I). Examined cells also expressed the CD34 marker that distinguished them from other mesenchymal stem cells. There was no evidence of significant sample contamination with hematopoietic cells, as the CD45 marker expression on cells examined after the first passage was low and was decreasing during the following passages.
| Passage no. | CD90 | CD44 | CD34 | CD45 |
|---|---|---|---|---|
| ASCsR | ||||
| P1 | 93.0 ± 5.7 | 95.8 ± 0.2 | 84.4 ± 1.1 | 16.0 ± 6.7 |
| P3 | 99.8 ± 0.1 | 96.8 ± 0.1 | 82.8 ± 0.4 | 15.4 ± 5.8 |
| P5 | 98.8 ± 0.1 | 97.7 ± 0.4 | 26.9 ± 2.8 | 10.1 ± 0.2 |
| ASCsPAL | ||||
| P1 | 86.4 ± 3.4 | 84.0 ± 10.7 | 91.0 ± 9.6 | 38.0 ± 2.3 |
| P3 | 88.6 ± 5.0 | 94.9 ± 1.3 | 56.5 ± 0.3 | 30.6 ± 2.8 |
| P5 | 98.1 ± 0.4 | 98.0 ± 0.1 | 44.1 ± 3.7 | 15.4 ± 3.4 |
| ASCsLAL | ||||
| P1 | 89.3 ± 3.5 | 93.7 ± 1.6 | 84.3 ± 1.3 | 14.7 ± 4.8 |
| P3 | 97.3 ± 0.1 | 97.9 ± 0.4 | 75.5 ± 0.1 | 6.3 ± 2.4 |
| P5 | 96.6 ± 4.0 | 95.4 ± 4.0 | 14.0 ± 0.5 | 5.2 ± 0.6 |
| Positive control | ||||
| P3 | 85.3 ± 4.9 | 95.9 ± 0.7 | 7.1 ± 0.6 | 10.1 ± 0.6 |
All analyzed cells were characterized by stable expression of CD90 and CD44 markers during prolonged culture (Table II). Expression of CD34 and CD45 markers was decreasing in subsequent passages. There was no difference in expression of CD44 and CD90 markers between all tested groups of cells.
| CD90 | CD44 | CD34 | CD45 | |
|---|---|---|---|---|
| ASCsR | ||||
| P1 vs. P3 | 0.2407 | 0.0871 | 0.4479 | 0.9381 |
| P3 vs. P5 | 0.2929 | 0.2196 | 0.0051* | 0.3221 |
| P1 vs. P5 | 0.2990 | 0.0827 | 0.0054* | 0.3403 |
| P3 vs. control | 0.0537 | 0.2196 | 0.0001* | 0.3241 |
| ASCsPAL | ||||
| P1 vs. P3 | 0.6385 | 0.2904 | 0.0363* | 0.0981 |
| P3 vs. P5 | 0.1197 | 0.0820 | 0.0427* | 0.0392* |
| P1 vs. P5 | 0.0402* | 0.2100 | 0.0231* | 0.0159* |
| P3 vs. control | 0.5605 | 0.4339 | 0.0001* | 0.0094* |
| ASCsLAL | ||||
| P1 vs. P3 | 0.0912 | 0.0664 | 0.0104* | 0.1577 |
| P3 vs. P5 | 0.8890 | 0.4674 | 0.0001* | 0.5932 |
| P1 vs. P5 | 0.1960 | 0.6432 | 0.0002* | 0.1090 |
| P3 vs. control | 0.0790 | 0.0755 | 0.0001* | 0.1666 |
- *Statistical significance (P < 0.05).
Expression of three pluripotency markers (hNanog, OCT3/4, and SOX2) was analyzed in cells after the first passage. Cytometric analysis did not show expression of selected pluripotency markers in examined cells (Fig. 6).
DIFFERENTIATION POTENTIAL OF DIFFERENT ASCs POPULATIONS
Potential of ASCs to differentiate into adipocytes was assessed on the basis of fat droplet presence in the cells’ cytoplasm. The size of ASCs after 8 days of culture in differentiation medium was significantly increased. Fat droplets were observed in the cytoplasm of ASCsLAL and ASCsPAL. After 14 days of culture in adipogenesis-inducing medium, differentiation and fatty vacuoles formation was confirmed by staining with Oil Red O solution (Fig. 7).
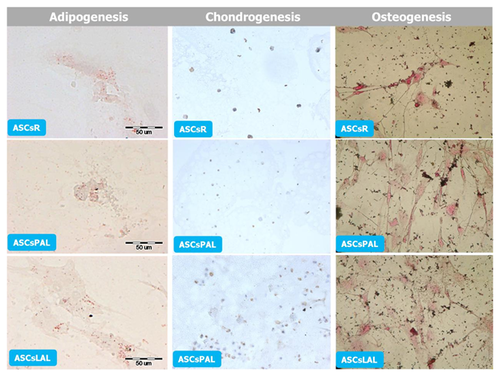
The chondrogenic potential of ASCs was evaluated by culture of micropellets in differentiating medium. After 14 days of culture, immunocytochemical analysis was performed and all tested ASCs showed expression of collagen type II (Fig. 7).
The induction of osteogenesis resulted in the change of ASCs morphology after 5 days of culture in the presence of differentiation factors. Spindle-shaped cells assumed characteristic for osteoblasts polygonal form. After 2 weeks, osteogenesis potential of all ASCs populations was confirmed by staining of extracellular matrix calcification (Fig. 7).
DISCUSSION
The issue of ready available sources for adult stem cells isolation has recently grown in importance, since these cells can become invaluable tools in the developing field of regenerative medicine. Collection of adipose tissue during surgical procedures such as, but not limited to, liposuction is relatively safe and does not cause much discomfort to the patient. However, the procedure used to harvest this tissue may have negative effect on the number and viability of isolated ASCs and thereby restrict their potential use in further therapy. In this study, we performed a robust, comparative analysis of ASCs derived using different methods (surgical resection vs. PAL vs. LAL) of adipose tissue isolation. The majority of studies conducted to date on ASCs refer to cells isolated from adipose tissue obtained by standard liposuction aspiration. In everyday clinical practice, however, less invasive procedures are becoming increasingly more popular, but our knowledge regarding their impact on ASCs quality is still very limited [Chung et al., 2013].
The biggest advantage of ASCs is the possibility of isolating high quantities of those cells at once. It is possible to isolate 5,000 to 3.5 million of cells from 1 g of adipose tissue [Aust et al., 2004; Mitchell et al., 2006; Yu et al., 2010; Guilak et al., 2010; Baer et al., 2013]. These differences in the number of isolated ASCs are caused mainly by donor characteristics, such as gender, race, body mass index, medical history, and the type of acquired adipose tissue (yellow/brown). Location of adipose tissue (subcutaneous/visceral fat), collection method, and culture conditions also plays a major role in the number of isolated cells [Olkowska-Truchanowicz, 2008].
The presented results confirm that adipose tissue is a rich and potent source of stem cells. The largest number of ASCs have been isolated from solid tissue, that is, 2.9 × 105 ± 2.4 × 105/1 g of fat tissue. There was no significant difference in the number of cells isolated from the tissue harvested by PAL (1.1 × 105 ± 0.8 × 105/1 ml lipoaspirate) and LAL (1.2 × 105 ± 0.7 × 105/1 ml lipoaspirate), but these results were significantly lower when compared with the number of cells isolated from solid tissue. It could be a result of adverse effects of the laser action during LAL or the infiltration solution used during PAL. Despite the smaller number of cells isolated from lipoaspirate, adipose tissue harvested during liposuction procedures was still a very rich source of adult stem cells in comparison to bone marrow [Pittenger et al., 2000]. Furthermore, taking into account considerable clinical benefits of liposuction compared to surgical resection, isolation of ASCs from lipoaspirate appears to be quite favorable for regenerative medicine purposes.
The clonogenic capacities of ASCs obtained due to these three methods also differed. In our analysis, clonogenicity was rated as 2.4%, 2.3%, and 0.9% for ASCsR, ASCsLAL, and ASCsPAL, respectively, which is a significantly better result compared to other authors [Bianco and Robey, 2004; Kastrinaki et al., 2008; Dmitrieva et al., 2012]. The greater expansion capability of ASCs was confirmed, as the doubling time was 72 and 144 h, respectively, for LAL and surgical resection. These findings differ from those obtained by Oedayrajsingh-Varma et al. [2006], who reported various results regarding cells isolated from solid tissue, where cell doubling time was 72 h and did not differ significantly from population doubling time of cells isolated from lipoaspirate harvested during tumescent liposuction. However, the results obtained for cells isolated from tissue harvested due to PAL, calculated as 96 h, are consistent with the results of other authors [Zuk et al., 2001; Oedayrajsingh-Varma et al., 2006; Jurgens et al., 2008]. It is well documented that the doubling time of ASCs population, in the logarithmic growth phase, ranges from 40 to 120 h depending on the donor age, type of fat (yellow whether brown), location (subcutaneous tissue whether visceral), culture conditions, density of seeding, composition of the culture medium or the type of surgical procedure used for the tissue collection [Zuk et al., 2001; De et al., 2003; Izadpanah et al., 2006; Mitchell et al., 2006]. Torio-Padron et al. [2010], in his research, shown no difference in the proliferation capacity of cells isolated from lipoaspirate and solid tissue, for which the resulting growth curves were similar.
In presented study, cell growth curves differed among the three studied groups. The adaptation phase was the longest for ASCsPAL. ASCsLAL were characterized by the longest plateau phase, which was not observed for ASCsR and ASCsPAL. The results obtained by other authors suggest that the differences in kinetics of ASCs growth and doubling time may be influenced by adipose tissue donor characetristics rather than its collection method [Van Harmelen et al., 2003; Lei et al., 2007; Frazier et al., 2013].
β-Galactosidase is the commonly used biomarker for senescent and aging cells. Increased activity of this enzyme was also observed in ASCs during long-term culture [Ding et al., 2013]. However, comparing to other adult stem cells, aging rate of ASCs is lower [Zhou et al., 2008]. We have confirmed an increase in β-galactosidase activity in subsequent passages of ASCs of all three tested groups. Analysis using both biochemical tests and cell staining for the presence of β-galactosidase shown that ASCsLAL underwent aging in the slowest rate. Low levels of β-galactosidase continued until, at least, the fifth passage, and morphology (shape and size) of ASCsLAL did not change significantly. Rapid senescence was observed in ASCsR. This may be associated with the donors characteristics [Roldan et al., 2011].
In recent years, many studies were aimed at the characterization of ASCs surface markers and comparing their expression on freshly isolated cells, as well as those maintained in culture in early and late passages [Gronthos et al., 2001; Zuk et al., 2001; Katz et al., 2005; Mitchell et al., 2006; Astori et al., 2007]. Unfortunately, to date, no unique marker confirming the presence of undifferentiated ASCs was identified. However, on the basis of available results, the International Society for Cellular Therapy, together with the International Federation for Adipose Therapeutics and Science, issued a joint statement with the minimum criteria defining the fraction of SVF and ASCs. On one hand, these criteria are similar to those identifying MSCs, and on the other they are intended to distinct this cell population from ASCs [Bourin et al., 2013]. According to these recommendations, ASCs should not express the hematopoietic markers (<2%), such as CD11b and CD45 and show a high expression of stromal markers (> 90%), such as CD13, CD73, CD90. In order to distinguish ASCs from bone marrow MSCs, it was advised to use two additional markers, that is, CD36 (GPIIIb), and CD106 (VCAM-1). According to literature reports ASCs, in contrast to MSCs, do not express CD106, while being CD36 positive [Katz et al., 2005; Maumus et al., 2011; Pachon-Pena et al., 2011]. Expression of CD34 in ASCs depends largely on the culture conditions. Generally, its expression is present during the early period of culture, and then decreases with further cell divisions [Mitchell et al., 2006; Maumus et al., 2011]. Increasingly, it is proposed that the basic characteristic phenotype of ASCs should include at least two positive and two negative markers in one analysis.
Recently, we performed the cell surface marker screening analysis by Lyoplate technology between adipose stem cells isolated during different liposuction method [Bajek et al., 2015]. We notice that the sample collection method did not significantly influence the expression of surface markers profile, at least after second passage.
In present study, the analysis of selected markers, CD90, CD44, CD34, CD45 expression, was conducted at the first, third, and fifth passages, to evaluate the phenotypic stability of ASCs in long-term culture. All tested groups, ASCsR, ASCsLAL, and ASCsPAL, were characterized by high (∼90%) expression of CD90 and CD44. Furthermore, surface markers expression was similar among all ASCs groups and was stable during various stages of culture. These results confirmed our previous findings [Bajek et al., 2015]. We perfomed additional molecular analysis, which also confirmed high expression of CD90 marker in ASCs. Contamination with hematopoietic cells was not detected, as CD45 expression was low, also in following passages. Expression of CD34 marker in ASCs was the subject of dispute for many years. Currently, the expression of this protein was confirmed in the subpopulations of ASCs, but it undergoes partial disappearance in later passages, depending on the culture conditions such as seeding density or the type of culture medium [Sengenes et al., 2005; Mitchell et al., 2006; Yoshimura et al., 2006; Suga et al., 2007; Rodeheffer et al., 2008; Maumus et al., 2011]. The flow cytometry analysis confirmed the decrease of CD34 expression in following passages, in all three groups of ASCs. Similar findings were previously reported by Mitchell et al. [2006]. Loss of CD34 expression may indicate on ASCs differentiation or in vitro conditions [Yoshimura et al., 2006; Suga et al., 2007; Traktuev et al., 2008].
Expression of ESC markers, Nanog, Oct3/4, and Sox2, was not detected in all three groups of ASCs with the use of flow cytometry. Guasti et al. [2012] also did not observe the expression of Sox2 in ASCs isolated from adult donors. There are some reports indicating on the lack of Nanog expression and low expression of Oct3/4 in cells isolated from adult donors, in contrast to the children donors [Lendeckel et al., 2004; Sun et al., 2009; Aoki et al., 2010; Sugii et al., 2010].
The multilineage differentiation potential is the hallmark of adult stem cells. We observed the possibility of ASCsR, ASCsLAL, and ASCsPAL to give rise to three distinct mesenchymal cell lineages.
The identification of ASCs in 2001 had a huge impact on research in the field of stem cells and regenerative medicine. Aforementioned cells were used for the first time in 2004 to regenerate a large, post-traumatic bone defect in the skull of a 7-year-old girl [Lendeckel et al., 2004]. However, the number of clinical trials evaluating the efficacy and safety of ASCs in clinical practice increases impressively, both in variety and numbers. These studies include diabetes treatment, fistulas, cardiovascular diseases, ischaemia, multiple sclerosis, augmentation of soft tissue, and bone defects. Unfortunately, there are still too little promising results that would confirm the real effectiveness of this type cell therapy. This is mainly due to insufficient knowledge regarding the ASCs and their biological properties, profile, mechanisms of regeneration, and their niches. Despite the enormous commitment of researchers all over the world, utility of ASCs still leaves a lot of unanswered questions, which make the better understanding the biology of adult stem cells necessary.
CONCLUSION
In summary, our findings demonstrate that method of ASCs collection affects some characteristics of these cells, such as number of isolated cells, clonogeneity, or doubling time. However, taking into account all of the above we assume, at the current state of knowledge, that the best method of ASCs collection for medical purposes is PAL due to high proliferation potential and slow senescence. Nevertheless, further research is necessary to improve our understanding of ASCs behavior during ex vivo expansion and long-term storage for their safe and effective use.
ACKNOWLEDGMENTS
Bienkowski Medical Center and Laser-Med. Centers are acknowledged for the supply of lipoaspirates.