Brain Activity Within Prefrontal Cortex: A Resting-State fNIRS Comparative Study in High-Functioning Autism Preschoolers and Typically Developed Peers
Funding: This work was supported by Chongqing Medical Scientific Research Project (Joint Project of Chongqing Health Commission and Science and Technology Bureau) (Grant Nos. 2022MSXM022 and 2022MSXM071).
Wenlong Song and Bin Zuo contributed equally to the work.
ABSTRACT
We applied functional near-infrared spectroscopy (fNIRS) technology to detect brain function within the prefrontal cortex in 23 typically developing (TD) preschool children and 48 children with high-functioning autism (HFA), aiming to observe the differences in brain function within the prefrontal cortex between the two groups. We found that the activation degree of channels 6–7-11 corresponding to the activation area of the right prefrontal lobe in the HFA group, is significantly higher than that in the Typical Development TD group. Moreover, the number and intensity of brain functional connectivity in the HFA group are significantly lower than those in the TD group. The active areas of the brain network in the HFA group are not as concentrated as those in the TD group. This demonstrates that fNIRS detection can serve as a potential biomarker for brain activity within the prefrontal cortex of preschool children with HFA.
1 Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous neuro-developmental disorder that begins in early childhood and persists throughout life. It is characterized by social deficits, restricted interests, stereotyped behavior, and alterations in sensory processing [1]. Approximately 1/100 children are diagnosed with ASD around the world [2, 3]. For a diagnosis of High-Functioning Autism (HFA), the subject had to meet the required criteria for autism based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders and also have a full scale IQ of at least 70 on a test of intelligence [4]. They are characterized by possessing simple basic abilities in normal language use, cognition, and social skills. Due to their relatively less severe core symptoms, they are often overlooked even by medical professionals. Past studies show that ASD children benefit from theory of mind behavioral intervention tasks to promote the development of competencies [5, 6]. However, the precondition for effectively completing the theory of mind task is to have the corresponding basic ability, and HFA children are the most suitable candidates [6, 7]. Therefore, it is crucial to early identify HFA children, which may assist in enhancing rehabilitation outcomes and improving prognosis. Despite significant progress in understanding the behavioral and cognitive aspects of HFA, the specific neuropathological changes and pathogenesis mechanisms remain somewhat unclear [8].
Now a days, the development of magnetic resonance imaging (MRI) technology has explored the abnormal changes in brain structure and function in HFA patients, trying to provide a more objective basis for early diagnosis [9-11]. Indeed, functional MRI is the gold standard for in vivo imaging of the human brain, but functional near-infrared spectroscopy (fNIRS) stands out for its high portability, robustness to noise, relatively low costs, and small size, bringing functional imaging into much more realistic environments [12, 13]. fNIRS is an optical neuroimaging technique that assesses cerebral activity based on hemodynamics, which is associated with changes in the transmission of low power near-infrared light directed through the scalp and skull into the brain [14, 15]. fNIRS has the capacity to measure different types of concentration changes in hemoglobin parameters, which provides a more comprehensive description of functional connectivity (FC). Moreover, fNIRS is quiet, comfortable, and insensitive to subject motion, which is well suited for special populations such as infants and patients. Finally, the portable and cost-effective features of fNIRS make it much easier to translate FC approaches from laboratory environments to clinical applications. Accordingly, fNIRS is becoming a promising imaging modality for RSFC assessment complementary to functional MRI in spite of its limited spatial resolution [16, 17].
Most of the higher and more complex motor, cognitive, and emotional behavioral functions are thought to be found primarily in the frontal lobes [17]. An increasing number of studies have found structural abnormalities related to ASD in multiple brain regions, especially in the frontal lobe [18, 19], and research shows that defects in prefrontal cortex function are associated with core clinical symptoms of ASD [20-23]. In the past decade, resting-state fNIRS has been increasingly used to depict the typical and atypical development of cortical FC and network topological features within the age range from neonates to adolescents [24]. Regions whose blood oxygen level-dependent (BOLD) signal fluctuations show a high degree of temporal correlation are presumed to constitute a tightly coupled neural network [25]. Current results demonstrate that the activation-deactivation dichotomy routinely observed in response to attention-demanding tasks is represented intrinsically in the resting human brain, demonstrable in the absence of any overt task or behavior [24]. Brain regions that are simultaneously active during the resting state show a positive temporal correlation in their associated BOLD signals, and these brain regions together form an intrinsic functional network [26] when describing the connectivity network of the brain in a spontaneous state. It is an endogenously mediated network that is activated during the resting state and when performing social/emotional tasks, but is inhibited when performing non-social tasks that require high cognitive demands [24-26]. Therefore, we speculate that exploring the mechanisms of prefrontal changes in ASD can provide a basis for the early diagnosis and precise treatment of children with ASD. To the best of our knowledge, there have been no reported studies using fNIRS to search for brain function markers of HFA.
The purpose of this study is to use fNIRS technology to investigate the abnormal prefrontal FC in children with HFA and typically developing (TD) children.
2 Materials and Methods
2.1 Participants
The ethics committee of our institution (Beibei Hospital Affiliated to Chongqing Medical University) approved this prospective study. After explaining the research protocol and objectives in person, written informed consent was obtained from the participants and their parents. From September 2022 to December 2023, a total of 48 children aged 4–7 years old with HFA were continuously recruited through the Department of Child Health Care to participate in this study at our center. The diagnosis of HFA was made by two qualified developmental behavioral pediatricians who were blinded to this study, based on the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders. In addition, the Autism Diagnostic Observation Schedule-2 (ADOS-2) and the Childhood Autism Rating Scale (CARS) were used to assess the current symptoms, so that the diagnosis of ASD could be confirmed by a trained and experienced psychometrician who was blinded to this study. Moreover, the participants were also examined using the Social Responsiveness Scale-2 (SRS-2), which can indicate the severity of HFA symptoms. To evaluate intelligence, we used the Wechsler Preschool and Primary Scale of Intelligence (WPPSI) to measure their Full Scale IQ (FS-IQ). In our study, all children with HFA had an FSIQ of 70 or above, which met the commonly used IQ threshold for HFA. The exclusion criteria for the HFA group included children with other developmental disorders, those suffering from severe diseases (e.g., a history of epileptic seizures, brain trauma, stroke), and those taking neuropsychiatric medications. At the same time, 23 TD children were continuously recruited, and their ages were carefully matched with those in the HFA group. The participants in the TD group were recruited from a kindergarten near our hospital. The FSIQ of TD children was also measured according to the Wechsler Intelligence Scale. For TD children, the exclusion criteria included a family history of ASD and/or the presence of language or developmental delay, other neurodevelopmental diseases, brain injury, and cerebrovascular diseases. All children were right-handed, native Chinese speakers, and had no visual or hearing problems. After completing the clinical assessment, the participants were invited to the laboratory for the fNIRS data collection session. The demographic and clinical characteristics of each experimental cohort are listed in Table 1.
| HFA (n = 48) | TD (n = 23) | t/x2 | p | |
|---|---|---|---|---|
| Age | 5.41 ± 0.70 | 5.55 ± 0.65 | 0.789 | 0.433 |
| Sex, n% | ||||
| Male | 43 (89.6) | 11 (47.8) | 14.888 | < 0.001* |
| Female | 5 (10.4) | 12 (52.2) | ||
| Head circumference | 52.10 ± 1.29 | 52.00 ± 1.24 | −0.322 | 0.749 |
| FS-IQ | 86.77 ± 8.16 | 102.65 ± 5.90 | 8.339 | < 0.001* |
| ADOS2-combined score | 10.15 ± 2.42 |
N/A |
N/A |
|
| ADOS2-communication | 4.25 ± 1.06 | |||
| ADOS2-social interaction | 8.29 ± 1.54 | |||
| CARS | 34.10 ± 2.62 | |||
| SRS-2 | 86.88 ± 8.03 | |||
2.2 fNIRS Acquisition
The experiment was conducted in a quiet and dimly lit room. Participants sat on a comfortable chair and were asked to close their eyes, stay awake, keep their bodies as still as possible, and avoid thinking about anything. This study used a fNIRS optical topographic system (employing a dual-wavelength combination of 695/830 nm, 22 channels, model ETG-ONE, manufactured by Hitachi Medical Corporation, Tokyo, Japan). Within the near-infrared spectral window, near-infrared light has strong penetrability, capable of penetrating tissues to a certain depth and reaching the cerebral cortex 20–30 mm beneath the skull. The probe was fixed to the scalp via a headcap, with a scanning frequency of 10 Hz. The system comprised 8 transmitting probes and 7 detecting probes, with a fixed distance of 30 mm between the transmitting and detecting probes. The headcap was generally rectangular, positioned in the prefrontal region, forming 22 measurement channels. Brain signal changes in the prefrontal cortex of the HFA group and TD children were measured in the resting state. To more accurately maintain the coverage area, we referred to the international 10–20 system positioning method to confirm that the lines of the red 12, blue 14, and red 17 optical fibers were located in the midline, and the probes in the next column were positioned on the line of Fp1-Fpz-Fp2 in the international electroencephalogram 10–20 positioning method, as shown in Figure 1.
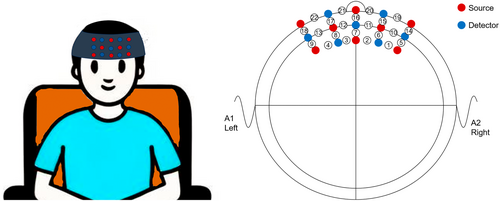
According to the modified Beer–Lambert law, the concentration changes of oxyhemoglobin and deoxyhemoglobin chromophores were calculated through the degree of infrared light attenuation. When the activation of a certain brain region increases, compared with the baseline period, an increase in oxyhemoglobin concentration and a decrease in deoxyhemoglobin concentration can be observed. The start time was set according to the measurement mode of the fNIRS system. A “ding” prompt sound was emitted at the beginning of the task, and 8 min of data were recorded. The probes were positioned according to the international 10–20 system, as shown in Figure 1.
2.3 fNIRS Data Processing
- Preparations Before Data Preprocessing First, import the collected raw light intensity MES data into the nirskit data conversion module. Using a 3 × 5 optode configuration with a total of 22 channels, convert the raw light intensity data into blood oxygen data and prepare for data preprocessing. Data preview can be performed before preprocessing, and the required preprocessing workflow can be added to observe the waveform after processing, thereby determining the preprocessing parameters.
- Data Preprocessing Workflow First-order drift removal and TDDR motion correction were selected, which effectively remove various jump noises and baseline drifts. For filtering, an IIR (infinite impulse response) filter was used. However, since short-separation channels for recording shallow-layer noise were not configured during the experiment, noise regression was not performed during preprocessing.
- Formal Data Analysis After Preprocessing First, calculate the FC at the individual level by computing the FC for each channel, outputting the corresponding brain FC matrix, and using it as input for subsequent analysis. After individual-level FC analysis, FC data for the ASD and TD groups were obtained, which were then tested to avoid false-positive results. Next, select the ROI (region of interest) channels (ch6, ch7, ch11). For ROI-to-whole-brain connectivity analysis, since multiple channels are selected as ROIs, an average time series is first generated, and then the FC between this average time series and each channel in the fNIRS data is calculated.
- Statistical Testing and Correction Independent sample t-tests were performed on the output brain FC matrices and ROI2Wholebrain data of the two groups, with FDR correction applied (p value set to 0.05). No covariates were added as none were present in the experiment. Apply masking was not selected, allowing multiple comparison correction across all channels. The test results showed that the PThrd of the brain FC matrix was 0.0411 for the ASD group and 0.0367 for the TD group, both significant. For ROI2Wholebrain, the PThrd was 0.0102 (ASD) and 0.0198 (TD), with all channels showing significant p-values after testing.
- Data Visualization After independent sample t-tests, data visualization was conducted for analysis convenience. The t-test results of brain FC matrices were imported into the visualization module, using the jet color map and manually adjusting the threshold to plot the FC matrices of the ASD and TD groups. The ROI2Wholebrain t-test results were then imported, with colors and thresholds unified between groups to generate 2D topographic images for analysis.
- 3D FC Mapping By adding spatial site information of each channel relative to the head and grouping by ROI regions, node and edge files were generated for 3D brain connectivity mapping. Using the BrainNet Viewer toolkit, a brain model was selected with node and edge information, and thresholds were adjusted to be identical for both groups to plot 3D FC maps.
2.4 Statistical Analysis
All statistical analyses were performed using SPSS version 22 software (International Business Machines Corporation, Armonk, New York, USA). A two-sided p-value < 0.05 was considered statistically significant. Statistical differences in demographic data, FS-IQ, and head circumference between the HFA group and the TD group were calculated. An independent samples t-test was used for continuous variables, and a chi-square test was applied for categorical features.
3 Results
3.1 Basic Demographic Characteristics of the Subjects
Table 1 shows that there were no significant differences in age and head circumference between the HFA group and the TD group. However, the FS-IQ of the TD group was significantly higher than that of the HFA group (p < 0.001). Males were more common in the HFA group (43 out of 48, accounting for 89.6%), while the proportion of males in the TD group (11 out of 23, accounting for 47.8%) was lower (p < 0.001).
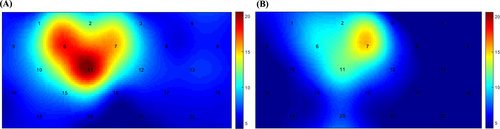
3.2 Multi-Channel Topographic Map
We used topographic maps to visually display the brain functional activation in the area covered by the headcap. A color scale was used to mark the activation levels of different regions, where colors closer to the red end indicate higher blood oxygen metabolism levels, and colors closer to the blue end indicate lower blood oxygen metabolism levels. Based on the coordinate information of channels with significantly higher blood oxygen metabolism levels, the relevant brain regions were determined by comparing with the Transcranial Brain Atlas (TBA) [29].
The TBA is a probabilistic mapping P(L|S) from scalp space to label space. To achieve this mapping, we need: First, mathematically define the scalp space S, that is, design a coordinate system suitable for the scalp; Second, establish a probabilistic mapping from scalp space to brain space, namely solve P(B|S), while considering the influence of individual differences; Finally, combine the scalp-brain mapping P(B|S) with the brain-label mapping P(L|B) to derive the scalp-label mapping P(L|S). As a probabilistic mapping P(L|S), the TBA supports transcranial brain imaging research in two different ways. In the forward application of this mapping, we can use P(L|S) to obtain label information from scalp positions, thereby clarifying the recording or stimulation targets of the placed devices. In the reverse application, we can use P(L|S) to estimate the scalp positions that can best record or stimulate specific target brain regions.
Analysis of topographic maps for the HFA group and TD group showed that blood oxygen metabolism levels were particularly prominent in Channels 6, 7, and 11 in both groups (Figure 2A,B). Comparison of topographic maps between the two groups revealed that blood oxygen metabolism levels in Channels 6, 7, and 11 were higher in the HFA group than in the TD group. Specifically, Channel 11 was the most active in the HFA group, followed by Channel 6 and then Channel 7; in the TD group, Channel 7 was the most active, followed by Channel 11 and then Channel 6. The activation regions clearly indicate significant differences in resting-state brain function between the two groups. Combined with MNI coordinate information, the coordinates corresponding to each channel are described in Table 2. By comparing with the TBA, it can be seen that the functional areas corresponding to Channel 6, Channel 7, and Channel 11 are the right prefrontal cortex region.
| Channel number | MNI_X | MNI_Y | MNI_Z |
|---|---|---|---|
| CH1 | 28.33 | 53.67 | 37.33 |
| CH2 | 11 | 62 | 36 |
| CH3 | −12 | 60.67 | 37 |
| CH4 | −30.67 | 51.67 | 34.67 |
| CH5 | 42.33 | 50.33 | 28.33 |
| CH6 | 21 | 67 | 25 |
| CH7 | −1.67 | 66 | 24.33 |
| CH8 | −22.67 | 66 | 23.67 |
| CH9 | −41.33 | 50.33 | 25.67 |
| CH10 | 32.33 | 63.33 | 14.33 |
| CH11 | 13.33 | 73 | 12.67 |
| CH12 | −14 | 72.67 | 12.33 |
| CH13 | −33.67 | 63 | 13 |
| CH14 | 48 | 54.67 | 2.33 |
| CH15 | 21 | 70.33 | 1.33 |
| CH16 | −2.67 | 70.67 | 0.33 |
| CH17 | −23.33 | 70 | 0.67 |
| CH18 | −44 | 56.33 | 1.33 |
| CH19 | 38.33 | 63.67 | −10.67 |
| CH20 | 13.33 | 71.33 | −11.33 |
| CH21 | −13.33 | 70.33 | −11.33 |
| CH22 | −36 | 62.67 | −10 |
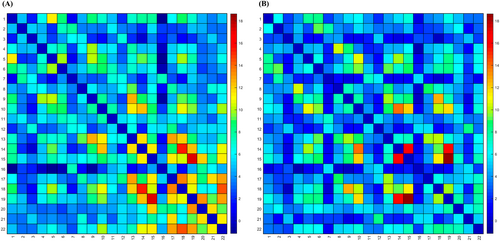
3.3 Visualization of Differences in Patient FC Matrices Between Groups
We presented the FC between channels through FC matrices. FC matrices serve as crucial tools for understanding how different brain regions collaborate. The degree of connectivity is represented by a color gradient, where the transition from red to blue indicates the correlation between channels from high to low. Results showed that in the HFA group (Figure 3A), high correlations were mainly observed in regions of Channels 15–19, 14–18, and 18–22. In the TD group (Figure 3B), high correlations were primarily distributed in Channels 15–19, 14–15, 14–19, 10–14, and 9–18. Notably, compared with the HFA group, the TD group exhibited stronger FC with a more concentrated distribution area.
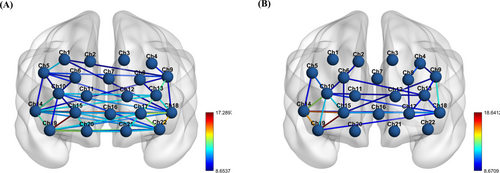
On the other hand, through brain network connectivity maps, the color of inter-channel connections can elucidate the brain network synergy between two points. The brain network connectivity maps of the two groups showed that active channel connections in the TD group (Figure 4B) were dominated by red and yellow, with the region concentrated between channels 10–14–15–19. In contrast, the HFA group (Figure 4A) had significantly fewer red and yellow lines representing tight inter-channel connections than the TD group, with a more dispersed distribution area. This result indicates that the active regions of the brain network in the HFA group were less concentrated than those in the TD group. In addition, although channels 18–12 are in the significantly activated region in the HFA group, there is no functional connection in the TD group.
4 Discussion
This study shows that fNIRS technology can observe differences in resting-state brain FC between TD children and HFA children. It also confirms that the prefrontal lobe of HFA children is more activated at rest than that of TD children, but the distribution of brain FC in HFA children is more dispersed than that in TD children, and the number and intensity of brain FC in the HFA group are significantly lower than those in the TD group.
By comparing the HFA and TD groups, we found that there were similar active regions in both groups. Our results indicate that the blood oxygen metabolism levels in Channels 6, 7, and 11 were higher in the HFA group than in the TD group, and Channels 6, 7, and 11 in the HFA group were more active. The channels 6, 7, and 11 correspond to the right prefrontal cortex region. Joshi G et al.'s [30] findings suggest that the architecture and strength of the default mode network FC are altered in HFA. The default mode network with the medial prefrontal cortex in the HFA group showed weaker correlations in comparison with healthy controls [30]. Previous studies suggest that during the observation of some tasks, frontal cortex activation is affected in HFA children using functional MRI [31, 32]. These research findings are similar to our results. The right prefrontal cortex has been shown to be associated with prosocial behavior [33], which is closely associated with general social skills deficits, and there are significant impairments in the measurements of working memory and processing speed [18]. A study designed to investigate the neural mechanisms of change following social skills interventions shows that the social cognitive skills group showed greater increases in activity in the medial prefrontal cortex, implicated in theory of mind, relative to the comparison group for both irony comprehension and gaze processing tasks [34]. These findings show that the ability of emotion recognition and theory of mind in the HFA may be related to the right prefrontal cortex.
Moreover, in the brain network connection diagrams of the two groups, the number of connections in the HFA group is significantly lower than that in the TD group, which may be related to the impaired brain functions of patients with ASD. In order to further understand the differences in brain activities between the two groups, we analyzed the differences in connectivity between channels. It is worth noting that the stronger functional connections in children with HFA mainly occur in Channels 15–19, 14–18, and 18–22; the stronger functional connections in TD children mainly occur in Channels 15–19, 14–15, 14–19, 10–14, and 9–18. These regions with obvious differences in connectivity are all located in the medial prefrontal cortex region and the dorsolateral prefrontal cortex region [29]. Studies have shown that patients with damage to the ventromedial prefrontal cortex will exhibit social isolation and apathy [35] and show a reduction in prosocial behavior in some social decision-making games [36]. From another perspective, in the brain network connection diagrams of the two groups, the number of connections in the HFA is significantly lower than that in the TD. Obviously, the results we observed are related to the changes in the function of the prefrontal lobe of the brain. In any case, the fNIRS technology reveals the differences in brain functions between the two groups during the resting state.
Previous fNIRS studies have shown that functional deficits in the prefrontal cortex are associated with ASD. Individuals with ASD exhibit atypical social behaviors and deficits in social cognition, such as impaired theory of mind and a lack of social interest [18]. Xiao et al. found that compared with TD children, children with HFA showed lower activation in the right prefrontal cortex when performing the “go/no-go” task and the Stroop task, which also linked the differences in executive functions to the prefrontal regions [37]. Funahashi et al. measured the activation changes in the prefrontal cortex and the temporal cortex of adolescents with ASD and their matched TD adolescents during listening and ignoring tasks. The results showed that there were significant activation differences in the prefrontal cortex between adolescents with ASD and those with typical development, but no such differences were found in the auditory brain regions [38]. This indicates that the lack of awareness of sounds in children with ASD may be due to deficits in attention control rather than auditory cortex dysfunction. Specifically, attention control ability may be one of the causes of non-social difficulties in ASD. Therefore, the dysfunction of the prefrontal cortex may be a developmental consequence resulting from other early deviations.
This study still has some limitations. Firstly, our sample lacks population diversity. Conducting a multicenter study with a larger sample size and involving people from multiple nationalities would be more representative. Secondly, although we randomly selected children from the HFA group and TD group as participants, it is obvious that there is a significant difference in gender ratio in the HFA group. It has been reported that the dominance of males in the autism population is a consistent finding. Finally, we cannot rule out the possibility of underestimating the intelligence level of children with HFA. Whether the WISC is a suitable measurement tool for assessing the cognitive status of children with HFASD is still controversial and worthy of further exploration in the future. Therefore, more carefully designed prospective studies using innovative methods are needed to clarify this issue.
5 Conclusion
In conclusion, we observed significant differences in signal channels in the prefrontal cortex region between preschool children with HFA and TD children. Moreover, the multi-channel topographic map showed a high correlation with children with HFA. The research results further indicate that the default network mode of children with HFA is relatively active in the right medial prefrontal cortex region, and the medial prefrontal cortex region may potentially serve as a new therapeutic target. These preliminary research findings may provide new insights into the potential pathophysiological basis of the autistic brain.
Author Contributions
Conceptualization, W.S. and Z.Z.; Methodology, W.S., B.Z., C.J. and Z.Z.; Resources, Z.Z.; Data Collection, W.S. and Z.Z.; Formal Analysis, W.S., B.Z., C.J. and Z.Z.; Writing – Original Draft Preparation, W.S.; Writing – Review and Editing, W.S., B.Z., C.J. and Z.Z.; Visualization, B.Z., C.J. and Z.Z.; Supervision, C.J.; Funding Acquisition, Z.Z. and C.J.; All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors are grateful to American Journal Experts (AJE) for their assistance with language editing. We are grateful for all of our colleagues from the Department of child healthcare in data collection and the participation of all the subjects in our study.
Ethics Statement
The study was approved by the Institutional Review Board of The Beibei Affiliated Hospital of Chongqing Medical University/The Ninth's People's Hospital of Chongqing (No. JY2021-4-14).
Consent
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




