Accuracy evaluation of Roche Accu-Chek Performa blood glucose meters at low glucose concentrations: A nine-year retrospective study
[Correction added on 28 September 2024, after first online publication: The text in Section 3.3 and Figure 3 caption had been changed from “100.00% (2979 out of 2978)” to “100.00% (2978 out of 2978)”.]
Abstract
Objective
To evaluate the accuracy of Roche Accu-Chek Performa glucose meters at a low glucose concentration of <5.55 mmol/L (100 mg/dL) over a 9-year period.
Methods
The accuracy of the Roche Accu-Chek Performa glucose meters at low glucose concentrations was evaluated using annual comparison data for 9 consecutive years from 2015 to 2023, according to the acceptability criteria specified in International Organization for Standardization (ISO) 15197:2013. Blood samples with low glucose concentrations of <5.55 mmol/L were prepared by incubation and glycolysis. The glucose concentration was detected using Roche Accu-Chek Performa glucose meters and a biochemical analyzer in the central laboratory.
Results
A total of 2978 pairs of comparison results from 211 glucose meters at a low glucose concentration of <5.55 mmol/L were retrospectively analyzed from 2015 to 2023. The clinical use duration spanned from 1 to 9 years and 40.76% (86 out of 211 glucose meters) had been used for more than 2 years. The correlation coefficient r between glucose meter measurements and laboratory reference values was 0.98 (p < 0.001). The mean according to Roche Accu-Chek Performa glucose meters was 0.05 mmol/L (0.9 mg/dL) higher than that of the biochemical analyzer (Z = −13.82, p < 0.0001). The results showed that 100.00% (211 out of 211) of the Roche Accu-Chek Performa glucose meters met the acceptability criteria specified in ISO 15197:2013. At a low glucose concentration of <5.55 mmol/L, 99.90% (2975 out of 2978) of the comparative data pairs in the error distribution fell within the range of ±0.83 mmol/L (15 mg/dL). Parkes consensus error grid analysis showed that 100.00% (2978 out of 2978) of comparative data pairs fell within region A.
Conclusions
This study demonstrated that Roche Accu-Chek Performa glucose meters successfully met the accuracy standards of ISO 15197:2013 for measuring blood glucose within the hypoglycemic range. Greater attention should be given to the performance of blood glucose monitoring systems in the low glycemic range, especially for patients with diabetes who are prone to hypoglycemia and require precise measurements.
Abbreviations
-
- BGMS
-
- blood glucose monitoring system
-
- CEG
-
- consensus error grid
-
- FDA
-
- U.S. Food and Drug Administration
-
- ISO
-
- International Organization for Standardization
-
- POC
-
- point-of-care
1 INTRODUCTION
Blood glucose monitoring systems (BGMSs) using point-of-care (POC) glucose meters have become increasingly common in hospital settings [1-3]. Maintenance of glycemic control in hospitalized patients is generally achieved by monitoring capillary glucose levels with POC glucose meters to guide treatment adjustment [4, 5]. On 12 July 2018, the U.S. Food and Drug Administration (FDA) cleared the first BGMSs for capillary blood glucose testing in critically ill hospitalized patients, which was a milestone in the management of these patients [6].
Misleading results obtained using POC glucose meters may alter clinical decisions in the care of individuals with diabetes [7]. Therefore, assessment of the accuracy of POC glucose meters is very important in clinical practice, especially at low blood glucose concentrations. The accuracy of POC glucose meters should be periodically evaluated by comparing glucose measurements with reference glucose values from the central laboratory. To ensure patient safety, it is necessary to confirm that blood glucose monitors are sufficiently accurate within the hypoglycemic range. Our previous study investigated the system accuracy of Roche Accu-Check Performa glucose meters across a concentration range of 1.1 mmol/L (19.6 mg/dL) to 33.3 mmol/L (594.6 mg/dL) [8]. We conducted the present study to further analyze the system accuracy of Roche Accu-Check Performa glucose meters at low blood glucose concentrations. The lower limit of detection of Roche Accu-Check Performa glucose meters for blood glucose was 0.6 mmol/L (10 mg/dL) and the low concentration of the acceptability criteria specified in ISO 15197:2013 was 5.55 mmol/L (100 mg/dL). Thus, we retrospectively analyzed the accuracy of Roche Accu-Chek Performa glucose meters at the low glucose concentrations of 0.6 mmol/L to 5.55 mmol/L using annual comparison data for 9 consecutive years from 2015 to 2023. The study could provide a comprehensive experimental data reference for the sustained use of blood glucose meters in the hospitals.
2 MATERIALS AND METHODS
2.1 Methods
This study was conducted using residual venous blood samples from clinical patients in Beijing Tsinghua Changgung Hospital (Beijing, China) from 2015 to 2023. The study was exempt from informed consent and was approved by the ethics committee of Beijing Tsinghua Changgung Hospital. The samples used in this study were residual heparin lithium-anticoagulated whole blood specimens from patients who underwent normal physical examination and patients with diabetes at our hospital. Blood sample inclusion criteria were as follows [9]: (1) samples from patients over 18 years old; (2) hematocrit of the sample was between 30.00% and 55.00%; and (3) blood sample volume was ≥2 mL. Blood sample exclusion criteria were as follows [9]: (1) samples from pregnant women; (2) contaminated blood samples; and (3) samples from patients with anemia. Samples with a low glucose concentration were prepared by incubation and glycolysis.
A total of 211 Roche Accu-Chek Performa glucose meters from multiple nursing stations in the hospital during 2015–2023 were included in this study. To evaluate the accuracy of Roche Accu-Chek Performa glucose meters at low glucose concentrations, the acceptability criteria specified in ISO 15197:2013 were applied [10]. This involved comparing the glucose results from the glucose meters with the reference glucose values from the biochemical analyzer in the central laboratory. The blood glucose concentrations of the samples were successively measured on each Roche Accu-Chek Performa glucose meter (glucose dehydrogenase method; Roche Diagnostics, Mannheim, Germany) and an Advia 2400 biochemical analyzer (Siemens; California, USA) implementing the glucose hexokinase method in the central laboratory within 10 min. The accuracy of the biochemical analyzer was verified using the Proficiency Testing Program provided by the China National Center for Clinical Laboratory. Comparisons were conducted by qualified nurses and technicians following the manufacturer's instructions to avoid measurement discrepancies caused by human operation. The accuracy acceptability of glucose meters was evaluated using the ISO 15197:2013 standard [10]. Parkes consensus error grid (CEG) analysis was used to assess the clinical accuracy of glucose meters [11].
2.2 Statistical analysis
MedCalc Statistical Software version 18 (MedCalc Software bvba, Ostend, Belgium) was used to study the correlation and method comparison analysis of glucose results between the glucose meters and biochemical analyzer. Inter-rater reliability testing was performed using IBM SPSS statistics version 22 (IBM Corp., Armonk, NY, USA) to study the alignment between the glucose results from the Roche Accu-Chek Performa glucose meters and the biochemical analyzer in the alignment matrix. The magnitude of bias between the two methods was compared using Bland-Altman plot analysis and paired samples Wilcoxon tests [12]. Weighted Cohen's kappa analysis was used for the alignment matrix between the glucose results from the Roche Accu-Chek Performa glucose meters and the biochemical analyzer. EP evaluator version 12.4 (Data Innovations LLC, Colchester, VT, USA) was used for the Parkes CEG analysis. A two-tailed p < 0.05 was considered statistically significant.
3 RESULTS
3.1 Glucose concentration distribution of blood glucose samples
To ensure the accuracy of the Roche Accu-Chek Performa glucose meters, an annual assessment was performed by comparing the glucose results obtained from these meters against reference values from the biochemical analyzer. For the yearly comparative data, we extracted measurements with low glucose concentrations below 5.55 mmol/L for inclusion in our research. We collected a total of 2978 comparative data pairs from 211 glucose meters from 2015 to 2023. The clinical use periods of the Roche Accu-Chek Performa glucose meters across various nursing stations spanned 1–9 years. Specifically, 40.76% (86 out of 211) of these meters had been in use for more than 2 years, and 13.74% (29 out of 211) had been in use for over 4 years.
The distribution of comparison data pairs used for accuracy evaluation across each concentration range is presented in Table 1. The minimum value, 20th percentile, 40th percentile, 60th percentile, 80th percentile, and the maximum value were 0.60, 2.23, 2.74, 3.33, 4.31, and 5.39, respectively. The reproducibility of blood glucose meter measurements was analyzed by different operators on different glucose meters on different days at the 40th percentile (2.74 mmol/L) and 80th percentile (4.31 mmol/L) of the laboratory-determined glucose values. The coefficient of variation, CV for reproducibility was found to be 2.22% at a glucose concentration of 2.74 mmol/L and 2.59% at 4.31 mmol/L, as shown in Table 2.
| Group | Blood glucose concentration (mmol/L) | Numbers of the comparison data pairs | Constituent ratio (%) |
|---|---|---|---|
| 1 | 0.60–2.23 | 606 | 20.35 |
| 2 | 2.24–2.74 | 590 | 19.81 |
| 3 | 2.75–3.33 | 612 | 20.55 |
| 4 | 3.34–4.31 | 602 | 20.21 |
| 5 | 4.32–5.39 | 568 | 19.07 |
- Note: Laboratory glucose results used for accuracy evaluation were distributed as follows: 0.60, 2.23, 2.74, 3.33, 4.31, and 5.39 mmol/L, representing the minimum value, the 20th percentile, 40th percentile, 60th percentile, 80th percentile, and the maximum value, respectively.
| Item | Reproducibility |
|---|---|
| 2.74 mmol/L | |
| Mean | 2.88 mmol/L |
| SD | 0.06 mmol/L |
| CV | 2.22% |
| n | 26 |
| 4.31 mmol/L | |
| Mean | 3.95 mmol/L |
| SD | 0.10 mmol/L |
| CV | 2.59% |
| n | 35 |
- Note: Reproducibility of Roche Accu-Chek Performa glucose meter measurements, performed by different operators on different meters across different days, was assessed at the 40th percentile (2.74 mmol/L) and 80th percentile (4.31 mmol/L) of the laboratory glucose results.
- Abbreviation: CV, coefficient of variation; n, number of glucose meters; SD, standard deviation.
3.2 Differences between glucose measurements obtained with glucose meters and biochemical analyzer
According to the method of accuracy evaluation outlined in ISO 15197:2013, a significant correlation was observed between glucose measurements obtained with the glucose meters (y) and the biochemical analyzer (x). The regression equation was y = 0.340 + 0.908 x, with a correlation coefficient of 0.98 (p < 0.001), as shown in Figure 1. The relationship between the results of the Roche Accu-Chek Performa glucose meters and the biochemical analyzer results was compared using Bland–Altman difference analysis. The comparison showed that the mean of the Roche Accu-Chek Performa glucose meters was 0.05 mmol/L (0.9 mg/dL) higher than that of the biochemical analyzer (Z = −13.82, p < 0.0001), giving a mean difference percentage of 3.4%. The differences in the mean of the two methods showed a downward trend, as shown in Figure 2. The Roche Accu-Chek Performa glucose meters yielded higher blood glucose readings than the biochemical analyzer at low concentrations and lower readings at high concentrations, as illustrated in Figure 2.
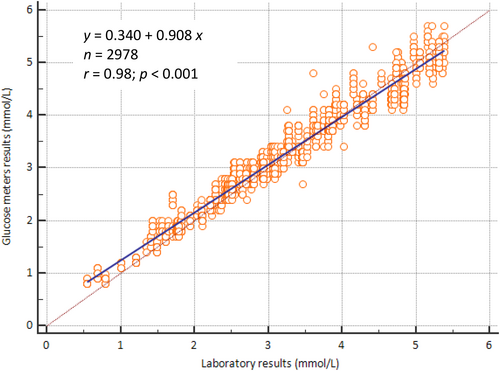
Correlation between glucose values obtained with glucose meters and biochemical analyzer. X-axis: glucose values from the biochemical analyzer (mmol/L). Y-axis: glucose values from Roche Accu-Chek Performa glucose meters (mmol/L). The regression equation was y = 0.340 + 0.908 x, with a correlation coefficient of 0.98 (p < 0.001). n, number of comparison data pairs from each glucose meter.
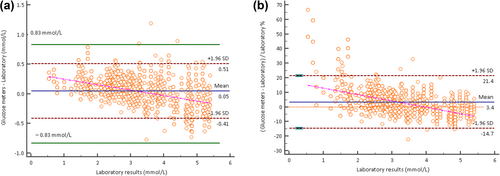
Bland–Altman plot showing a graph of the difference against the mean difference between laboratory reference values and glucose meter results. The magnitude of bias between the two methods was compared using a paired samples Wilcoxon test. (a) Mean difference = 0.05 mmol/L (0.9 mg/dL), Z = −13.82, p < 0.0001. Green line at ±0.83 mmol/L (15 mg/dL): acceptability criteria according to ISO 15197:2013 for glucose concentrations below 5.55 mmol/L (100 mg/dL). (b) Mean difference% = 3.4%. Pink line: regression line of the mean difference between methods. SD, standard deviation.
When the Roche Accu-Chek Performa glucose meters were assessed against the reference glucose values from the biochemical analyzer at glucose concentrations less than 5.55 mmol/L, 100.00% (211 out of 211) of glucose measurements from the meters fell within the specified range of ±0.28 mmol/L (5 mg/dL). In a similar evaluation of the comparison data pairs against the reference glucose values at glucose concentrations less than 5.55 mmol/L, 77.50% (2309 out of 2978), 97.80% (2911 out of 2978), and 99.90% (2975 out of 2978) of the comparative data pairs fell within the ranges of ±0.28 mmol/L (5 mg/dL), ±0.56 mmol/L (10 mg/dL), and ±0.83 mmol/L (15 mg/dL), respectively.
The alignment between glucose results from the Roche Accu-Chek Performa glucose meters and the biochemical analyzer is shown in the alignment matrix in Table 3. The inconsistencies were all within only one category. The matrix table revealed that 7.69% [229 (15 + 15 + 73 + 126) out of 2978] of glucose meter results (blue background) were one category level lower than the laboratory results, 74.48% [2218 (443 + 432 + 482 + 451 + 410) out of 2978] (green background) fell within the same equivalent category as the laboratory results, and 17.83% [531 (163 + 143 + 115 + 78 + 32) out of 2978] (yellow background) were one category level higher than the laboratory results. Weighted Cohen's kappa was 0.682 (95% confidence interval: 0.662–0.702) (p < 0.001).
| Lab results | Glucose results of the Roche Accu-Chek Performa glucose meters | Total | Kappa | U | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.60–2.23 | 2.24–2.74 | 2.75–3.33 | 3.34–4.31 | 4.32–5.39 | 5.40–5.54 | |||||
| 0.60–2.23 | 443 | 163 | 0 | 0 | 0 | 0 | 606 | 0.682 | 75.18 | <0.001 |
| 2.24–2.74 | 15 | 432 | 143 | 0 | 0 | 0 | 590 | |||
| 2.75–3.33 | 0 | 15 | 482 | 115 | 0 | 0 | 612 | |||
| 3.34–4.31 | 0 | 0 | 73 | 451 | 78 | 0 | 602 | |||
| 4.32–5.39 | 0 | 0 | 0 | 126 | 410 | 32 | 568 | |||
| 5.40–5.54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Total | 458 | 610 | 698 | 692 | 488 | 32 | 2978 | |||
3.3 Parkes consensus error grid analysis
Parkes CEG analysis was used to evaluate the accuracy of the Roche Accu-Chek Performa glucose meter as a risk-based approach. In total, 100.00% (2978 out of 2978) of comparative data pairs fell within region A Parkes CEG analysis (Figure 3).
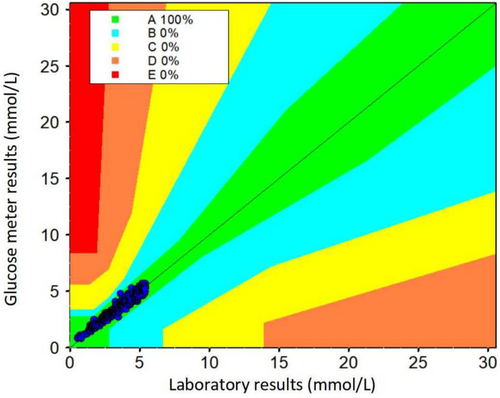
Parkes CEG analysis of the accuracy of the Roche Accu-Chek Performa glucose meter. X-axis: glucose values from the biochemical analyzer (mmol/L); Y-axis: glucose values from Roche Accu-Chek Performa glucose meters (mmol/L). Region A: No effect on clinical action. Region B: Altered clinical action but little or no effect on clinical outcome. Region C: Altered action likely to affect the outcome. Region D: Significant medical risk. Region E: Could have dangerous consequences. In total, 100.00% (2978 out of 2978) of comparative data pairs fell within region A using Parkes CEG analysis. CEG, consensus error grid.
4 DISCUSSION
Hypoglycemia is a prevalent and serious acute complication of diabetes mellitus. Clinicians and nurses require precise tools for the early detection, prevention, and treatment of hypoglycemia within the spectrum of low blood glucose levels to avoid or reduce the risk of associated complications. Studies have reported that improved glycemic control is associated with superior clinical outcomes [7, 13, 14]. However, it is often challenging to maintain high accuracy in the hypoglycemic range using blood glucose monitoring technology. There is increasing concern regarding the accuracy and reliability of glucose meters [8, 15, 16]. Patient safety should be a primary concern in terms of the possible negative effects of recurring or prolonged hypoglycemia.
To evaluate the accuracy of Roche Accu-Chek Performa glucose meters, we compared an assessment comparing glucose results obtained using these meters against reference values from a biochemical analyzer [8]. Several studies evaluating current BGMSs have reported considerable differences in performance in the low-glucose range [12, 17, 18]. This study was conducted using annual comparison data for 9 consecutive years from 2015 to 2023 according to the acceptability criteria specified in ISO 15197:2013. A total of 2978 pairs of comparison results from 211 Roche Accu-Chek Performa glucose meters at the low glucose concentration of <5.55 mmol/L were retrospectively analyzed. The CV for reproducibility of the blood glucose meter measurements conducted by different operators on different glucose meters and on different days was found to be 2.22% at a glucose concentration of 2.74 mmol/L and 2.59% at 4.31 mmol/L. This indicated that the Roche Accu-Chek Performa glucose meters exhibited good reproducibility at low blood glucose levels and maintained consistent performance across inter-batch and long-term day-to-day measurements.
According to the ISO 15197:2013 guideline, 95% of blood glucose measurements should be within 0.83 mmol/L (15 mg/dL) for blood glucose <5.55 mmol/L (100 mg/dL). Our study showed that 100.00% (211 out of 211) of Roche Accu-Chek Performa glucose meters and 99.90% (2975 out of 2978) of the comparative data pairs fell within the range of ±0.83 mmol/L (15 mg/dL) according to ISO 15197:2013. Parkes CEG analysis showed that 100.00% (2978 out of 2978) of the comparative data pairs fell within region A. These results indicated that the Roche Accu-Chek Performa glucose meters well met the criteria of ISO 15197:2013.
Bland–Altman plots in this study showed that the mean value of the Roche Accu-Chek Performa glucose meters was 0.05 mmol/L (3.4%) higher than that of the biochemical analyzer at blood glucose concentrations between 0.60 mmol/L and 5.39 mmol/L; the differences were statistically significant using paired samples Wilcoxon tests (Z = −13.82, p < 0.0001). We divided the glucose concentration range of 0.60–5.39 into two intervals (0.60–2.73 and 2.74–5.39) based on the 20th percentile (2.74 mmol/L) and comparative analysis was conducted for each interval. The findings showed that the mean value of the Roche Accu-Chek Performa glucose meters was 0.01 mmol/L (0.1%) lower than that of the biochemical analyzer at blood glucose concentrations between 2.74 mmol/L and 5.39 mmol/L; the differences were not statistically significant using paired samples Wilcoxon tests (Z = 1.52, p = 0.13). The mean value of Roche Accu-Chek Performa glucose meters was 0.08 mmol/L (8.4%) higher than that of the biochemical analyzer at blood glucose concentrations between 0.60 mmol/L and 2.73 mmol/L; the differences were statistically significant using paired samples Wilcoxon tests (Z = −26.25, p < 0.0001). It is recommended that whenever a blood glucose measurement from Roche Accu-Chek Performa glucose meters falls below 2.74 mmol/L, a laboratory measurement should be conducted immediately to verify the POC results. To avoid overdose, caution is needed when using a blood glucose measurement of less than 2.74 mmol/L to adjust a patient's medication dose. Our study findings raise awareness regarding the accuracy of blood glucose meters at low concentrations [19]. Notably, the system accuracy of glucose meters varies considerably; therefore, performance verification of glucose meters before clinical application is essential [15, 20].
Several factors may influence the results of comparison between glucose meters and central laboratory methods. (1) Glycolysis is generally not a concern when using glucose meters, but it can become important when using central laboratory methods owing to the metabolism of glucose by blood cells at a rate of 5%–7% per hour in vitro [21]. In this study, blood glucose concentrations were first measured on glucose meters; then, the sample was transferred to a biochemical analyzer for blood glucose concentration testing. The time difference between the measurements may partially account for the mildly higher glucose results observed with the Accu-Check glucose meters. (2) The Roche Accu-Chek Performa glucose meter measures blood glucose using glucose dehydrogenase-based test strips. It has been reported that oxygen tension has a minimal effect on glucose oxidase-based test strips but does not significantly affect glucose measured using glucose dehydrogenase-based test strips [22]. Thus, greater attention is needed when using glucose meters that feature oxygen-independent test strips, which are recommended for critically ill patients and those with high or unpredictable blood oxygen levels [23]. (3) Other factors that contribute to the discrepancies include inter-lot variability in glucose meter reagent strips, reagent stability, equipment wear and tear at a time level, and sample transportation.
This study had several limitations. First, we used venous blood samples instead of capillary blood samples for the comparative analysis because we encountered difficulties in procuring capillary whole blood samples or commutable proficiency testing items. Second, glucose concentrations decrease depending upon the baseline number of red blood cells, white blood cells, and monocytes in the sample [24]. We did not incorporate these influencing factors into our sample exclusion criteria. Because this was a comparative paired study where samples were measured using both glucose meters and a biochemical analyzer within 10 min, the effect on both methods was considered to be equivalent.
In summary, the Roche Accu-Chek Performa glucose meters in our study could accurately reflect patients' blood glucose levels and had good accuracy and precision in monitoring the low blood glucose range, meeting the ISO 15197:2013 standard. From a clinical standpoint, greater attention should be given to the performance of BGMSs in the low-glycemic range, especially for patients with diabetes who are prone to hypoglycemia and require precise measurements.
AUTHOR CONTRIBUTIONS
Zhipeng Zhao and Runqing Li analyzed the data and prepared the first draft of the manuscript. Lina Zhang, Tengjiao Wang, Xiuying Zhao, Song Yang, Ning Han, and Dong Zhu participated in the conception and design of the study. Runqing Li constructively revised the manuscript. Zhipeng Zhao, Runqing Li, and Tengjiao Wang participated in data collection and organization. All authors commented on previous versions of the manuscript and approved the final version.
ACKNOWLEDGMENTS
The authors are grateful to the staff of the Laboratory Medicine Department, Nursing Department, Clinical Administration Department, and Instrument Department of Beijing Tsinghua Changgung Hospital for their efforts in this study.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




