High-throughput screening and biological display technology: Applications in molecular imaging
Abstract
Molecular imaging plays important roles in many fields, including disease diagnosis, therapeutic efficacy evaluation, intraoperative imaging guidance, drug metabolism monitoring, and patient selection for appropriate treatment. As a key component, the targeting ligand determines the specificity, affinity, and in vivo performance of molecular imaging probes. In this review, high-throughput screening and biological display platforms for the discovery of ligands applicable to molecular imaging are briefly reviewed. Basic information on ligand development for molecular imaging is first introduced, followed by a presentation of various selection platforms and typical or iterative cases. The features, advantages, limitations, and application scope of screening and display platforms are compared and discussed. Last, a basic selection strategy and a perspective for protein-based ligands are provided.
Abbreviations
-
- CT
-
- computed tomography
-
- EGFR
-
- epidermal growth factor receptor
-
- Fab
-
- fragment antigen-binding
-
- FACS
-
- fluorescence-activated cell sorting
-
- FITC
-
- fluorescein isothiocyanate
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HER3
-
- human epidermal growth factor receptor 3
-
- MB
-
- microbubbles
-
- MIP
-
- maximum intensity projection
-
- MRI
-
- magnetic resonance imaging
-
- OMP
-
- out membrane proteins
-
- PCR
-
- polymerase chain reaction
-
- PD-L1
-
- programmed death ligand 1
-
- PET
-
- Positron emission tomography
-
- scFv
-
- single-chain fragment variable
-
- SPECT
-
- single-photon emission computed tomography
-
- VEGFR
-
- vascular endothelial growth factor receptor
1 INTRODUCTION
Molecular imaging is a noninvasive method used to visualize biochemical processes and changes in biomarker profiles at the cellular or molecular level in living subjects [1]. In contrast to conventional diagnostic methods, molecular imaging has increased measurement accuracy and provides crucial information for the early diagnosis of diseases. Molecular imaging provides both qualitative and quantitative information, which allows clinical researchers to understand the pathological mechanism and distribution of diseases, thus facilitating the development of suitable personalized treatment plans for patients. Molecular imaging is a significant tool for disease diagnosis, therapeutic efficacy evaluation, intraoperative imaging guidance, drug metabolism monitoring, and patient selection for appropriate treatment [2]. Generally, molecular imaging is achieved with imaging instruments and molecular probes [3]. Numerous imaging platforms have been invented since the 1950s, including positron emission tomography (PET), single photon emission computed tomography (SPECT), computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and near-infrared imaging [4]. In addition to developing imaging contrast agents (e.g., radionuclides, fluorescent dyes, and nanomaterials), the rational design of molecular probes is important to fully exploit the potential of different imaging instruments. Molecular probes can be divided into two classes: targeting and nontargeting probes [5]. Nontargeting probes mainly accumulate in the target via the enhanced permeability and retention effect of vessels, which inevitably result in high background signals and heterogeneous distribution, thereby lowering the imaging performance [6]. To solve these shortcomings, probes can be functionalized with targeting ligands, which specifically interact with disease-related antigens, receptors, transporters, and enzymes, thereby improving the tissue-targeting efficiency and in vivo imaging performance.
The targeting moiety can be either natural or artificial. Natural moieties containing cargos usually attach to cell receptors or transporters (such as serotonin and dopamine), natural peptides (such as bombesin, gastrin, and cholecystokinin), and exendin analogs. Unfortunately, there is currently only a limited number of natural moieties, which is insufficient to tackle the wide variety of tumor biomarkers [7]. However, bioengineering can be combined with high-throughput screening [8], a technique used to automatically test thousands of samples for binding activity toward one or many targets in parallel. As a result, various artificially engineered ligands can be displayed on the surface of different vectors, such as viral, prokaryotic, and eukaryotic cells, as well as cell-free systems, which greatly expand the diversity of ligands and improve the efficiency of ligand development [9, 10].
In this review, we mainly focus on the use of high-throughput screening and display bioengineering for the selection of targeting ligands applicable to molecular imaging. We also present a comparison of the features as well as advantages and disadvantages of different screening platforms (Table 1). Last, we provide some perspectives and a basic selection strategy for protein-based ligands.
| Selection platform | Library size | Supported ligands | Selection cycle | Presentation efficacy | Support in vivo selection or not? | Candidates identification method | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Phage display | 1011–12 | Small protein: peptides, protein scaffolds, nanobody, antibody fragments | Few weeks | High | Yes | Elisa | High compatibility with various ligands and screening conditions |
Preference bias resulting from different proportions of codons Potential loss of candidates Lack of PTM |
| Homologous presentation | ||||||||
| Bacterial surface display | 1011–12 | Small protein and full-length antibody | High | No | FACS | Supporting liquid phase selection | Poor stability for large fusion ligands | |
| Ease of preparation and affinity maturation | ||||||||
| Yeast surface display | 109 | High | No | Ease of preparation and affinity maturation | Smaller library size | |||
| Ribosome and mRNA display | 1012–15 | Low | No | Huge library size | Ribosome competes with ligands | |||
| Compatible with toxic protein and unnatural amino acids | Instability of mRNA, monovalent presentation | |||||||
| Suitable for molecule evolution | Potential linker effect |
2 LIGAND DEVELOPMENT FOR MOLECULAR IMAGING
2.1 Ligand categories
To date, high-throughput screening and display bioengineering have been extensively and effectively used to develop various peptide- and protein-based ligands for molecular imaging. As shown in Figure 1, these ligands can be broadly categorized as linear peptides, cyclic peptides, protein scaffolds, nanobodies, and antibodies.
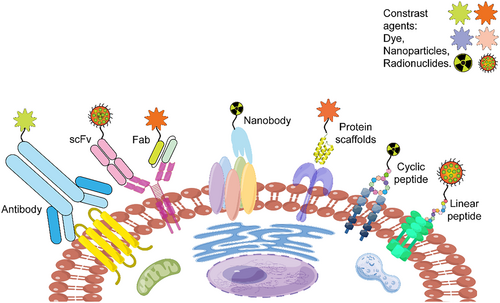
Peptide- and protein-based ligands for molecular imaging (produced via Figdraw online tools, ID: ARAAP84447).
Linear polypeptides (3–5 kDa) are short, small proteins composed of <50 amino acids attached by covalent bonds. They are characterized by ease of preparation and modification, high cellular uptake/tissue penetration, relatively fast pharmacokinetics, and safe immunological profiles, and furthermore, they can be identified using mature screening platforms, which are benefits for imaging applications [11].
Cyclic peptides, originally designed as alternatives to linear peptides, are mainly formed via disulfide bonds, unnatural amino acids, and carbonic anhydrase catalysis. Cyclic peptides generally have a higher stability and longer half-life than linear peptides. Until now, the Food and Drug Administration (FDA) has approved 20 cyclic peptides either for cancer therapy and diagnosis or as antibiotic and antifungal drugs [12].
Protein scaffolds (4–20 kDa), considered alternatives to antibodies, have a rigid tertiary structure and several randomized sites. Protein scaffolds are generally more stable physicochemically than polypeptides. Representative protein scaffolds, such as affibody, knottin, anticalin, adnectin, and DARPins®, have been extensively investigated in preclinical research for clinical imaging or therapy [13].
The nanobody (12–15 kDa) derived from the camelid antibody VHH domain has a long CDR3 region with a high affinity for hidden epitopes. The nanobody does not require pairing of heavy and light chains, making it suitable for biological display systems. Additionally, the nanobody has demonstrated high potential for drug delivery, disease therapy or diagnosis, in vitro detection kit development, and crystallization research [14].
Antibodies (150 kDa) and antibody derivatives, such as Fab, scFv, and minibody (25–75 kDa), are pioneering affinity ligands that play important roles in targeted therapy or detection. Currently, over 100 monoclonal antibodies and eight antibody fragments have been approved by the FDA for marketing or clinical trials [15].
2.2 High-throughput screening using biological display systems
Biological display systems have been systematically developed for more than 30 years and have been broadly used for high-throughput ligand selection and other fields (Figure 2) [16]. With gene recombinant technologies, the structure of natural ligands can be conveniently imitated and reshaped by incorporating functional tags, groups, and linkers. Additionally, biological display systems ensure strict expression regulation and easy library amplification at low cost as well as rapid information decoding of candidates via polymerase chain reaction (PCR) and sequencing. Currently, representative display platforms are phage, bacteria, yeast, ribosome, mRNA, and mammalian cell display systems [17, 18].
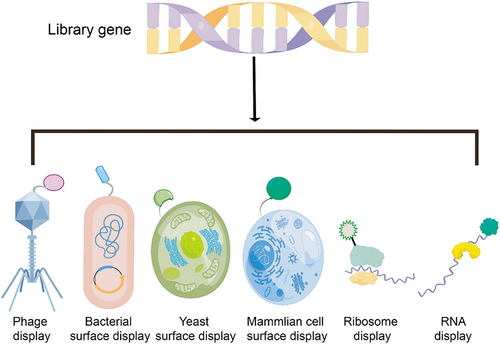
Types of surface display systems (produced by Figdraw online tools, ID: URTIR338d3).
2.3 Signal detection
Monitoring the interactions between ligands and targets provides physical information for candidate selection. Generally, candidates from virus-based phage display platforms interact with solid-phase immobilized target molecules. Subsequently, enzymatic luminescence signals are detected and recorded via a microplate reader. Cell-based selection platforms, such as bacterial and yeast, are relatively convenient. Because monoclonal candidates presented on the cell surface are multivalent, the intensity of the fluorescence signal corresponds to the strength of the interaction between candidates and targets during fluorescence-activated cell sorting (FACS). More importantly, FACS directly collects single-cell signals, which leads to greatly reduced false positives compared with cascade amplification signals of cumbersome enzyme-linked immunosorbent assay (Elisa) [8]. Subsequently, surface plasmon resonance (SPR) or bio-layer interferometry (BLI) platforms are used to accurately analyze dynamic changes in the molecular interaction and measure the ligand affinity. More importantly, the microplates and microarray chips of libraries or target molecules can be integrated with the above physical detection instruments to establish a high-throughput screening platform, which enables rapid and automatic screening of thousands of samples for affinity toward one or more molecules in parallel [19].
3 BIOLOGICAL DISPLAY PLATFORMS AND IMAGING APPLICATIONS
3.1 Phage display
In 1985, Smith et al. were the first to present a 57-mer peptide on the surface of a filamentous phage via insertion of a foreign gene into coat protein III of the filamentous bacteriophage. The phage represents a perfect vector for exogenous protein presentation, and because phage display has various advantageous features, including easy library construction via genetic engineering, strict regulation of expression, and high infectivity and propagation of the phage, it has been further developed in many fields since its establishment [20].
Although many phage display systems and formats are available, the filamentous phage and Podoviridae phage are most commonly used because of the high compatibility of the long foreign fragment [21]. The M13 bacteriophage is a rod-shaped nonlytic phage with a 6.4-kB single-stranded genomic DNA and 11 functional proteins. The fusion-protein length and amount may prevent phage rearrangement, infectivity, and propagation if a foreign gene is directly inserted in the genome. The phagemid vector and helper phage combination display modality (3 + 3 and 8 + 8) can be devised for independent display without interference to the phage functionality. The high-copy PVIII capsid protein, which is used for multivalent and small proteins, and the low-copy PIII capsid protein, which is used for low valency and large proteins, are the main presentation display systems of filamentous phage platforms. Because of the differences in the presentation valency, PVIII and PIII systems tend to generate high avidity and affinity ligands, respectively [22]. T7 is a lytic phage with an icosahedral capsid head and a 40-kB double-stranded genomic DNA. A fusion protein gene is commonly inserted at the c-terminal of coat protein gp10B for surface display [23].
The process of hit discovery follows library design and construction, phage recovery, bio-panning with targets, and function testing and sequencing of candidates. As the most popular system for peptide and protein-based ligand development, phage display has achieved great success not only for imaging but also for therapeutic applications [21].
3.1.1 Linear peptides
Linear peptides derived from combinatorial phage libraries are pioneer ligands. Normally, linear peptide sequences with lengths ranging from 5 to 20 AA are continually randomized in the NNK format and cloned into various phagemid vectors. Dozens of phage-derived peptides have been tested for imaging or therapeutic applications thus far [24].
The early phage-display derived HER2 specific peptide, KCCYSL (295 nM), has a CCY motif that is similar to HER2 native ligands. The 111In-DOTA (GSG)-KCCYSL probe emits a peak tumor signal to blood signal at 2 h post injection in a mouse tumor model. More importantly, KCCYSL does not competitively bind with trastuzumab, in contrast to the other HER2-binding peptide, LTVSPWY, which is an advantage for cancer therapy monitoring [25, 26]. Recently, Paola and colleagues decorated tetrameric KCCYSL peptides with a liposome to increase the uptake of the peptides in HER2-positive BT-474 cells to the Herceptin antibody level. This liposome-HER2 peptide complex carrying the (C18)2DTPA (Gd) agent emits a gradually improved signal in the liver and kidneys of healthy C57BL/6 mice 15–30 min after injection, demonstrating its potential for MRI [27]. Additionally, folate receptor alpha (FRα) modulates folate transportation and is overexpressed in many adenocarcinomas. Xing and coworkers identified C7 peptides (MHTAPGWGYRLS) active against FRα from a Ph.D.-12 phage display peptide library, and using ex vivo tissues, they showed that FITC-C7 emits a 3.3-times higher fluorescence signal in the tumor than in the ovary, demonstrating the targeting ability of FITC-C7 [28].
The frequently used EGFR-binding peptide, GE11 (YHWYGYTPQNVI, KD = 22 nM), was initially screened from a phage library. More recently, GE11-based fluorescent polymer probes have been found to have no antibody interference with the ability to localize to the tumor site 4 h after injection, demonstrating its good targeting ability and potential value in fluorescence-navigated surgery [29]. Another phage-derived peptide (KCCFPAQ, KD = 70 nM), labeled with FITC and selected to target sessile serrated adenoma, has favorable specificity and a 2.43-fold stronger fluorescence signal in colonic mucosa than in normal mucosa during an endoscopic examination [30]. CD44v6 is a typical biomarker of gastric cancer. He et al. recently identified a CD44v6-binding ELT peptide (ELTVMGYYPGMS, KD = 611.2 nM) from a Ph.D.-12 phage display peptide library. The FITC-ELT probe emits a four-times stronger imaging signal in the test group than in the control group 3 h after injection and was rapidly cleared within 4 h after administration [31].
3.1.2 Cyclic peptides
Linear peptides have disadvantages, including sensitivity to enzyme degradation, rapid clearance from the kidneys, terminal negative charges preventing internalization, and flexible structure leading to reduced binding ability, which impede their applications. Cyclic peptides are designed to mitigate these limitations, making them better suited for in vivo applications [32]. Introducing cysteine at both ends or in the discontinuous format to produce a disulfide bond is the most common strategy for cyclizing peptides identified from the phage display [33]. Although disulfide bonds are unstable under intracellular reducing conditions, the Fasan group has improved the stability of disulfide bonds by using an amber stop codon to integrate a cysteine-reactive O2beY amino acid, which allows the formation of an irreversible thioether bond with a proximal cysteine residue [34]. Another cyclization method using chemical group substitution has also been reported [35].
The RGD motif has been identified as an integrin binding sequence and has paved the way for designing an integrin homing peptide phage library. KIn et al. reshaped a phage library-derived cyclic peptide iRGD (CRGDKRGPDC), to Cy5.5-iRGDC-Pt (IV) fluorescent probe, good tumor imaging 1 h post injection and tumor suppression efficacy has shown in mice model [36]. Moreover, a C-MET targeting peptide, GE-137 (AGSCYCSGPPRFECWCYETEGT, KD = 3 nM), obtained from M13 phage selection was used to generate GE-137-Cy5 probes to conduct colonoscopy of 15 patients. Colonoscopy conducted in the fluorescence mode enabled the detection of 22 lesions, which were inaccessible in the white-light mode as well as the visualization of 94% of hyperplastic lesions and 100% of serrated polyps [37]. Another cyclic peptide, ZD2 (CTVRTSADC, KD = 11 μM), selected from a phage library to bind to extra-domain B fibronectin, was used to generate the SPECT probe 99mTc-HYNIC-ZD2, which has a high tumor uptake 2 h after injection and is mainly cleared via the kidneys [38].
More recently, the Liu group identified Glypican-3 targeting cyclic peptide F3 (CGGGGGGGC) using a Ph.D.-C7C phage display library. In a PET imaging study, the probe labeled with 68Ga emits a peak signal 1 h after administration and is mainly cleared via the urinary system [39]. The blood-brain barrier (BBB) is a well-known obstacle to the treatment of brain diseases. The Li group recently reported an in vivo phage selection-derived cyclic peptide, PMK (PMKSHTN), which can cross the BBB. Cy5-PMK emits a selective peak signal in the brains of nude mice 30 min after injection [40].
3.1.3 Protein scaffolds
Affibody (6.5–7 kDa), a typical example of protein scaffolds, is derived from Staphylococcal protein A and contains a three-helix residue and 13 mutable residues. Since the first affibody phage display library was constructed in 1995, more than 300 relevant protein scaffolds active against over 50 different targets have been reported, some of which have shown favorable results in clinical imaging and therapeutic applications [41].
The HER2 biomarker is broadly expressed on Luminal B and HER2-positive breast cancer cells. The Wikman group implemented phage selection to target the HER2-extracellular domain and selected the first anti-HER2 affibody ZHER2/neu:4, which has an affinity of approximately 50 nM. 125I- His6-ZHER2/neu:4 emits specific imaging signals in the SKBR-3 cell line [42]. To further improve the HER2 affibody affinity while retaining specificity, Nilsson's team applied the amino acid preference of mutable residues and built a new phage library based on seven previously reported ZHER2 candidates. Specifically, the 13Y, 14W, 28R, 32R, and 35Y positions were fixed with R/K and Q/T representing positions 10 and 11, respectively. The remaining sites, 9, 17, 18, 24, 25, and 27, were coded by the NNG/T format. The affinity maturation library was used to further specify the scope of potential ligands, and its smaller library size was used to reduce diversity loss during the electro-transformation process. Interestingly, the resulting affibody, ZHER2:342 (KD = 22 pM), has an almost 2200-fold greater affinity than ZHER2/neu:4 [43]. Moreover, DOTA-ZHER2:342-pep2 labeled with either 111In or 68Ga (ABY-002) is the first affibody-based clinical PET probe with the ability to delineate HER2-expressing metastatic breast cancer lesions of patients, which represents a clinical imaging precedent for protein scaffold molecules [44].
ABY-002 exhibits high partial accumulation in the liver. Therefore, Feldwisch conducted amino acid substitution to further improve affibody thermostability and lower non-specificity to IgM. A second-generation affibody library with position changes (S33K, N3A, F5Y, N6A, N23T, N43E, A42S, A46S, and A54S) was used to produce a new HER2 affibody, ABY-025, which is mainly cleared via the kidneys and exhibits better imaging performance than ABY-022 (NCT01858116 and NCT01216033) [45, 46]. Feldwisch's study demonstrates that the substitution of position 33 of the affibody can lower its non-specificity binding, representing an additional mutable site to further improve library diversity. Using a combination of this discovery and a gradient-site strategy, Hackel's group built a new affibody yeast display library, of which the details are discussed in the following section [47].
3.1.4 Nanobody
The camelid antibody-derived nanobody is an appealing targeting moiety for imaging and diagnosis owing to its small molecular size, high thermostability, and high affinity. Generally, genes of natural or immunized nanobodies can be directly cloned into a phagemid vector for nanobody library construction. However, humanization optimization may necessitate lowering the immunogenicity at the expense of structural stability [48]. As an alternative, a synthetic nanobody library, with a larger capacity and lower cost, does not require animal immunization and is compatible with toxic and pathogenic antigens. The prerequisites for constructing a synthetic library are a stable scaffold and specific coding complementarity-determining regions (CDRs) for further gene cloning. In this respect, the most intensively investigated scaffolds are mature cAbBCII10 and NbBCII10FGLA, which are already humanized and very stable, with no need for disulfide bonds. Moreover, the length polymorphism of CDR3 expands the library diversity, and the CDR3 coding sequence (3–28 aa) abides by the native nanobody preference to balance the diversity while improving structural stability, solubility, and correct folding [49].
Recently, Li's group identified a CDH17-binding nanobody, E8 (KD = 70.3 nM), using the native nanobody phage library. E8-IR800 emits the strongest fluorescence signal at the tumor site of an MKN45 mouse model 12 h after injection, which is nearly four-fold greater than that observed in the control group (Figure 3). The E8-PE38 toxin complex also efficiently inhibits tumor growth and prolongs mouse survival [50]. Another PD-L1-specific nanobody, Nb16 labeled with Cy7, exhibits selective and high tumor accumulation in vivo, and the ex vivo imaging signal of this nanobody is 2.7-fold greater than that of the PD-L1 monoclonal antibody [51].
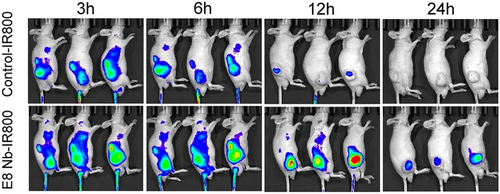
In vivo near-infrared imaging using E8 Nb-IR800 probes (bottom) and control probe IR800 (top) at different time points (3, 6, 12, and 24 h). E8 Nb-IR800 probes emit stronger tumor signals, persisting for 12–24 h, than IR800 (n = 3). This research was originally published in JNM. Rogers(s). Preclinical Evaluation of an Engineered Single-Chain Fragment Variable-Fragment Crystallizable Targeting Human CD44. J Nucl Med. 2021; 62(1):137–143. © SNMMI. Ref. [50].
3.1.5 Antibody fragments
The Geyer group designed a phage display Fab library with a framework derived from the HER2 monoclonal antibody trastuzumab with diversified CDR L3 and H3 [52]. Using this library, they acquired an HER3-targeting Fab, which was reshaped to scFv, diabody, scFv-CH3, scFv-Fc, and IgG for further IRDye800CW labeling. In vivo imaging evaluation of these six antibody-based probes demonstrated that the molecular weight difference influences the bio-distribution and clearance time, while the affinity is improved with the bivalent fragment. The smallest probe, scFv, emits a single peak at 4 h post injection and is cleared at 6 h post injection. The other probes emit signals with a minimal background fluorescence at 24 h post injection, and IgG and scFv-Fc emit the strongest signals, which last for 72 h [53].
Using a naïve phage display library, Lebeau's group also selected a FAP-specific scFv, which was engineered to a full-length antibody, B12 IgG. In a prostate cancer xenograft model, B12 IgG-IRDye-800cw emits a specific signal that is more than three times greater than that of the control group 24 h after injection [54]. Another phage-selected CD44-binding scFv was evaluated using PET [55]. Specifically, bivalent scFv-Fc-CD44 was generated with a more than 200-fold improvement in affinity. [89Zr]-DFO-scFv-Fc-CD44 localizes to the tumor tissue 1 day after administration with a high uptake (~56% injected dose/g), and the SUVmax ratio (scFv-Fc-CD44 vs. control) increases two- to seven-fold from days 1 to 7 (Figure 4).
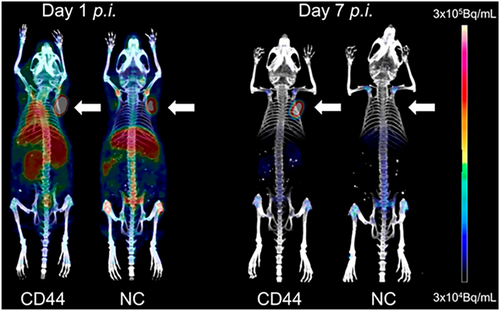
PET using [89Zr]-DFO-scFv-Fc-CD44 (left) or [89Zr]-DFO-scFv-Fc-GPA (right) from days 1 to 7. The tumor xenografts are indicated by the white arrow. The bivalent CD44-binding scFv PET probes emit signals with a low background at day 7 compared with the control group. Reprinted with permission from Ref. [55].
3.2 Bacterial display
The phage display system is limited by the potential interference of helper phages, which accounted for 10% in secreted virions, long panning cycle, and lack of ability to monitor the binding strength during the selection process. To tackle these limitations, the bacterial surface display was invented by anchoring ligands to cell membrane proteins.
The OMP and LPXTG motif fusion systems are the mainstream surface platforms for Gram-negative Escherichia coli and Gram-positive staphylococcus bacteria, respectively. Generally, the ligand library gene is inserted into bacterial display vectors and expressed, which is stringently controlled by the arabinose promoter. After incubation and FACS, the candidate genes are then extracted and transformed to new host cells for the next round selection [18]. The biggest advantage of the bacterial surface display is the multivalent presentation, which enables monitoring of the ligand-binding strength via the fluorescence intensity during FACS for better ligand affinity maturation [56]. However, large ligands may display poorly because the two-layer membranes function as a barrier, and fusion ligands may reduce outer membrane integrity, cell vitality, and library size [20]. Despite these disadvantages, full-length antibody presentation of the NlpA-ZZ fusion protein via the bacterial display has been reported [57]. The bacterial surface display is mainly applied to improve ligand affinity for molecular imaging.
Skerra's group employed the bacterial display for the initial selection of human HSP70-binding anticalin primary candidates (KD = 13 nM). They constructed a mutated bacterial library via error-prone PCR and performed another cycle of selection, which produced the BGG10I clone (KD = 391 pM), with an affinity improvement of nearly 33-fold. The specific PET signal was detected at the tumor site 24 h after the injection of [89Zr]-labeled Anticalin-PAS200 [58]. Löfblom's group combined phage and staphylococcal display systems and selected a HER3 affibody Z05416 with nanomolar affinity. Next, they conducted alanine scanning and established a second-generation staphylococcal surface display library. Z08698 with an affinity of 21 pM was obtained via FACS [59, 60]. PET of [68Ga]-(HE)3-Z08698-NODAGA with a hydrophilic tag has an increased tumor uptake but lowered blood and hepatic uptakes in BxPC-3 xenografts [61].
Löfblom's group used the same strategy to obtain several VEGFR2-binding affibodies with nanomolar affinity, which were then engineered to biparatopic affibody construct ZVEGFR2_Bp2 (ZVEGFR2_22-(S4G)-ZVEGFR2_40-(S4G)3-ABD035), which has an inhibitory potency similar to that of ramucirumab (Figure 5a). [111In]-NODAGA-ZVEGFR2-Bp2, with picomolar affinity (KD = 33 ± 18 pM), emits a strong SPECT signal in the MS1 tumors of mice at 2 h post injection (Figure 5b). Moreover, the signal ratio of tumor-to-blood, tumor-to-muscle, and tumor-to-brain reached 11, 15, and 78, respectively. An intracranial imaging signal was observed in the glioblastoma orthotopic model at 2 h after the injection of 4 or 40 μg of [111In]-NODAGA-ZVEGFR2-Bp2 and 4 μg of [111In]-NODAGA-Ztag-Ztaq. The 4-μg ZVEGFR2-Bp2 group shows a two-fold higher tumor-to-cerebellum signal ratio than the others, and the specificity is consistent with the results of macroautoradiography and hematoxylin and eosin staining (Figure 5c,d) [62-64].
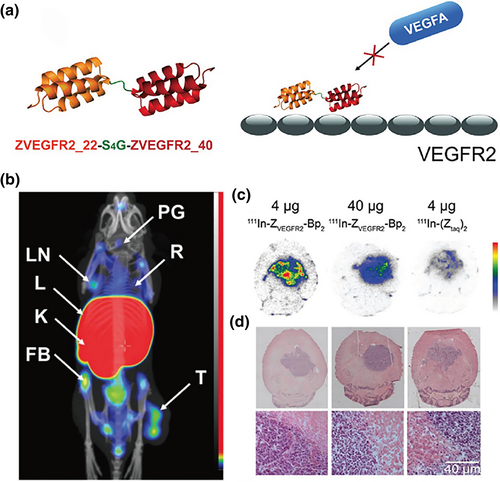
Affibody-based imaging probe development. (a) Structure of biparatopic affibody construct ZVEGFR2_Bp2. (b) SPECT imaging and biodistribution of [111In]-NODAGA-ZVEGFR2-Bp2 in BALB/C nu/nu mice bearing MS1 tumors. Mice were injected with 4 μg of [111In]-NODAGA-ZVEGFR2-Bp2 and euthanized 2 h pi. FB, femur and bone; K, kidneys; L, liver; LN, lymph node; PG, pineal gland; R, ribs; T, tumor. (c) Macroautoradiography and (d) H&E staining of brain slices of mice bearing intracranial GL261 tumors. Animals were injected with 4 μg (left column) or 40 μg (middle column) of [111In]-NODAGA-ZVEGFR2-Bp2 or 4 μg of [111In]-NODAGA-Ztag-Ztaq (right column) and were euthanized 2 h pi. Reprinted with permission from Ref. [64].
3.3 Yeast surface display
The yeast surface display emerged in 1997 as an alternative to the phage display to mitigate the disadvantage of the lacking PTM (post-translational modification) of the prokaryotic system. Following the discovery of glycosylphosphatidylinositol anchored surface proteins, including Agα1p, Aga2p, flocculin Flo1p, Cwp1p, Cwp2p, Sed1p, Tip1p, YCR89w, and Tir1, two typical yeast platforms based on agglutinin Aga1–Aga2 and flocculin Flo1p were established. Commonly, a ligand library gene is cloned into the following sites: (i) either or both terminals of the Aga2 gene of Saccharomyces cerevisiae, (ii) the C-terminal of the adhesive domain of the Flo1p gene, or (iii) the N-terminal of the Flo1p gene, the C-terminal of which contains a GPI anchoring gene [65]. In contrast to the phage display, the yeast display technique not only simplifies the library preparation and quantification but also reduces the selection cycle and improves the accuracy with FACS compatibility. However, the yeast growth cycle is longer than the bacterial growth cycle, and over-glycosylation may impair protein folding and function. Although the yeast display platform is mainly applied to affinity maturation of the antibody–antigen interaction, development and optimization of imaging ligands based on this system have come to the fore [66].
Hackel's group identified a CD276-binding affibody, ABYB7-H3 (KD = 310 ± 100 nM), from a yeast display library, the affinity of which was further improved to 0.9–20 mM by employing a helix-walking strategy. The ABYB7-H3 ultrasound imaging probe modified with microbubbles (MB), MBABY-B7-H3, emits a strong in vivo signal (8.4 ± 3.3 a.u.) in breast orthotopic tumors, which is nearly five-fold greater than that observed in the control group [67]. They also engineered an EGFR-binding GP2 molecule via the yeast display platform and FACS. The optimized ligand, GαE35, exhibits an improved charge distribution and high affinity (KD = 8.8 ± 0.6 nM). 64Cu-NODAGA-GαE35 accumulated in A431 cells emits a good PET signal (4.7 ± 0.5%ID/g), and it has a low unspecific uptake of 0.6 ± 0.1%ID/g by MDA-MB-435 cells [68].
Abbott's group developed a yeast display library and identified the scFv-C9 antibody, which specifically binds to bisecting N-glycans of tumor cells. An in vivo study of scFv-C9 magnetic bead complexes showed a selective and relatively stable signal in A1847 xenograft tumors [69]. Willmann's team employed yeast surface display and FACS to select a Thy1-targeting scFv to prepare an ultrasound imaging probe. MBThy1-scFv has a high signal in the orthotopic pancreatic ductal adenocarcinoma (5.32 ± 1.59 a.u.) of mice, which is nearly nine times greater than that in the normal pancreas (0.67 ± 0.71 a.u.) [70].
Cochran et al. constructed a yeast surface display library of knottin peptides based on the EETI-II 2.5D scaffold, and they isolated a predominate candidate, 3–4C, with an affinity of 2 nM toward αvβ3 integrin after 9 rounds of FACS (Figure 6a). Knottin 3–4C was further reshaped to 3–4A with two RGD integrin binding loops. Compared with the parent knottin, 3–4A shows a nearly three-fold increase in binding ability to U87 cells, two-fold increase in binding ability to K562-αvβ5 cells, and similar affinity to K562-αvβ3 cells. Moreover, 64Cu-DOTA-knottin 3–4A has properties that are favorable for imaging with a rapid tumor uptake of 3.51% ± 0.83%ID/g at 1 h post injection, tumor-to-blood signal ratio of 27 ± 3, and tumor-to-muscle signal ratio of 31 ± 7 at 4 h post injection in U87MG xenograft mice [71]. Another 68Ga-NOTA-3-4A PET probe was further evaluated for its ability to diagnose vulnerable atherosclerotic plaques. This probe has a high specific plaque uptake of 6.67 ± 1.44 and a favorable plaque-to-normal artery signal ratio of 15.88 at 1 h post injection in a mouse model (Figure 6b–e) [72].
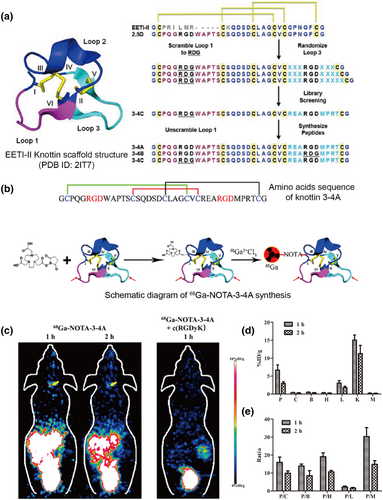
Screening of knottin peptide-based molecular probes for PET. (a) Structure of the knottin scaffold of Ecballium elaterium trypsin inhibitor (PDB ID: 2IT7), the process of library design, and candidate sequences. Reprinted with permission from Ref. [71]. (b) Knottin 3–4A sequence and synthesis scheme of 68Ga-NOTA-3-4A PET probe. (c) Coronal PET images of a mouse model with carotid atherosclerotic plaque at 1 and 2 h post injection of 68Ga-NOTA-3-4A (left) and at 1 h post coinjection of 68Ga-NOTA-3-4A and c (RGDyK) blocker (right). (d) Quantification of 68Ga-NOTA-3-4A accumulated in plaque and other selected organs and tissues at 1 and 2 h post injection. (e) Quantification of plaque-to-other organ and tissue signal ratios at 1 and 2 h post injection. B, brain; C, normal vessel wall; H, heart; K, kidney; L, liver; M, muscle; P, plaque. Reprinted with permission from Ref. [72]. Copyright © 2019, American Chemical Society.
Gambhir et al. also developed an αvβ6-binding knottin, R01-MG, via yeast surface display selection. No adverse effects were observed for healthy human volunteers who were administered [18F]FP-R01-MG-F2, which mainly cleared via the kidneys with low lung and liver uptakes (SUVmean < 1) (Figure 7a). Moreover, in female patients with pancreatic cancer, [18F]FP-R01-MG-F2 could be detected throughout the tumor (ROI = ~8000 mm3, SUVmean = 6.2) and had a low liver uptake (SUVmean = 0.9). [18F]FDG only localized to the biliary stent margin (ROI = ~3000 mm3, SUVmean = 4.1) and showed a liver uptake (SUVmean = 2.9). Therefore, the knottin-based PET probe has good specificity and high contrast image for tumor diagnosis (Figure 7b,c). Additionally, in a pilot study of six patients with idiopathic pulmonary fibrosis (IPF), the [18F]FP-R01-MG-F2 probe demonstrated a potential value for the detection of IPF, although more histological data are needed for further verification [73].
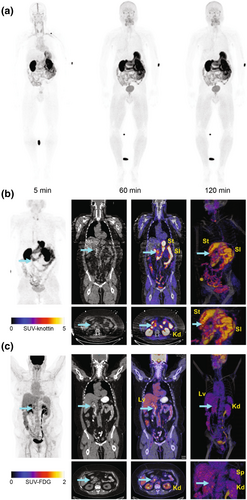
Clinical translation study of the knottin-based PET probe. (a) Images from whole-body MIP PET using [18F]FP-R01-MG-F2 of a healthy volunteer (50-year-old male), showing the biodistribution of the PET tracer at ∼5, 60, and 120 min post injection. (b) PET using [18F]FP- R01-MG-F2 and (c) [18F]FDG of a patient with pancreatic cancer. Images of MIP, CT, PET/CT (axial and coronal), and volume-rendered PET/CT (full and magnified) are shown from left to right. The liver, kidneys, small intestines, stomach, and spleen are denoted Lv, Kd, SI, St, and Sp, respectively. The cyan arrow points to the tumor. Reprinted with permission from Ref. [73]. CT, computed tomography; MIP, maximum intensity projection; PET, positron emission tomography.
3.4 Ribosome display
The ribosome display method was initially developed in 1994 by Mattheakis and coworkers to solve the low transformation efficiency of the phage display system [74]. The ribosome display system comprises three components, namely library gene template synthesis, an in vitro transcription–translation system, and affinity selection. Specifically, the library DNA cassette contains the ligand gene, and the C-terminal without a stop codon ensures the formation of the mRNA-ribosome-ligand complex. The prokaryotic E. coli S30, eukaryotic wheat germ, and rabbit reticulocyte lysate systems are generally used for ligand production with a selection process similar to that of the phage system. mRNA is then eluted and reverse transcribed into cDNA for the next round of library preparation or sequencing [75].
Because the ribosome display is independent of a cell expression system, it excludes the gene transformation step, thereby efficiently expanding the library size and eliminating the amplification bias from the difference in cell growth rates. Moreover, toxic or protease-sensitive ligands are produced, which further ensure the library diversity, while site-directed mutation can be easily realized via error-prone PCR for further molecular evolution. However, some obstacles remain. For example, there is a need to reduce the mRNA instability via an RNAse inhibitor and terminal hair-pin structures. Moreover, the ribosome of the complex may compete with the ligand to interact with the target molecule, and a ligand with a large molecular size may not be efficiently presented [76].
Currently, the ribosome display is popular for scFv selection, and some protein scaffolds developed for imaging have been reported [77]. For example, the thrombus plays a significant role in stroke. Jaroslav's group selected a fibrin clot-binding ABD molecule, D7, using a ribosome display platform and demonstrated the strong binding between fibrin fibers and D7 molecules using confocal and electron microscopy. The D7H2 liposome can penetrate an in vitro flow thrombus model to a depth of 50–100 μm after a 20-min incubation, demonstrating the potential value of D7 for in vivo diagnostic imaging of thrombi [78].
The inflammatory biomarker VCAM-1 is widely expressed on the endothelial cell surface. Kaufmann's team recently reported VCAM-1-binding DARPins® molecules selected from a ribosome display. Compared with MB, MB1732_F8 and MB1730_C7 can produce ultrasound imaging signals with a four-fold enhancement in murine hind-limb inflammation models [79].
3.5 mRNA display
The mRNA display system was reported in 1997 by the Roberts group. The selection on the mRNA display platform is similar to that on a yeast display system. Specifically, the mRNA library comprises synthetic DNA templates (T7 promoter-start codon-ligand gene-deoxyadenosine spacer). mRNA is acquired after in vitro translation and then modified with an aminoacylated tRNA mimic puromycin to the mRNA C-terminal for gene–protein phenotype connection, and the mRNA-linker-protein mixture is then incubated with target molecules, followed by the regular screening process [80].
The puromycin linker is too small to influence ligand–target interactions, and the gene–protein phenotype linkage ensures stringent selection and decreases the diversity loss. Unnatural amino acids or chemical modification are also suitable for functionality improvement and structure reshaping [81]. Although the mRNA display platform has been rarely used for imaging applications, ligands selected from an mRNA display platform have been developed for biomedical applications [82].
By selecting a PD-L1 binding affibody, M1, with nanomole affinity, the Millward group recently showed that the M1-Cy5 fluorescent probe can localize to the tumor site after 1 h, with a signal that is nearly three times stronger than that of the HER2 affibody probe, in an EL4 tumor model. The M1 affibody can also inhibit the PD-L1 signaling pathway at micromolar concentrations, further demonstrating its therapeutic potential. Millward and coworkers also selected the HER2 affibody and autophagy protein LC3 targeting ligands [83].
Robert's team integrated an unnatural amino acid into an mRNA display library to improve the stability of the ligand scaffold. They selected a HER2-targeting cyclic peptide, SUPR4 (KD = 76 ± 30 nM) with 500- and 3700-fold improvement in serum stability and protease resistance, respectively. The SUPR4-Cy5 probe gradually accumulates in the tumor from 4 to 24 h and has a low hepatic uptake at 48 h in the subcutaneous HER2-positve tumor model [84]. The group also used the same strategy to develop a PD-L1 targeting linear peptide, SPAM (KD = 67 nM), with a binding site that overlaps with that of natural PD-1 and Atezolizumab, although the in vivo study remains to be conducted [85].
In addition to ligands targeting receptors, ligands active against other targets can be developed using mRNA display platforms. For example, Matsumoto's group recently designed a macrocyclic peptide mRNA library and selected HIP-8 to inhibit the hepatocyte growth factor. 64Cu-labeled HiP-8-PEG11 rapidly localizes to the tumor within 20 min, with clearance from the liver, for tumor imaging at 90 min post injection in HGF knock-out tumor-bearing mice [86].
4 CHALLENGES AND PERSPECTIVES
Although many selection platforms are currently available, the following factors should be considered for better ligand development: (i) Target immobilization is related to the epitope exposure rate during molecular interactions. Generally, to improve specificity, liquid phase selection can present more functional epitopes than conventional solid immobilization screening. Additionally, candidates are easily sorted with liquid phase selection. (ii) Candidate biocompatibility and specificity are important and generally depend on the target’s phenotype, while combinatory selection against different targets, such as recombinant proteins, cells, and tissues, may improve the in vivo performance of the ligands. (iii) Binding affinity is crucial and determines the strength of interactions between target molecules and ligands. The binding dynamics from SPR or BLI can provide more details; for example, a curve revealing rapid association and slow disassociation indicates stable binding, which may be a preferable ability of imaging ligands.
The size of libraries, efficiency of ligand presentation, and compatibility of biological display systems are critical from a comprehensive perspective. Despite the enormous sizes of ribosome and mRNA libraries, they have many shortcomings, including instability of mRNA, ribosome competition with targets, and poor presentation efficiency of large molecules, such as Fab. In contrast, the phage library is generally larger than cell-based libraries because of the high transformation efficiency of bacterial to fungal and mammalian cells. Although the bacterial surface library has the same transformation efficiency as the phage library, the large fusion protein resulting in OMP fragility may reduce the cell vitality and real library size. Yeast systems are favorable for affinity maturation, and the mammalian surface display is mainly suitable for full-length antibodies, although it has a small library size and is somewhat expensive. Therefore, the phage display platform still holds the primary position in protein-based ligand screening for molecular imaging.
A generalizable strategy can be considered a reference for ligand discovery and imaging probe development. Specifically, first-generation candidates are produced via phage display screening against targets with different phenotypes. The affinity can be further matured by constructing a second-generation cell display library based on the amino acid preference of mutable sites of primary candidates. Moreover, additional mutable sites can be identified via alanine scanning and CDR walking for improving ligand specificity and library diversity. Finally, the stringent panning process is crucial and can be completed using an automatic high-throughput screening platform. The selection cycle, false-positive clones, and manual errors can be greatly reduced to improve efficiency and accuracy.
5 CONCLUSION
Overall, high-throughput screening and biological display technology play significant roles in ligand library construction and ligand presentation, which, in combination with other screening and verification platforms, can offer promising ligands. High-throughput screening platforms can identify biocompatible ligands, which can be used as contrast agents with good targeting efficiency and enhanced tumor uptake, leading to reduced signal interference for improved disease diagnosis and monitoring. Because high-affinity ligands can evade affinity loss from modification via appropriate chemical conjugation methods, favorable imaging signals can be acquired under extremely low administration doses, which further ensure safety from the viewpoint of dosimetry and metabolism. The above advantages promote the clinical translation of targeting ligand-based probes, exemplified by some promising progress in current clinical research using PET/CT, MRI, and NIR imaging modalities. Although there are many important disease targets with no available ligands, it is expected that the above selection platforms will help us to overcome these obstacles and bring us more novel targeting ligands in future.
AUTHOR CONTRIBUTIONS
Renli Luo: Writing – original draft (lead). Hongguang Liu: Writing – review & editing (supporting). Zhen Cheng: Writing – review & editing.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




