FSOCA-induced switchable footpad skin optical clearing window for blood flow and cell imaging in vivo
Abstract
The mouse footpad for its feature of hairlessness provides an available window for imaging vascular and cellular structure and function in vivo. Unfortunately, the strong scattering of its skin limits the penetration of light and reduces the imaging contrast and depth. Herein, an innovative footpad skin optical clearing agent (FSOCA) was developed to make the footpad skin transparent quickly by topical application. The results demonstrate that FSOCA treatment not only allowed the cutaneous blood vessels and blood flow distribution to be monitored by laser speckle contrast imaging technique with higher contrast, but also permitted the fluorescent cells to be imaged by laser scanning confocal microscopy with higher fluorescence signal intensity and larger imaging depth. In addition, the physiological saline-treatment could make the footpad skin recover to the initial turbid status, and reclearing would not induce any adverse effects on the distributions and morphologies of blood vessels and cells, which demonstrated a safe and switchable window for biomedical imaging. This switchable footpad skin optical clearing window will be significant for studying blood flow dynamics and cellular immune function in vivo in some vascular and immunological diseases. Picture: Repeated cell imaging in vivo before (a) and after (b) FSOCA treatment. (c) Merged images of 4 h (cyan border) or 72 h (magenta border) over 0 h. (d) Zoom of ROI in 4 h (yellow rectangle) or 72 h (red rectangle).
1 Introduction
With the developments of laser and optics, various optical imaging approaches are becoming the primary tools for intravital imaging over the last decades. The advantages in terms of superior spatiotemporal resolution, multichannel parallel detection, and time-lapse dynamic monitoring permit us to monitor blood flow dynamics 1-5, tracking angiogenesis, tumor growth and interventional treatment 6-8, etc. for small living animals.
Skin, as the outermost organ of the body, is an inevitable barrier to perform intravital optical imaging, whose strong scattering characteristic limits the penetration of light into tissue. In order to resolve this problem, a dorsal skin chamber window based on surgical operation was developed, which has been widely used in tumor studies and pharmacokinetics 9-11, etc. It was well known that surgical operations were always accompanied with bleeding and changes of the normal physiological environment during process of model establishment 10, 11. Recently, the rapidly developed tissue optical clearing (TOC) 12-18 technique provides an alternative solution through topical application of optical clearing agents (OCAs) on the dorsal skin, which demonstrated its powerful capacity in optimizing the imaging performances, including the imaging depth, resolution, contrast and sensitivity, of various optical imaging modalities, e. g. laser speckle contrast imaging 19-22, photoacoustic microscopy 23, 24, optical coherence tomography 25, 26, confocal microscopy 27, 28, two-photon microscopy 29, 30 and flow cytometry 31, 32, etc.
Compared with the dorsal skin, footpad skin is more convenient for intravital optical imaging due to its characteristic feature of hairlessness 33. As a classic physiological site 34, the footpad has been widely used to study the immune function of immonocytes, e. g. tracking of cellular recruitment and migration at the single-cell level using microscopical imaging 34-37. Unfortunately, both the imaging contrast and imaging depth suffered from its strong scattering, thus the investigations on immunological events were usually limited to the superficial tissue only 38, 39. As is known, the TOC technique could improve the imaging quality efficiently, but up till now, the optical clearing of footpad skin has not been reported. Actually, the footpad skin has its distinctive structure, e. g. thicker stratum corneum and no hair follicles 33, which makes the penetration of OCAs into the dermis of footpad skin more difficult, and results in the fact that previous skin OCAs cannot make the footpad skin transparent enough.
In this work, we report an innovative footpad skin optical clearing agent (FSOCA), which could make the mouse footpad skin much more transparent quickly by topical application. Both the in vitro and in vivo experiments were performed to evaluate the optical clearing efficacy after footpad skin was treated by FSOCA. Laser speckle contrast imaging (LSCI) and laser scanning confocal microscopy (LSCM) were employed to image the cutaneous blood flow and fluorescent cells through the intact or cleared footpad skin, respectively, and the improvements in LSCI contrast, fluorescence signal intensity and imaging depth were quantified. In addition, the safety of a retreatment-induced switchable footpad skin optical clearing window was also assessed.
2 Materials and methods
2.1 Chemical agents and animal preparations
In this study, two kinds of optical clearing agents were used. One is the dorsal skin optical clearing agent (DSOCA) we developed before, i. e., the mixture of saturated fructose solution (78.9 %, wt/wt), polyethylene glycol-400 and thiazone with the volume ratio of 11:8:1 20. The other is called as footpad skin optical clearing agent (FSOCA), which includes a higher volume ratio of the saturated fructose solution, and a little ethanol, dimethyl sulfoxide, polyethylene glycol-400 and thiazone.
C57BL/6 mice expressing GFP under the control of endogenous Cx3cr1 locus, abbreviated as Cx3cr1-GFP mice, were purchased from Jackson Laboratory (B6.129P-Cx3cr1tm1Litt/J, Stock No. 005582) and reproduced in the specific pathogen-free animal facility of WNLO-HUST. In the footpad skin, the main fluorescence-labeled cells are monocytes. About seven weeks old, male Cx3cr1-GFP mice were anesthetized with the mixture of 2 % α-chloralose and 10 % urethane (8 mL/kg) via intraperitoneal injection. The residual impurities on the footpad skin were removed with depilatory cream. All animal care and all experimental procedures were performed according to the guidelines of HUST and the Experimental Animal Management Ordinance of Hubei Province, P. R. China, and have been approved by the Institutional Ethics Committee of HUST.
2.2 In vitro experiments
To evaluate the optical clearing efficacy, fresh footpad skin samples (N=12) obtained from Cx3cr1-GFP mice were randomly divided into two groups and treated by the two kinds of optical clearing agents, respectively, for 20 min with an interval of 5 min. Images of the 1951 United States Air Force (USAF) resolution test target beneath the skin samples were taken at each time point by a CCD camera (Pixelfly USB, PCO, Germany) (CCD pixel size: 6.45×6.45 μm) attached to a stereomicroscope (SZ61TR, Olympus, Japan). The Camware software (PCO, Germany) was used to control the devices during acquisition and storage of the image datasets.
Here, Rayleigh's criterion was used to determine the maximum resolution of the USAF target beneath skin samples. The intensity profile across each region of interest (ROI) was analyzed, and the resolution was calculated by indentifying the smallest bar spacing in which intensity peaks and valleys could be distinguished. If the intensity of the valley region, Imin, is less than or equal to 8/π2 (∼0.81) times the maximum intensity of the peaks, Imax, then the bars can be resolved, and the resolution can be obtained correspondingly (each bar indicates a specific resolution) 40.
2.3 LSCI for cutaneous blood–flow imaging
Here, the laser speckle contrast imaging (LSCI) system was applied to monitor the cutaneous blood flow 41. Briefly, a He−Ne laser beam (λ=632.8 nm, 3 mW) expanded by a collimating lens was used to illuminate the cutaneous areas of interest. A sequence of raw speckle images can be recorded by the aforementioned CCD camera mounted on the stereomicroscopy.
After mice (N=10) were placed on the experimental platform, the raw speckle images through the intact footpad skin were acquired. Then, the footpad was placed in a self-made reservoir followed by topical application of the FSOCA on footpad skin in vivo to keep the footpad skin immersed into the FSOCA, and the raw speckle images were taken at 5-min interval for 20 min. After imaging was finished, the FSOCA was gently removed and the physiological saline solution was used for recovery 13, 42, and then the raw speckle images were taken. The laser speckle temporal contrast analysis method was used to deduce the relative blood flow velocity 43. The FSOCA was topically applied on the footpad skin again at 4 h, 24 h, 48 h, and 72 h, and the raw speckle images were taken immediately after FSOCA treatment for 20 min at each time point to evaluate whether there is any influence of FSOCA on cutaneous blood flow distribution.
2.4 LSCM for fluorescence imaging
Fluorescence imaging was performed using the laser scanning confocal microscopy (LSCM) (LSM-710, Zeiss, Germany). Mice (N=10) were additionally anesthetized with 1.5 % isoflurane (0.6 L/min air) during experiments. After the footpad was mounted on the experimental platform, GFP-expressed monocytes were excited by a 488-nm laser and the fluorescence emission was recorded using a 500–550 bandpass filter. The ZEN software (Zeiss, Germany) was used to control the various hardware devices during acquisition, processing, and storage of the image datasets. Z-stack images were continuously acquired using a 20×/0.8 objective with a 2-μm space. Herein, the depth at which the superficial autofluorescence cannot be observed in the imaging area was considered zero. Then, mice were taken down from the experimental platform, and the footpad was topically treated by the FSOCA. About 15 min later, the footpad was wiped gently, and fluorescence imaging at the same region was performed once again. After imaging was finished, the FSOCA was gently removed and the physiological saline solution was used for recovery 13, 42. The FSOCA was topically applied on the footpad skin again at 4, 24, 48, and 72 h, to evaluate whether there is any influence of FSOCA on monocytes.
2.5 Histopathologic examination
To explore the influences of repeated FSOCA treatment on cutaneous microstructure, the footpad skin of interest was cut off after it was treated by FSOCA at each aforementioned time point. Then, the skin samples were fixed in 4 % neutral paraformaldehyde, dehydrated and embedded in paraffin. After cutting into 4-μm sections, the samples were stained with hematoxylin and eosin (H&E) and Masson, respectively. The former can illustrate the cutaneous microstructure and cellular distribution, and the latter can show the collagen alignment in dermis.
3 Results
3.1 FSOCA-induced optical clearing of footpad skin in vitro
Figures 1a and 1b show the typical white-light images of footpad skin before, 5 min, 10 min, 15 min and 20 min after treatment with DSOCA and FSOCA, respectively. It can be seen that, initially, the footpad skin is turbid and the USAF target was completely concealed. After the footpad skin samples were, respectively, immersed into DSOCA and FSOCA, the USAF target beneath the footpad skin samples can be seen gradually. However, the footpad skin becomes more transparent after treatment by FSOCA at each time point than the DSOCA does, which permits us to distinguish the USAF target much more clearly.
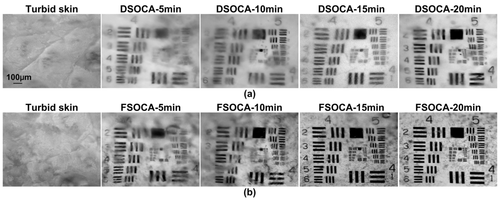
DSOCA- and FSOCA-induced time-dependent in vitro footpad skin optical clearing.
Furthermore, the resolutions through the footpad skin after treatment by DSOCA and FSOCA respectively for 5 min, 10 min, 15 min and 20 min were calculated, as shown in Table 1. This indicates that the resolution can achieve 14.65±2.76 μm and 7.38±0.87 μm after treatment by DSOCA and FSOCA, respectively, for 15 min. Meanwhile, the resolution has no noticeable changes when the treatment time reaches 20 min, they are 13.72±2.47 μm and 7.06±0.61 μm, respectively. Thus, this indicates that FSOCA has better footpad skin optical clearing ability than the DSOCA does, and the optimal optical clearing efficacy can be obtained after FSOCA treatment for 15 min.
Resolution (μm) |
Agents Time |
DSOCA |
FSOCA |
|---|---|---|---|
|
5 min |
27.5 ± 2.36 |
17.1 ± 1.3 |
|
10 min |
19.8 ± 3.5 |
12.5 ± 1.5 |
|
15 min |
14.65 ± 2.76 |
7.38 ± 0.87 |
|
20 min |
13.72 ± 2.47 |
7.06 ± 0.61 |
3.2 FSOCA-induced footpad skin optical clearing for blood flow and cell imaging in vivo
Figure 2 shows the typical results of footpad skin in vivo in conditions of intact treatment with FSOCA for 5 min, 15 min and 20 min, and recovery with physiological saline. It can be seen that, initially, the footpad skin is turbid, and it is difficult to distinguish the blood vessels and blood flow distribution. However, after treatment with FSOCA for 5 min, the cutaneous blood vessels and blood flow distribution can be observed. Even the blood flow of the small branch vessels can be monitored by the LSCI technique with superior imaging resolution and contrast after FSOCA treatment for 15 min. In addition, after treatment with physiological saline, the footpad skin could recover to the turbid status, and the blood vessels and the blood flow distribution can only be distinguished hazily.
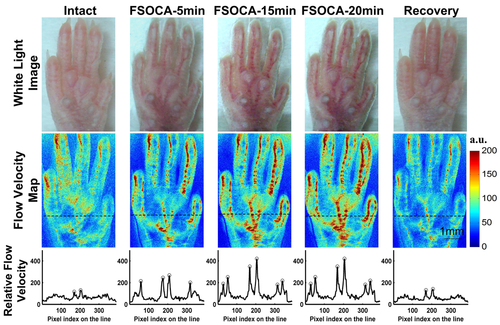
White-light images (top row), relative blood flow velocity maps (middle row) and profiles of blood-flow index along the dashed line (bottom row) through the intact, FSOCA-5 min, FSOCA-15 min, FSOCA-20 min, and recovered footpad skin. The circles indicate the blood vessels of interest.
Furthermore, the profiles of blood-flow index along the dashed line in the speckle velocity maps were also plotted. In the profiles of blood-flow index, each upward peak indicates a blood vessel whose blood flow information can be resolved. We can see that, for intact footpad skin, only the upward peaks reflecting the blood flow of the two large vessels can be distinguished. However, after treatment with FSOCA for 5 min, two more upward peaks increase observably, which indicates that the blood flow of other two more blood vessels can be resolved clearly. After treatment with FSOCA for 15 or 20 min, the peak values increase further, and the blood flow of all the six blood vessels can be distinguished with higher imaging contrast. However, physiological saline-treatment can make the footpad skin recover to the initial state again, and only blood flow of the two large vessels along the dashed line can be barely distinguished.
To assess whether FSOCA could also optimize the performance of cell imaging in vivo, fluorescence-labeled cells, namely monocytes, were imaged with confocal microscopy. Figure 3a shows the maximum intensity projection (MIP) images and intensity profiles along the horizontal white line through the intact and transparent footpad skin, respectively. The results show that through the intact footpad skin, only sparse monocytes can be distinguished, and the fluorescence signal intensity is relatively weak. However, for transparent footpad skin, we observe a remarkable increase in the number of monocytes, and fluorescence signal intensity increases 3.5-fold in average, compared to the turbid footpad skin. In addition, the improvement in imaging depth was also evaluated. Figure 3b demonstrates the typical depth-resolved fluorescence images through the intact skin and the transparent skin. It can be seen that, at the superficial layer of footpad skin, monocytes can be detected through both the intact and transparent skin. However, when the imaging depth increases to 40 μm or more, it is difficult to detect the fluorescence signal of monocytes through the intact skin, while the cells can still be distinguished with acceptable fluorescence signal intensity when the imaging depth reaches 90 μm through the transparent footpad skin. That is, the imaging depth is improved by 1.3-fold in average.
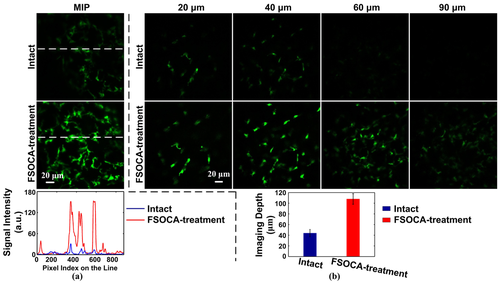
(a) Maximum intensity projection (MIP) maps imaged by confocal microscopy through the intact (top row) and transparent footpad skin (middle row), and profiles of fluorescence signal intensity along the dashed line (bottom row). (b) Depth-resolved fluorescence images (the first two rows) and the imaging depth (the third row) before and after FSOCA treatment. Data are expressed as mean ±SEM.
3.3 FSOCA-implemented repeated imaging and safety evaluation
Repeated imaging is essential, especially for monitoring the blood-flow dynamics during the occurrence and development of some vascular diseases, tracking recruitment and migration of immunocytes during inflammations, etc. Figures 4a and b show the distribution information of blood vessels and blood flow of footpad skin before and after repeated FSOCA treatment in vivo at 0, 4, 24, 48, and 72 h, respectively. This indicates that, before FSOCA treatment, the distribution of blood vessels and blood flow in footpad skin can only be distinguished hazily. However, after FSOCA treatment, the distribution of blood vessels can be imaged clearly, and the blood flow of the small branch vessels can be monitored with higher resolution.
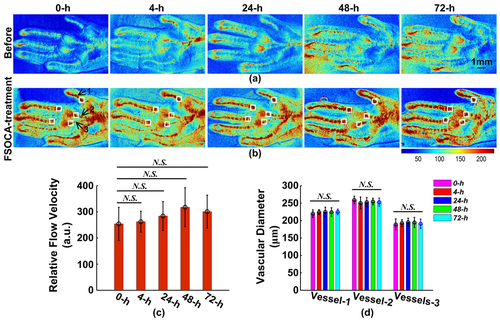
Repeated blood-flow imaging in vivo before (a) and after (b) FSOCA treatment. (c) the averaged relative flow velocity from the five white rectangles in (b). (d) the diameters of blood vessels pointed with the three black arrows in (b). Data are expressed as mean ± SEM. N. S. refers to no significance, Mann–Whitney test.
In addition, the relative blood flow velocities in white rectangles located in Figure 4 b were calculated and averaged as the relative flow velocity at that time point, as shown in Figure 4 c. By comparing the distribution of blood vessels and blood flow, it indicates that there is no significant change during repeated FSOCA treatment in vivo. Furthermore, the vascular diameters of the blood vessels pointed with the black arrows located in Figure 4 b were also calculated, as shown in Figure 4. This demonstrates that the vascular diameter also has no significant changes during repeated FSOCA treatment in vivo.
Figures 5a and b show the distributions of monocytes before and after repeated FSOCA treatment at 0, 4, 24, 48, and 72 h, respectively. This demonstrates that FSOCA treatment can evidently enhance the fluorescence signal intensity of monocytes. By merging the images at 4 h or 72 h together with that at 0 h, Figure 5 c demonstrates that the distributions of monocytes at 4 h (cyan border) is almost the same as that at 0 h, and that at 72 h (magenta border) also has no noticeable changes in distributions of monocytes. Moreover, Figure 5 d shows the zoomed morphological structure of monocyte interested at 4 h (yellow rectangle in 5b) and 72 h (red rectangle in 5b), which indicates that the morphology of monocytes at 4 h and 72 h has no noticeable changes.
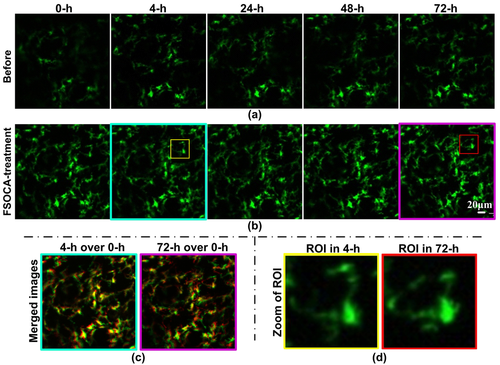
Repeated cell imaging in vivo before (a) and after (b) FSOCA treatment. (c) Merged images of 4 h (cyan border) or 72 h (magenta border) together with that at 0 h. (d) Zoom of ROI in 4 h (yellow rectangle) or 72 h (red rectangle).
Moreover, Figures 6a and b demonstrate the microstructure and the collagen alignment of footpad skin before and after repeated FSOCA treatment at 0, 4, 24, 48, and 72 h, respectively. The results with H&E staining show that the cutaneous structure is almost integrated at 0 h and 4 h, while at 24 h, 48 h and 72 h, the stratum corneum becomes loosened, but no inflammation cells infiltrate into the epidermis and dermis. The results with Masson staining demonstrate that the collagen in dermis is arranged densely before and after repeated FSOCA treatment. This means that repeated FSOCA treatment does not induce any observable histopathologic or microstructural changes in footpad skin.
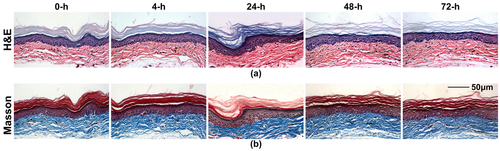
H&E stains (a) and Masson stains (b) of footpad skin before and after repeated FSOCA treatment.
4 Discussion and conclusion
The above results indicate that the calculated resolution of USAF target through the in vitro footpad skin treated by FSOCA is much higher than that with DSOCA, which proves that the former can make footpad skin more transparent than the latter. In addition, after topical application of FSOCA on footpad skin in vivo, the performances of the cutaneous blood-flow imaging and cell imaging are greatly improved. Compared with DSOCA, FSOCA includes a little ethanol and dimethyl sulfoxide besides the components of DSOCA. Here, the role of ethanol and dimethyl sulfoxide is penetration enhancer. Moreover, FSOCA includes a higher volume ratio of fructose, the higher volume ratio of fructose gives better hygroscopicity and higher osmotic pressure that could induce a much quicker water flux. Undoubtedly, these characteristics are quite helpful for inducing footpad skin optical clearing since the corneous layer of footpad skin is much thicker than that of skin in other parts of the body, besides the fact that footpad skin has no hair follicles.
Till now, intravital optical imaging approaches still depend largely on surgery-established imaging windows for visualization of blood vessels and cells in situ 9, 36. However, the surgery itself might adversely produce a local, and sometimes even systemic proinflammatory stimulus 35, which may directly impair the behaviors of cells. Moreover, even a transient drying of surgically exposed tissues might induce massive microvascular dysfunction 15. However, the relatively transparent mouse ear was an alternative to avoid the problems mentioned above, but the strong scattering of its skin still significantly suppressed the performances of various optical imaging techniques. Thus, the widely used skin OCA, e. g. glycerol, was used to optimize the imaging performances to some extent 39, 44, 45. Compared with the glycerol, when the ear skin was topically treated with the DSOCA we proposed before, it induced many more enhancements in signal intensity and imaging depth for in vivo flow cytometry 31.
Compared with the ear, the mouse footpad provides a much more convenient tissue environment for imaging cellular function with intravital microscopy because of its feature of hairlessness and proximity to draining lymph nodes 34, 36, 46, 47, but the investigations were usually restricted to the superficial layer only 48. Due to the distinctive structural feature of footpad skin, the predeveloped DSOCA cannot make the footpad skin transparent enough. Herein, a newly proposed FSOCA was used, and the in vitro experimental results indicated that the imaging resolution through FSOCA-treated footpad skin was much higher than that through DSOCA-treatment, which verified that FSOCA could make footpad skin more transparent than DSOCA.
The performances of blood flow and cell imaging in vivo were greatly improved after topical treatment of FSOCA, and the morphological structures of blood vessels and cells could be distinguished more clearly. Similar results were demonstrated by Enfield et al., in which a ∼2-fold increase in the magnitude of the vessel density was detected by correlation mapping optical coherence tomography after application of high-concentration fructose gel (86.9 %, wt/wt) on volar forearm of human for 40 min. They speculated that the increasing blood flow to the region of OCA treatment might induce a vessel dilation or erythema, which had not been demonstrated 25. In this work, repeated FSOCA treatment cannot induce any obvious changes in blood-flow distribution, cellular morphology, and collagen alignment except that the stratum corneum became loosened (see Figures 4–6). We also found that treatment of FSOCA seemed to increase the relative blood flow velocity significantly (see Figure 2). However, we cannot conclude that there must be changes in the absolute blood flow velocity based on measurements of LSCI. It is well known that LSCI is only valid for measuring relative velocity. Tissue optical clearing could decrease the static speckles and improve the imaging contrast, which enhanced the sensitivity of LSCI for blood flow velocity. However, the measured velocity would increase obviously for the stable flow velocity 49.
In addition, Tuchin et al. 50 and Kolesnikov et al. 51 found that topical treatment of OCAs (30 % and 40 % glucose, glycerol, propylene glycol, Polyethylene glycol-600) on human skin could induce different degrees of dehydration, and claimed that the skin transparent window existed from 10 to 22 min after OCA application. In our study, we applied a self-made reservoir to keep the surface of skin immersed into the abundant reagent, thus the transparent skin window could be maintained for longer times to meet the need of imaging. Therefore, there were no obvious changes in blood-flow distribution after skin became transparent in this work (see Figure 4) and previous investigations 19, 42.
In summary, an innovative FSOCA was developed to make footpad skin in vivo transparent by topical application. Moreover, repeated FSOCA treatment did not induce observable adverse effects on blood-flow distribution, cellular morphology, and cutaneous microstructure except for stratum corneum. Therefore, the optical clearing method demonstrates a switchable footpad skin optical clearing window, which provides a solution for repeatable blood flow and cell imaging. It not only allows the cutaneous blood flow distribution to be monitored by the LSCI with higher contrast, but also permits the LSCM to image fluorescent cells much deeper and with higher fluorescence signal intensity. The superior imaging depth will make it possible to track the cellular events that happen in much deeper skin, and the improved fluorescence signal intensity permits the laser power of excitation light to be reduced and thus decreases the risk of phototoxicity. We believe that combining this switchable footpad skin optical clearing window with some intravital imaging will deliver new insights for investigating blood-flow dynamics and cellular immune function in vivo, which will be significant for developing and evaluating the outcomes of some new therapeutics to some vascular and immunological diseases.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 31571002), the Science Fund for Creative Research Group of China (Grant No. 61421064), the Seed Project of WNLO-HUST, and the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University.
Author biographies
Please see Supporting Information online.




