Effects of Photobiomodulation Application on Glutathione-Related Antioxidant Defense System in Rabbit Eye Tissues
Funding: This work was supported by Zonguldak Bülent Ecevit Üniversitesi and Hacettepe Üniversitesi.
Abstract
Photobiomodulation (PBM) has emerged as a potentially effective therapeutic approach to modulate cellular functions. This study aimed to examine the impact of PBM on reactive oxygen species (ROS), lipid peroxidation, and glutathione-related antioxidant defense systems in rabbit eye tissues. A polychromatic light source with an intensity of 2.6 J/cm2/min was used for PBM treatment in New Zealand White rabbits for 12 min. The PBM group (n = 8) received treatments every 2 days for a total of 12 sessions, whereas the control group (n = 8) did not undergo any PBM light exposure during the same period. The application of PBM significantly elevated ROS-mediated glutathione levels, along with increased activities of glutathione peroxidase and reductase, particularly in corneal tissue (p ≤ 0.05). In conclusion, PBM treatment effectively enhances antioxidant defense mechanisms in the eye, particularly in corneal tissue, suggesting its potential as a therapeutic strategy for managing oxidative stress-related ocular conditions.
1 Introduction
PBM or phototherapy has emerged as a promising application in the area of medical research, offering a noninvasive approach to modulate cellular functions [1]. PBM facilitates tissue healing and alleviates pain, while also diminishing inflammation, often utilizing a low-power light source such as a laser or LED. PBM exposure does not cause a noticeable temperature increase in the treated tissue because its power is generally under 500 mW. Thus, PBM is considered a noninvasive and safe treatment since it does not result in a notable alteration in the overall tissue architecture [2, 3].
In PBM applications, various signaling pathways are triggered by ROS, cyclic adenosine monophosphate, nitric oxide, and Ca2+ through the absorption of photons by cells [3, 4]. Those molecules initiate the activation of transcription factors, which can potentially stimulate elevated gene expressions associated with cell migration and growth, anti-inflammatory, anti-apoptotic proteins, and antioxidant enzymes [3]. Furthermore, it is established that ROS are generated at a minimal amount during regular mitochondrial metabolism [5, 6]. Different cellular mechanisms have evolved to detect increased ROS levels, activating transcription factors and prompting the synthesis of antioxidant defenses [7].
Recent studies have increasingly explored the therapeutic potential of PBM in ocular tissues, demonstrating promising results across various eye conditions. Notably, PBM has been investigated for its potential benefits in treating age-related macular degeneration (AMD), dry eye disease, and diabetic retinopathy. PBM has been shown in several studies to reduce drusen volume and improve visual acuity in patients with AMD. It is suggested that PBM may help manage AMD by enhancing both functional and anatomical outcomes without causing adverse side effects [8-10]. PBM therapy has also been shown to reduce inflammation, enhance tear production, and improve the overall health of the ocular surface. The mechanism involves stimulating the meibomian glands and reducing ocular surface inflammation, leading to symptomatic relief in patients [11, 12]. In addition, various studies suggest that PBM may benefit diabetic retinopathy by reducing retinal inflammation and preventing further damage to retinal cells. The anti-inflammatory and neuroprotective effects of low-level light therapy are pivotal in maintaining retinal function and structure [13-15].
The Glutathione-related antioxidant defense system is crucial for protecting against oxidative stress, which is involved in various diseases. Glutathione, an antioxidant molecule, plays a crucial role in maintaining the antioxidant defense system and cellular redox balance. Oxidative stress arises from a disparity in ROS generation and the capacity of cells to neutralize them. The antioxidant defense system associated with glutathione functions as a strong barrier, managing the delicate balance of oxidants and antioxidants within the context of cells [16-18].
The dysregulation of the antioxidant protective system has been associated with various illnesses ranging from neurological disorders to cancer including ophthalmology [19, 20]. Previous studies have demonstrated the general benefits of PBM in reducing oxidative stress and promoting tissue repair [4, 21, 22]. Although it is known that the redox balance is disrupted and oxidative stress increases in many eye diseases, there is a lack of studies investigating the potential of methods such as PBM to strengthen the antioxidant defense system in the eye tissues [23, 24]. In this investigation, our objective was to assess the impacts of PBM application on the glutathione-related antioxidant defense system in various rabbit eye compartments, including the cornea, lens, and vitreous. We reported the impact of PBM on ROS production, levels of lipid peroxidation and glutathione, as well as the functions of enzymes related to glutathione in rabbit eye tissues for the first time in this study.
2 Materials and Methods
2.1 Materials
A light emitter (Collagentex, Tanses Technologies, Montreal, Canada) was used for photobiomodulation treatments. Unless explicitly mentioned in this article, all chemicals and agents were procured from Millipore Sigma (St. Louis, MO, USA).
2.2 Animals and Experimental Design
Sixteen male New Zealand White rabbits weighing between 2.5 and 3.5 kg, were sourced from the Experimental Animals Breeding and Research Center of Hacettepe University (Ankara, Turkey). Ethical approval for the animal studies (approval number: 2021/01) was obtained from the Hacettepe University Animal Ethical Committee prior to initiation. The animals were housed in conventional conditions, maintaining a temperature of 21°C°C with relative humidity fluctuating between 30% and 70%, under a light and dark cycle set at 12 h with unrestricted food and water. The rabbits were randomly assigned and categorized into two groups the control (n = 8) and the PBM group receiving PBM treatment (n = 8). The PBM was applied using a polychromatic light source (Collagentex; Tanses Technologies) featuring two key components: a specially doped quartz plasma arc lamp, and a bandpass filter and reflector system. This setup harnesses 685 W of power to produce visible and infrared light within the 590–1500 nm wavelength range [25, 26]. The source can provide a relatively uniform intensity across its spectrum, ensuring that no particular wavelength is disproportionately intense, which can be important for experiments requiring balanced exposure. The polychromatic light source with an intensity of 2.6 J cm2 −1 min−1 was administered from a distance of 20 cm throughout 12 min, resulting in a total dose of 31.2 J/cm2 for a single PBM session. PBM was applied to the PBM group at 2-day intervals for 12 days, while the control group was prevented from any PBM light exposure. Sacrifice and tissue sampling were conducted on the twelfth day following the completion of PBM.
2.3 Sample Preparation
The collected eyes were washed and divided into three parts: cornea, lens, and vitreous fluid. Lens and corneal tissues were weighed, and the volume of vitreous fluid was measured. The tissues and vitreous fluid were homogenized in cold 100 mM phosphate buffer included 1 mM EDTA (pH 7.0). The obtained homogenates were divided into two parts. One part of them was centrifuged at 10 000 × g, 4°C°C for 10 min and supernatants were taken for enzyme activities, The remaining portion was separated without centrifugation for the measurements of ROS, lipid peroxidation and glutathione levels.
2.4 ROS Assay
The ROS levels in cornea, lens, and vitreous fluid homogenates were measured using 2′,7′-dichlorofluorescein diacetate (DCFH-DA) prepared in Tris–HCl buffer (pH 7.4) at 10 mM. We added 20 μL of homogenate and 200 μL of DCFH-DA to each sample in a 96-well black plate. The mixture was incubated in darkness at 37°C°C for 40 min. ROS levels were then measured using a SpectraMax M2 spectrophotometer (Molecular Devices, San Jose, CA) with excitation/emission wavelengths of 485/525 nm. ROS production was expressed as a percentage compared to the control, adjusted for tissue weight and vitreous fluid volume.
2.5 Determination of Lipid Peroxidation and Glutathione Levels
After the eye tissues were weighed, they were homogenized using an Ultra Turrax tissue homogenizer in cold 100 mM phosphate buffer (pH: 7.0) containing 1 mM EDTA. The obtained homogenates were divided into two portions to determine the levels of lipid peroxidation and glutathione (GSH). The level of lipid peroxidation was determined directly in the homogenate by a previously described method involving the measurement of thiobarbituric acid-reactive substances formed at 95°C [27].
For the determination of GSH level, the other portion of the homogenate was centrifuged at 10 000 × g, 4°C for 15 min. The resulting supernatants were deproteinated with the 10% trichloroacetic acid solution and centrifuged at 10 000 × g, 4°C for 15 min to eliminate precipitated proteins. The total GSH level was quantified using a kinetic assay, where GSH catalytically reduces 5,5′-dithiobis (2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid (TNB), and the oxidized form of GSH (GSSG) is regenerated by glutathione reductase and nicotinamide adenine dinucleotide phosphate [28]. GSH and lipid peroxidation levels were expressed in nmol per g tissue.
2.6 Glutathione S-Transferase Assay
Glutathione transferase (GST) activity was assessed by monitoring the initial rate of absorbance change at 340 nm on a Spectramax M2 microplate reader (Molecular Devices) according to the procedure outlined by Habig et al. [29]. The enzymatic assay was conducted in 100 mM phosphate buffer at pH 6.5, supplemented with 1 mM EDTA, 1 mM GSH, and 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) at 30°C. As a result of the reaction, the production of GS-DNB conjugate absorbs at 340 nm. The rate of absorption increase is directly correlated with the GST activity present in the sample. The molar absorption coefficient for CDNB is 9.6 mM−1 cm−1, and GST activity was presented as nmol.min−1 mg−1 protein.
2.7 Glutathione Peroxidase Assay
Glutathione peroxidase (GPx) activity was analyzed indirectly through a coupled reaction with glutathione reductase (GR). GSSG, formed during the reduction of hydrogen peroxide by GPx, is regenerated to its reduced state through the action of glutathione reductase (GR) and NADPH. The conversion of NADPH to NADP+ results in a decrease in absorbance at 340 nm. The rate of this decrease is directly related to the GPx activity present in the sample [30]. The enzymatic assay was performed in the 100 mM potassium phosphate buffer (pH: 7.0), 0.2 mM NADPH, 1 mM GSH, 1 mM EDTA, 4 mM sodium azide, 100 U/mL glutathione reductase enzyme, and 0.1 mM hydrogen peroxide. Enzyme activity was assessed by monitoring the reduction in absorbance at 340 nm for 10 min using the SpectraMax M2 microplate reader. The GPx activity unit was defined as the quantity of enzyme catalyzing the oxidation of 1 μmol of NADPH in 1 min under the specified conditions. The molar absorption coefficient for NADPH is 6.22 mM−1 cm−1 and GPx activity was exhibited as pmol min−1 mg−1 protein.
2.8 Glutathione Reductase Assay
GR activity was assessed by observing the oxidation of NADPH in the presence of GSSG, following the Stall method [31, 32]. The reaction mixture comprised 100 mM sodium phosphate buffer (pH 7.4), 1 mM GSSG, and 0.2 mM NADPH. The reduction in absorbance of NADPH at 340 nm was recorded over a 10-min period using a Spectramax M2 microplate reader (Molecular Devices) at 30°C. One unit of activity (U) was defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of NADPH per minute under these conditions. The molar absorption coefficient for NADPH is 6.22 mM−1. cm−1 and GR activity was exhibited as nmol min−1 mg−1 protein.
2.9 Statistical Analysis
The statistical analysis was conducted using IBM SPSS Statistics 22 software. The Shapiro–Wilk test was utilized to determine the normal distribution of the samples [33]. Data with normal distribution were assessed using the Student's t-test, whereas data without normal distribution were analyzed using the Mann–Whitney test. All results were presented as mean ± standard error of the mean (SEM), and significance was defined as p-value ≤0.05.
3 Results
3.1 Evaluation of ROS and Lipid Peroxidation Levels
The impact of PBM application on ROS production in the cornea, lens, and vitreous of the eye is illustrated in Figure 1. Although an elevation in ROS was observed in the cornea and vitreous of PBM treated group, only the rise in ROS was determined to be significant in the vitreous (p ≤ 0.05). Conversely, the ROS level in the lens tissue of PBM treated group did not change compared to the control.
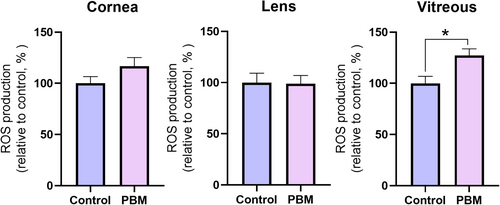
To assess the safety of applying PBM to eye tissues, malondialdehyde (MDA) levels in the cornea, lens, and vitreous were investigated. The findings revealed no significant difference in MDA levels across all three tissues of PBM treated group compared to the control group (Figure 2).
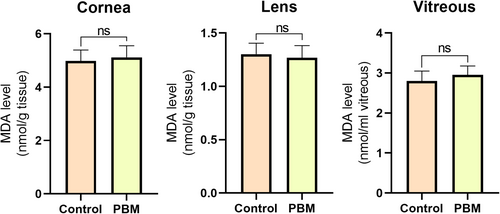
3.2 Effects of Photobiomodulation on Glutathione Levels
To assess the effects of PBM application on the antioxidant defense system in rabbit eyes, the level of GSH and the activities of GSH-related enzymes were investigated in eye tissues. The changes in GSH levels in the cornea, lens, and vitreous were demonstrated in Figure 3. A significant increase in GSH levels was observed in the cornea and vitreous of the PBM-treated group compared to the control (p ≤ 0.05). However, the elevation in GSH levels in the lens did not reach significance.
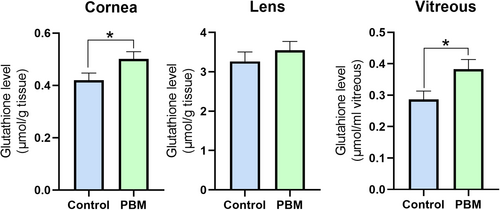
3.3 Assesment of Glutathione-Related Enzyme Activities
GSTs are a group of Phase II detoxification enzymes responsible for attaching GSH to various internal and external electrophilic compounds within cells. The GST activities were measured in cornea, lens, and vitroeus for assessing the impact of PBM application on the eye. There were no significant differences observed in GST activities between PBM and control groups (Figure 4).
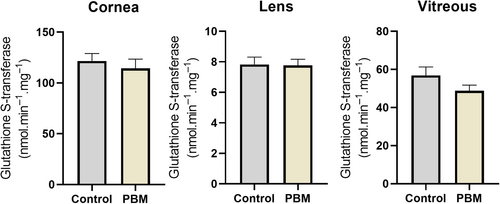
GPx plays a vital role in preventing oxidative stress and maintaining redox balance that, thereby influencing a wide array of cellular processes. In the present study, the application of PBM significantly elevated glutathione peroxidase activity in the cornea (p ≤ 0.01) (Figure 5).
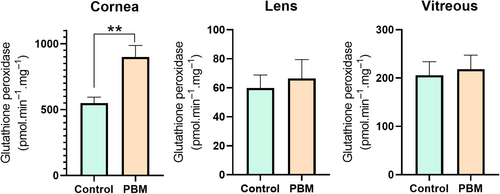
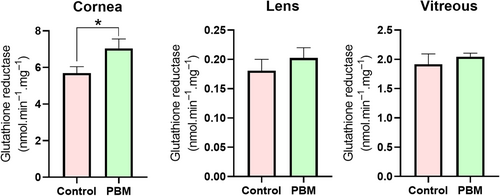
GR is a key player in the recycling and regeneration of GSH, a tripeptide molecule essential for cellular protection against oxidative stress. The utilization of PBM was observed to significantly enhance glutathione reductase activity in the corneal tissue (p ≤ 0.05) (Figure 6).
3.4 Impacts of Photobiomodulation on the Glutathione-Related Antioxidant Defense System
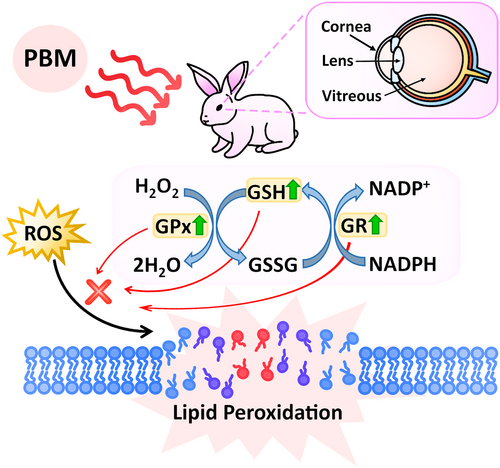
As a result, the effects of PBM application on the GSH-related antioxidant defense system were determined in various rabbit eye compartments, including the cornea, lens, and vitreous in this study (Figure 7). Detailed statistical differences are demonstrated in the Data S1. Briefly, PBM treatment significantly increased ROS-mediated GSH levels, along with increased activities of GPx and GR, particularly in corneal tissue. Overall, the hypothesis proposed that elevated levels of ROS are effectively neutralized by an intricate antioxidant defense system involving GSH, GPx, and GR. GSH, a major antioxidant, directly scavenges ROS, while GPx catalyzes the reduction of hydrogen peroxide and lipid peroxides by using GSH as a substrate, converting it into its oxidized form, GSSG. Subsequently, GR regenerates GSH from GSSG using NADPH as a reducing agent. This coordinated activity of GSH, GPx, and GR helps in maintaining cellular redox balance and prevents lipid peroxidation, thereby protecting cellular membranes from oxidative damage.
4 Discussion
PBM, a treatment method that uses low-level light exposure, has received increased attention in recent years due to its potential therapeutic effects in a variety of biological systems [1, 34]. PBM is currently under investigation as a potential therapeutic strategy for conditions linked to oxidative stress, encompassing neurodegenerative disorders, cardiovascular diseases, and skin conditions [35-37]. The interaction of PBM and ROS is crucial for a variety of therapeutic applications. ROS are characterized as dual-faced mediators: advantageous in low concentrations and detrimental in high concentrations; beneficial during short exposures but harmful during long-term exposures [38, 39]. In this study, it was observed that PBM application increased ROS production in the cornea and vitreous. However, it is important to highlight that the elevated ROS levels did not reach a harmful level, as verified through the measurement of lipid peroxide levels. The prevention of lipid peroxidation might be attributed to the activation of antioxidant defense systems, triggered by the rise in ROS within the cornea and vitreous. It is known that various cellular mechanisms evolved to recognize elevated levels of ROS and stimulate transcription factors, leading to the synthesis of additional antioxidant defenses [7]. There are no studies in the literature specifically measuring ROS levels with PBM treatment in ocular tissues such as the cornea, lens, and vitreous. However, a study conducted on human corneal cells demonstrated that PBM increases ROS levels, thereby triggering wound healing [40]. Another study suggests that PBM application suppresses oxidative stress in the retinal pigment epithelium [41].
Variations in the PBM response among different tissues like the cornea, lens, and vitreous can be attributed to several factors. The cornea is a transparent, avascular tissue composed mainly of collagen fibers and keratocytes. Its structure allows light to penetrate easily, which may influence how PBM is absorbed and utilized. The lens is also transparent but has a denser protein composition (crystallins). This density can affect how light is scattered and absorbed, potentially altering the PBM response. The vitreous body is a gel-like substance composed of water, collagen, and hyaluronic acid. Its different physical properties compared to the cornea and lens could impact how PBM light is transmitted and absorbed. In this study, it is considered that the application of PBM may have been more effective on the cornea and vitreous body than on the lens due to their tissue composition and structure.
GSH, the most abundant antioxidant molecule, plays a central role in maintaining redox balance within cells. In the present study, the application of PBM was observed to elevate GSH levels in eye tissues. Various studies are reporting the use of PBM in the treatment of neurodegenerative diseases typically results in increased GSH levels [36, 42]. However, there is a lack of research investigating the impact of PBM treatment on GSH levels specifically in eye tissue. On the other hand, it is known that the oxidative stress level increases in the pathogenesis of eye diseases including age-related macular degeneration, cataracts, and glaucoma [24, 43]. In this context, it is thought that PBM application may contribute to treating eye diseases by increasing the level of GSH antioxidant molecules.
GSH is involved in detoxification processes within the cell as a potent scavenger of ROS. The antioxidant function of GSH renders it susceptible to oxidation during the neutralization of ROS via GPx. Once oxidized, GSH becomes GSSG. To maintain an optimal cellular redox state, GSSG must be efficiently converted back to GSH through GR [18, 44-46]. Hence, the activities of GPx and GR, along with the levels of glutathione, are crucial contributors to antioxidant defense systems. Limited studies are showing the effect of PBM application on antioxidant defense systems. One study reported that PBM application increases protein expressions of the antioxidant defense mechanism in cell lines [47]. Our study represents the pioneering investigation into the alterations in GSH levels and associated enzyme activities resulting from the in vivo application of PBM in eye tissue. In the present study, it was demonstrated that the application of PBM increased the activities of GPx and GR, particularly in the corneal tissue. The significant rise in enzyme activities in the cornea could be attributed to the higher exposure to PBM in this area. Conversely, the lens tissue exhibited the least responsiveness in terms of GSH and GSH-related enzyme activities through PBM application. This observation is presumed to be caused by the lens tissue's rigid and protected structural features. Last but not least, it is crucial to note that there was no significant change observed in the activity levels of GST. The activity of GST is primarily influenced by elevated oxidative stress levels and/or the presence of xenobiotics [48-50]. In the context of this study, it is hypothesized that the increases in ROS were effectively neutralized by GSH, GPx, and GR, preventing lipid peroxidation—an oxidative stress parameter. Consequently, this prevention of significant lipid peroxidation may account for the lack of a notable change in GST activity.
It is important to compare our findings with existing studies on PBM in nonocular tissues, highlighting both the similarities and differences to evaluate the broader implications of our results. Other studies on PBM in nonocular tissues show improved tissue repair, reduced inflammation, and enhanced cellular functions, consistent with the benefits highlighted in the present study [51-53]. Studies also commonly reported the role of ROS in mediating these therapeutic effects [51]. However, differences are observed in the specific parameters used, such as the exact wavelengths, intensities, and durations of light exposure, which can lead to varying outcomes across different tissue types and conditions [51, 53]. For instance, while some studies focus on dermatological applications, others investigate neural or muscular tissue, each requiring tailored PBM protocols [51, 53].
PBM can modulate antioxidant defense mechanisms through several key cellular signaling pathways. PBM has been shown to activate the nuclear factor erythroid 2–related factor 2 (NRF2) pathway. NRF2 is a transcription factor that regulates the expression of antioxidant proteins. When activated by PBM, NRF2 translocates to the nucleus and binds to the antioxidant response element in the DNA, promoting the expression of genes involved in the antioxidant defense, such as heme oxygenase-1 and NAD(P)H oxidoreductase 1 [51, 54]. On the other hand, PI3K/Akt pathway plays a crucial role in cell survival and metabolism. PBM can activate the PI3K/Akt signaling pathway, which in turn can enhance the activation of NRF2. The PI3K/Akt pathway is also involved in reducing oxidative stress by promoting the survival of cells under oxidative conditions [51]. In addition, mitogen-activated protein kinases (MAPKs) are involved in transmitting extracellular signals to the nucleus. PBM can modulate this pathway, which includes the ERK, JNK, and p38 MAPKs. Activation of these kinases can lead to the induction of antioxidant response elements and reduce oxidative stress in cells [51, 54]. Moreover, PBM can activate SIRT1, a NAD + -dependent deacetylase that plays a key role in cellular stress responses. Activation of SIRT1 by PBM leads to deacetylation of various transcription factors and coactivators, enhancing the expression of antioxidant genes and improving mitochondrial function [54]. Furthermore, PBM can modulate the NF-κB pathway, which is involved in the inflammatory response. NF-κB is typically activated by oxidative stress, and its inhibition by PBM can reduce inflammation and oxidative damage in cells [54].
The potential clinical implications of our findings for the treatment of ocular diseases are significant. The observed increase in ROS-mediated GSH levels and the activities of GPx and GR suggest that PBM could enhance antioxidant defenses in the cornea. This enhancement may help in the management of oxidative stress-related ocular conditions, such as keratoconus, corneal dystrophies, and dry eye syndrome [55-57]. Furthermore, the reduction of oxidative damage could potentially improve healing outcomes in postsurgical corneal recovery and reduce the progression of age-related ocular diseases. In the present study, we acknowledged that the use of a single dosage and application length of PBM limits the generalizability of our findings. Future studies would benefit from exploring different dosages and application lengths to determine the optimal PBM parameters for enhancing antioxidant defense mechanisms in ocular tissues. Additionally, including a larger sample size and examining the long-term effects of PBM would provide a more comprehensive understanding of its therapeutic potential.
5 Conclusion
This study demonstrates the potential for unraveling the complicated connection between PBM and essential cellular components associated with oxidative stress such as ROS and lipid peroxidation levels and antioxidant defense systems in rabbit eye tissues. The findings might not only provide a better understanding of PBM's therapeutic potential in ocular health but might also open the path for new approaches aimed at preventing or treating ocular illnesses caused by oxidative stress.
Author Contributions
Mehmet Ozcan: conceptualization, methodology, investigation, validation, visualization, writing – original draft, writing – review and editing. Ayse Burus: methodology, investigation, visualization. Etkin Boynuyogun: methodology, investigation. Mert Calis: methodology, investigation. Figen Ozgur: methodology, investigation. Yasemin Bayazit: methodology, investigation, review and editing.
Ethics Statement
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Hacettepe University Animal Experimentations Local Ethics Board (approval number: 2021/01).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




