Long-term prognostic impact of cardiovascular comorbidities in patients with prostate cancer receiving androgen deprivation therapy: A population-based competing risk analysis
Abstract
Our study investigated how adverse cardiovascular outcomes are impacted by cardiovascular comorbidities in patients with prostate cancer treated by androgen deprivation therapy (ADT). Using prospective, population-based data, all Hong Kong patients with prostate cancer who received ADT during 1 January 1993 to 3 March 2021 were identified and followed up for the endpoint of cardiovascular hospitalization/mortality until 31 September 2021, whichever earlier. Multivariable competing risk regression was used to compare the endpoint's cumulative incidence between different combinations of major cardiovascular comorbidities (heart failure [HF], myocardial infarction [MI], stroke and/or arrhythmia), with noncardiovascular death as competing event. Altogether, 13 537 patients were included (median age 75.9 [interquartile range 70.0-81.5] years old; median follow-up 3.3 [1.5-6.7] years). Compared to those with none of prior HF/MI/stroke/arrhythmia, the incidence of the endpoint was not different in those with only stroke (subhazard ratio [SHR] 1.06 [95% confidence interval (CI): 0.92-1.23], P = .391), but was higher in those with only HF (SHR 1.67 [1.37-2.02], P < .001), arrhythmia (SHR 1.63 [1.35-1.98], P < .001) or MI (SHR 1.43 [1.14-1.79], P = .002). Those with ≥2 of HF/MI/stroke/arrhythmia had the highest incidence of the endpoint (SHR 1.94 [1.62-2.33], P < .001), among whom different major cardiovascular comorbidities had similar prognostic impacts, with the number of comorbidities present being significantly prognostic instead. In conclusion, in patients with prostate cancer receiving ADT, the sole presence of HF, MI or arrhythmia, but not stroke, may be associated with elevated cardiovascular risks. In those with ≥2 of HF/MI/stroke/arrhythmia, the number of major cardiovascular comorbidities may be prognostically more important than the type of comorbidities.
Graphical Abstract
What's new?
While androgen deprivation therapy has been associated with adverse cardiovascular outcomes in patients with prostate cancer, how adverse cardiovascular outcomes are impacted by cardiovascular comorbidities remains unclear. In this Hong Kong population-based study of 13 537 patients covering the 1993 to 2021 period, a history of heart failure, myocardial infarction and arrhythmia, but not stroke, was associated with co-existing cardiovascular conditions, as well as a higher risk of cardiovascular events on follow-up. However, in patients with more than two major co-existing cardiovascular conditions, the number of cardiovascular comorbidities may have greater prognostic importance than the type of comorbidities.
Abbreviations
-
- ADT
-
- androgen deprivation therapy
-
- ARSI
-
- androgen receptor signaling inhibitor
-
- CDARS
-
- Clinical Data Analysis and Reporting System
-
- CI
-
- confidence interval
-
- HF
-
- heart failure
-
- HFA-ICOS
-
- Heart Failure Association-International Cardio-Oncology Society
-
- ICD-9
-
- International Classification of Diseases, Ninth revision
-
- ICD-10
-
- International Classification of Diseases, Tenth revision
-
- IQR
-
- interquartile range
-
- MI
-
- myocardial infarction
-
- PCa
-
- prostate cancer
-
- SHR
-
- subhazard ratio
1 INTRODUCTION
Androgen deprivation therapy (ADT) involves pharmacological or surgical suppression of androgen activity, and has long been a cornerstone of prostate cancer (PCa) treatment.1 While the efficacy of ADT for treating PCa is undoubted, the past decade has seen studies demonstrating an association between ADT and adverse cardiovascular outcomes. Ever since the landmark study by Keating et al,2 ADT has been shown to be associated with increased risks of myocardial infarction, arrhythmia, stroke, heart failure and cardiovascular mortality, with many also demonstrating other aspects of adverse cardiometabolic outcomes.3
Given the rising prevalence of PCa and the important role played by ADT in PCa treatment, increasing attention has been given to the adverse cardiovascular effects of ADT. In the 2022 European Society of Cardiology Guidelines on cardio-oncology, one of the first major societal guidelines in cardio-oncology, a separate section was dedicated to the surveillance for ADT-related cardiotoxicity.4 The same guideline recommended the use of the Heart Failure Association-International Cardio-Oncology Society (HFA-ICOS) risk assessment tool for cardiovascular risk stratification of cardio-oncology patients, in which numerous cardiovascular risk factors were designated categories that reflect their relative prognostic significance for cardiotoxicity related to a specific class of anticancer medications.4, 5 However, this important risk assessment tool did not include such designations for ADT. Indeed, little is known about the relative impact of different cardiovascular risk factors on adverse cardiovascular outcomes. Similarly, studies of relationships between different cardiovascular risk factors in patients receiving ADT are lacking. These represent important gaps in the understanding and stratification of cardiovascular risk and burden in these patients. Therefore, we aimed to investigate the interrelationship between different major cardiovascular comorbidities, and their impact on cardiovascular outcomes in patients with PCa receiving ADT.
2 MATERIALS AND METHODS
Our study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guideline.
2.1 Source of data
Data were collected from the Clinical Data Analysis and Reporting System (CDARS), a population-based administrative database that prospectively records basic demographics, diagnoses, laboratory tests and medical procedures of all patients attending public hospitals and clinics in Hong Kong, which cover the entire territory of Hong Kong and serve an estimated 90% of the population.6 Diagnoses are encoded by the International Classification of Diseases, Ninth revision (ICD-9) codes regardless of the time of data input, as ICD-10 codes have not been implemented in CDARS to date. Mortality data were collected from the linked Hong Kong Death Registry, a governmental registry of all Hong Kong citizens' death records. Both databases have been used extensively in research, and have been shown to have good coding accuracy and data completeness.7-13
2.2 Patient population
Adult (aged 18 years old or above) patients with PCa who received ADT between 1 January 1993 to 31 March 2021 were identified. ADT included medical castration and bilateral orchiectomy (BO). There were no exclusion criteria. This cohort has been published earlier.14
The following baseline characteristics were recorded: age, type of androgen deprivation therapy, comorbidities (hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, chronic liver disease, stroke, myocardial infarction (MI), heart failure (HF) and arrhythmias (atrial fibrillation, ventricular tachycardia or ventricular fibrillation)) and use of medications or prior procedures (radiotherapy, chemotherapy, radical prostatectomy, androgen receptor signaling inhibitor [ARSI], dihydropyridine calcium channel blockers, metformin, sulphonylurea, dipeptidyl peptidase-4 inhibitors, glucagon-like peptide-1 receptor agonist, insulin, antiplatelet, anticoagulant and corticosteroid).
2.3 Follow-up and outcomes
All patients were followed up from the date of ADT initiation until 31 September 2021, death or the primary endpoint, whichever occurred earlier. The primary endpoint was a composite of cardiovascular hospitalization and cardiovascular mortality. Cardiovascular hospitalizations were identified by ICD-9 codes listed in Table S1, while cardiovascular mortality was identified by ICD-9 or ICD-10 codes listed in Table S2.
2.4 Major cardiovascular comorbidities and their relative impact
We focused on prior history of HF, MI, stroke and arrhythmia as the major cardiovascular comorbidities of interest. These comorbidities were chosen among broader cardiometabolic conditions, such as hypertension and diabetes mellitus, as (a) we had shown that these accounted for >75% of cardiovascular hospitalizations in patients with PCa receiving ADT,14 (b) they are clinically more severe conditions that are likely to lead to substantially worse morbidity and (c) the inclusion of broader cardiometabolic comorbidities would have created too many combinations of comorbidities with limited sample sizes, preventing any meaningful analysis. Patients were categorized into the following groups by the presence of these major cardiovascular comorbidities for comparisons: none of HF/MI/stroke/arrhythmia, HF only, MI only, stroke only, arrhythmia only and ≥2 of HF/MI/stroke/arrhythmia. To explore the effects of stroke subtypes, we carried out a post hoc analysis in which patients with stroke only were split into those having had only ischemic stroke, only hemorrhagic stroke or both ischemic and hemorrhagic stroke.
As these comorbidities commonly overlap, we further explored the relative impact of each major cardiovascular comorbidity in those with overlapping comorbidities (ie, with ≥2 major cardiovascular comorbidities). This was done by comparing patients with both a condition of focus and any of the “nonfocus” conditions against patients with ≥2 of the “nonfocus” conditions. For instance, to explore the relative impact of HF, we compared patients with HF and any of MI/stroke/arrhythmia against those with ≥2 of MI/stroke/arrhythmia.
2.5 Statistical analyses
Continuous variables were expressed as medians with interquartile ranges (IQRs). There was no missing value due to the nature of database and variables used. All variables used for multivariable adjustments were determined based on clinical expertise. The number of major cardiovascular comorbidities was compared between groups using multivariable Poisson regression adjusting for all recorded baseline variables, with the ratio of cardiovascular comorbidity counts and corresponding 95% confidence intervals (CIs) as summary statistics. Noncardiovascular mortality constituted a competing event for the primary outcome. As Kaplan-Meier curves over-estimate cumulative incidences in the presence of competing events,14, 15 the cause-specific cumulative incidence of the primary endpoint was estimated and visualized using the Aalen-Johansen estimator.14, 15 The 3-, 5- and 10-year cause-specific cumulative incidences were estimated for each group (none of HF/MI/stroke/arrhythmia, HF only, MI only, stroke only, arrhythmia only and at least two of HF/MI/stroke/arrhythmia). The cumulative incidence of the primary endpoint was quantitatively compared between groups using multivariable Fine-Gray competing risk regression, with subhazard ratios (SHRs) and 95% CIs as summary statistics and adjusting for all collected baseline variables. All reported SHRs and corresponding CIs are adjusted estimates.
As the exploratory analysis for the relative impact of each major cardiovascular comorbidity focused on patients with ≥2 of these comorbidities, the corresponding Fine-Gray regressions were additionally adjusted for the number of major cardiovascular comorbidities present to account for potential imbalances in the number of comorbidities between groups. Furthermore, in view of the results from the exploratory analysis, a post hoc analysis was conducted to explore the prognostic effects of the number of major cardiovascular comorbidities on the primary endpoint among patients with ≥2 of these comorbidities. Because very few patients had all four of these comorbidities, this post hoc analysis compared those with two of these comorbidities against those with ≥3 of these comorbidities.
Fine-Gray regression was separately performed in two prespecified and one post hoc subgroup analyses. First, to clarify any impact that heterogeneity in the type of ADT may have on the observed effects, subgroup analysis was performed for each type of ADT (medical castration, bilateral orchidectomy and both; all patients received bilateral orchidectomy after medication castration, mostly because, until recently, the local reimbursement system did not subsidize medical castration and bilateral orchidectomy was often performed as a cost-saving long-term alternative to medical castration). On the other hand, as cancer staging and histology were not available from the database used, we used ever-prescription of ARSI or chemotherapy as a surrogate for metastatic disease, as these were only indicated in metastatic PCa.16, 17 The second subgroup analysis thus stratified patients by whether they were ever prescribed ARSI or chemotherapy, as a surrogate of whether they had metastatic disease. The third, post hoc subgroup analysis explored the effects of metabolic dysfunction or hypertension on our findings, with stratification for the presence of hypertension, diabetes mellitus or dyslipidemia. Due to the small sample sizes for each risk category, interactions between subgroups were not tested. Additionally, due to the relatively small number of patients with ≥2 major cardiovascular comorbidities, these subgroup analyses were not performed for the exploratory analysis of the relative impact of each comorbidity in those with overlapping comorbidities.
Two-sided P < .05 were considered statistically significant. All analyses were performed on Stata version 16.1 (StataCorp LLC, College Station, Texas).
3 RESULTS
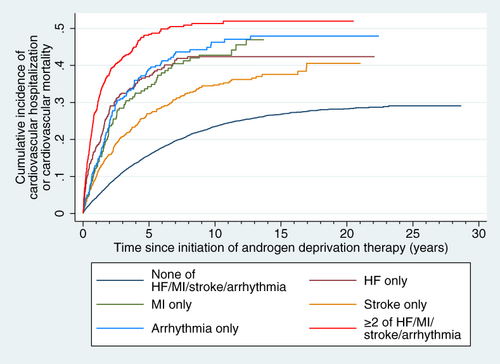
Altogether, 13 537 patients were included (median age 75.9 [IQR 70.0-81.5] years old; Table 1). Most patients (11 102, 82.0%) had none of prior HF/MI/stroke/arrhythmia, 357 (2.6%) had prior HF only, 240 (1.8%) had MI only, 968 (7.2%) had prior stroke only, 319 (2.4%) had arrhythmia only (602 [4.5%] had only atrial fibrillation, 22 [0.2%] had only ventricular tachycardia or fibrillation and 8 [0.1%] had both atrial fibrillation and ventricular tachycardia or fibrillation) and 494 (3.6%) had ≥2 of HF/MI/stroke/arrhythmia. Among patients who had one to three of the four major cardiovascular comorbidities, patients who had HF, MI or arrhythmia had significantly more major cardiovascular comorbidities than those who did not have each of these conditions (P < .001 for all; Table 2), but not for those who had stroke (P = .303). The number of patients with each possible combination of major cardiovascular comorbidities are shown in Table S3.
| Number of patients, N | 13 537 |
|---|---|
| Age, years | 75.5 ± 8.5 |
| Medical castration, N (%) | 8178 (60.4) |
| Bilateral orchidectomy, N (%) | 6593 (48.7) |
| Ischemic stroke, N (%) | 1052 (7.8%) |
| Hemorrhagic stroke, N (%) | 241 (1.8%) |
| Myocardial infarction, N (%) | 427 (3.2) |
| Heart failure, N (%) | 695 (5.1) |
| Arrhythmia, N (%) | 632 (4.7) |
| Hypertension, N (%) | 3624 (26.8) |
| Diabetes mellitus, N (%) | 2886 (21.3) |
| Dyslipidemia, N (%) | 1270 (9.4) |
| Chronic kidney disease, N (%) | 452 (3.3) |
| Prior radiotherapy, N (%) | 493 (3.6) |
| Prior radical prostatectomy, N (%) | 3735 (27.6) |
| Prior chemotherapy, N (%) | 61 (0.5) |
| Ever received chemotherapy or ARSI, N (%) | 5116 (37.8) |
| Dihydropyridine CCB users, N (%) | 5396 (39.9) |
| Metformin users, N (%) | 1480 (10.9) |
| Sulphonylurea users, N (%) | 1744 (12.9) |
| DPP4 inhibitor users, N (%) | 150 (1.1) |
| GLP1 receptor agonist users, N (%) | 2 (0.0) |
| Insulin users, N (%) | 722 (5.3) |
| Antiplatelet users, N (%) | 2962 (21.9) |
| Anticoagulant users, N (%) | 458 (3.4) |
| Corticosteroid users, N (%) | 2342 (17.3) |
- Abbreviations: ARSI, androgen receptor signaling inhibitor; CCB, calcium channel blocker; DPP4, dipeptidyl peptidase 4; GLP1, glucagon-like peptide-1.
| Category | Number of major cardiovascular comorbidities, N (%) | Ratio of cardiovascular comorbidity counts [95% confidence interval] | P-value | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| With HF | 357 (51.9) | 255 (37.1) | 76 (10.9) | 1.44 [1.34-1.56] | <.001 |
| Without HF (reference) | 1527 (90.7) | 148 (8.8) | 8 (0.5) | ||
| With MI | 240 (57.1) | 130 (31.0) | 50 (11.9) | 1.34 [1.22-1.47] | <.001 |
| Without MI (reference) | 1644 (84.3) | 273 (14.0) | 34 (1.7) | ||
| With stroke | 968 (80.1) | 187 (15.5) | 54 (4.5) | 1.04 [0.97-1.12] | .303 |
| Without stroke (reference) | 916 (78.8) | 216 (18.6) | 30 (2.6) | ||
| With arrhythmia | 319 (51.0) | 234 (37.4) | 72 (11.5) | 1.39 [1.27-1.52] | <.001 |
| Without arrhythmia (reference) | 1565 (89.6) | 169 (9.7) | 12 (0.7) | ||
- Abbreviations: HF, heart failure; MI, myocardial infarction.
3.1 Impact of cardiovascular comorbidities
Over a median follow-up of 3.3 [1.5-6.7] years, 3225 (23.8%) met the primary endpoint and 6662 (49.2%) died of noncardiac causes without meeting the primary endpoint; 9113 (67.6%) died in total. Of the 3225 patients who met the primary endpoint, 1 had cardiovascular mortality and 3224 had cardiovascular hospitalization (among whom 670 [4.9% of all patients; 20.8% of those who had cardiovascular hospitalization] went on to have cardiovascular mortality). Cumulative incidence of the primary endpoint is shown in Figure 1, and each group's estimated 3-, 5- and 10-year cumulative incidences of the primary endpoint are summarized in Table S4. Compared to patients who had none of prior HF/MI/stroke/arrhythmia, patients with only prior HF (SHR 1.67 [95% confidence interval (CI): 1.37-2.02], P < .001), prior arrhythmia (SHR 1.63 [1.35-1.98], P < .001) or prior MI (SHR 1.43 [1.14-1.79], P = .002) had significantly higher incidence of the primary endpoint, but not those who only had prior stroke (SHR 1.06 [0.92-1.23], P = .391). Those who had ≥2 of HF/MI/stroke/arrhythmia had the highest incidence of the primary endpoint (SHR 1.94 [1.62-2.33], P < .001). In post hoc analysis, patients with stroke only did not have significantly different incidence of the primary endpoint regardless of the type of stroke (ischemic stroke: SHR 1.07 [0.92-1.24], P = .408; hemorrhagic stroke: SHR 1.10 [0.77-1.56], P = .598; both ischemic and hemorrhagic stroke: SHR 0.93 [0.53-1.63], P = .808), compared to those without any of major cardiovascular comorbidities.
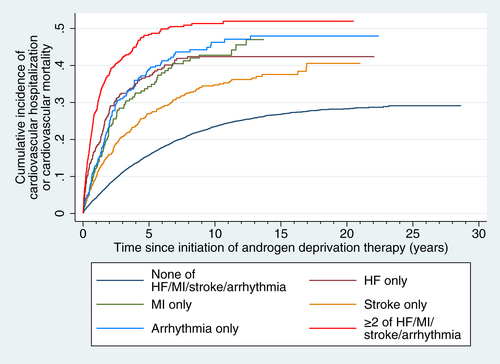
Among the 1881 patients with only one of the major cardiovascular comorbidities, HF (SHR 1.51 [1.21-1.89], P < .001), arrhythmia (SHR 1.39 [1.11-1.73], P = .004) and MI (SHR 1.31 [1.03-1.66], P = .026) were all associated with significantly higher incidences of the primary endpoint compared to those with only stroke.
3.2 Subgroup analysis
Subgroup analysis by the type of ADT (Table 3) showed mostly consistent trends in patients who received only medical castration (N = 6944) or only bilateral orchidectomy (N = 5116), with those having ≥2 of HF/MI/stroke/arrhythmia having the highest incidence of the primary endpoint, and with only HF, MI and arrhythmia being associated with significantly higher incidence of the primary endpoint when compared to those without any of HF/MI/stroke/arrhythmia. However, no significant association was observed in the 1234 patients who received both medical castration and bilateral orchidectomy.
| Major cardiovascular comorbidities | Medical castration (N = 6944) | Bilateral orchidectomy (N = 5359) | Both medical castration and bilateral orchidectomy (N = 1234) |
|---|---|---|---|
| None of HF/MI/stroke/arrhythmia | 1 (reference) | 1 (reference) | 1 (reference) |
| HF only | 1.65 [1.25-2.17], P < .001 | 1.77 [1.31-2.38], P < .001 | 1.15 [0.56-2.38], P = .704 |
| MI only | 1.36 [1.01-1.84], P = .045 | 1.65 [1.13-2.42], P = .009 | 1.02 [0.39-2.64], P = .972 |
| Stroke only | 1.01 [0.83-1.23], P = .915 | 1.20 [0.96-1.51], P = .109 | 0.74 [0.44-1.23], P = .243 |
| Arrhythmia only | 1.76 [1.36-2.28], P < .001 | 1.67 [1.22-2.30], P = .002 | 0.52 [0.22-1.22], P = .133 |
| ≥2 of HF/MI/stroke/arrhythmia | 1.81 [1.43-2.29], P < .001 | 2.38 [1.75-3.24], P < .001 | 1.34 [0.61-2.94], P = .463 |
- Abbreviations: HF, heart failure; MI, myocardial infarction.
On the other hand, while MI and arrhythmia remained to be associated with significantly higher incidences of the primary endpoint regardless of whether patients had metastatic disease (ie, prescribed ARSI or chemotherapy; Table S5), such association for HF was significant in those who did not have metastatic disease (ie, never prescribed ARSI or chemotherapy), but only approached significance in those with metastatic disease (P = .059). Having ≥2 of HF/MI/stroke/arrhythmia remained to be associated with the highest incidence of primary endpoint regardless of whether patients had metastatic disease.
In the post hoc subgroup analysis by the presence of hypertension, diabetes mellitus or dyslipidemia, findings in both subgroups were largely consistent with the main analysis (Table S6), although the association for patients with only MI among those with hypertension, diabetes mellitus or dyslipidemia only approached statistical significance (P = .055).
3.3 Relative impact of each comorbidity in patients with overlapping comorbidities
The relative impact of each specified major cardiovascular comorbidity were explored among the 494 patients with ≥2 of HF/MI/stroke/arrhythmia, with cumulative incidences of the primary endpoint stratified by different combinations of cardiovascular comorbidities (Figure 2). HF, MI, stroke and arrhythmia did not have significantly different impact on the incidence of the primary endpoint (Table 4). Post hoc analysis suggested that compared to those with two of HF/MI/stroke/arrhythmia, those who had ≥3 of these comorbidities had significantly higher incidence of the primary endpoint (SHR 1.42 [1.02-1.97], P = .037).
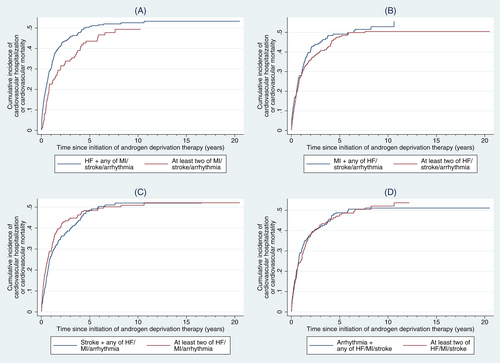
| Comparator (comorbidity of interest highlighted) | Reference group | Subhazard ratio [95% CI] | P-value |
|---|---|---|---|
| HF + any of MI/stroke/arrhythmia (N = 338) | ≥2 of MI/stroke/arrhythmia (N = 156) | 1.15 [0.86-1.55] | .343 |
| MI + any of HF/stroke/arrhythmia (N = 187) | ≥2 of HF/stroke/arrhythmia (N = 307) | 0.99 [0.73-1.34] | .934 |
| Stroke + any of HF/MI/arrhythmia (N = 248) | ≥2 of HF/MI/arrhythmia (N = 246) | 0.91 [0.69-1.19] | .490 |
| Arrhythmia + any of HF/MI/stroke (N = 313) | ≥2 of HF/MI/stroke (N = 181) | 0.98 [0.70-1.37] | .924 |
- Note: Subhazard ratios displayed were adjusted for all recorded baseline variables and the number of major cardiovascular comorbidities present.
- Abbreviation: CI, confidence interval.
4 DISCUSSION
This prospective, population-based study had three main findings. First, the presence of HF, MI and arrhythmia, but not stroke, were associated with more cardiovascular comorbidities. Second, in those with only one of HF/MI/stroke/arrhythmia, HF, MI and arrhythmias conferred similarly elevated cardiovascular risks compared to those without any of these conditions, but stroke was not associated with any significant increase in cardiovascular risks, regardless of the type of stroke. Third, in those with ≥2 of HF/MI/stroke/arrhythmia, these conditions may have similar impact on cardiovascular risks, and the overall number of comorbidities was likely a significant prognosticator for cardiovascular events.
The first finding was within expectations. Most intuitively, HF, MI and arrhythmia are all cardiac disorders with many similarities in the underlying pathophysiological pathways, most of which are directly cardiac in nature. In contrast, although atherosclerosis and thromboembolism from underlying atrial fibrillation represent some pathophysiological overlap between stroke and HF/MI/arrhythmia, intracranial pathologies and mechanisms leading to bleeding diathesis are likely to contribute significantly to stroke, but not HF/MI/arrhythmia. The extent of similarities between stroke and HF/MI/arrhythmia are thus not comparable to that between HF, MI and arrhythmia.
For our second finding, it was not surprising that patients with HF, MI or arrhythmia only had significantly higher incidence of the primary endpoint than those without any of the major cardiovascular comorbidities. The cardiovascular effects of ADT are mediated by several different mechanisms, including changes in body composition and circulating adipocytokines, insulin resistance, disordered lipid metabolism, hypercoagulability, endothelial dysfunction and immune activation,18 which are also pathophysiological pathways underlying HF, MI and arrhythmia. A prior history signifies probable disorders in these regards, thereby driving the observed associations. These associations were mostly consistent regardless of the type of ADT received, with the lack of associations in the subgroup who received both medical castration and bilateral orchidectomy likely due to the small sample size. Interestingly, the association for HF only approached significance in those who were ever prescribed ARSI/chemotherapy. This may have been because many chemotherapeutic agents have direct cardiotoxic effects unrelated to atherosclerosis and inflammation which underlie most cases of HF at baseline19—the lack of overlap in pathophysiology of chemotherapy-related cardiovascular events and prior HF could have diluted the association.
It is less clear why patients with stroke only did not have significantly different incidence of the primary endpoint compared to those without any of the major cardiovascular comorbidities, regardless of the type of stroke. A probable explanation may be that significant physical disability and immobility are not uncommon in patients with major stroke, which are likely to deter clinicians from initiating ADT in these patients. Therefore, the observed patients with only stroke likely had relatively mild stroke, implying a less prominent association with underlying atherosclerotic burden and inflammatory activity which thus did not confer any significant increase in the risk of adverse cardiovascular outcomes. This was further supported by our finding that patients with a history of stroke did not have significantly different number of major cardiovascular comorbidities than those without stroke (Table 2).
The third finding of our study showed that the overall number of comorbidities, instead of the exact type of comorbidities, may be more important for cardiovascular prognostication. This is clinically important. Although both the HFA-ICOS cardiovascular risk stratification tool and the European Society of Cardiology Guidelines recommended using general cardiovascular risk scores such as the SCORE2 or SCORE2-OP risk calculators for patients receiving ADT,4, 5 these were developed for the general population, and thus do not include existing cardiovascular comorbidities in the models, and were not validated in patients with cancer undergoing cardiotoxic cancer therapies.20, 21 This lack of evidence was also reflected in the above guideline recommendations which were only based on expert consensus. Although this may be a reasonable compromise given the absence of tools developed specifically for cardiovascular risk stratification in patients receiving ADT,4 the epidemiology and natural history of PCa imply that a substantial portion of patients with PCa undergoing ADT would be at relatively old age with pre-existing cardiovascular comorbidities, who are the ones in the most need for specialized cardio-oncology referrals and follow-ups, but who are unaccounted for by most, if not all general cardiovascular risk calculators. Our finding thus complements these recommended cardiovascular risk stratification tools, allowing clinicians to rapidly identify high-risk patients from an easy count of their cardiovascular comorbidities. This should facilitate timely referral of high-risk patients to specialists and optimize distribution of healthcare resources.
Moving forward, our findings should prompt and facilitate further research into the cardiovascular risk stratification of patients with PCa undergoing ADT. Although our findings may complement existing cardiovascular risk stratification tools as a rough, qualitative guidance of the risks associated with the studied cardiovascular comorbidities, they were certainly not meant for quantitatively estimating cardiovascular risks. Much work remains to be done in this regard, and more comprehensive multivariable modeling with internal and external validation is necessary for developing an accurate, representative and actionable cardiovascular risk score for the captioned patients. Our findings may inform these future studies in terms of variable selection and modeling choices. In addition, we noted that despite an increasing understanding of the adverse cardiovascular risks of ADT, very little has been done to understand the baseline cardiovascular risk profile in patients receiving ADT. With our study, we hoped to inspire more in-depth studies of the risk profile and interactions between different cardiometabolic risk factors in these patients.
4.1 Strengths and limitations
Using data from a prospectively recorded population-based database, our study is representative of the clinical practice in Hong Kong, a modern Asian metropolitan, and our findings are likely generalizable to many other Asian cohorts. The population-based nature also allowed a sizeable cohort, reinforcing the validity of our findings. Furthermore, we carefully considered and modeled for the important issue of competing risks in our study. Competing risks are critically important in cardio-oncology studies, as many patients die of cancer or noncardiovascular causes before experiencing cardiovascular events. By recognizing this issue and modeling for it using appropriate statistical models, we likely avoided significant biases in estimates that would have occurred if other methods not accounting for competing risks were used.
Nonetheless, our study is not without limitations. First, owing to the nature of the database used, cancer staging and histology were not available. Nonetheless, ADT is unlikely to be used for nonadvanced disease.16, 17 Additionally, we attempted to mitigate this by using prescription of ARSI or chemotherapy as a surrogate for metastatic disease14, 16, 17, 22 and performing a subgroup analysis accordingly. The results appeared qualitatively similar for most risk groups with all CIs showing substantial overlaps, supporting that the associations were likely to be independent of whether the PCa is metastatic or not.
Second, owing to the observational nature of our study, unmeasured and residual confounders may be present. For instance, due to the nature of the database used, some important cardiovascular risk factors, such as smoking, metabolic syndrome and obesity, could not be accounted for. We adjusted for the widest range of relevant risk factors available to us, which should sufficiently account for a significant proportion of confounders. Nonetheless, further studies with greater data granularity are required to verify our findings. Third, the database used (CDARS) did not allow individual adjudication of data. However, CDARS has been demonstrated to have good data completeness and accuracy,7 and all data were input by treating clinicians independent of the authors.
5 CONCLUSIONS
In patients with PCa receiving ADT, the sole presence of a history of HF, MI and arrhythmia, but not stroke, were associated with the presence of more major cardiovascular comorbidities. The sole presence of HF, MI or arrhythmia, but not stroke, may be associated with significantly elevated cardiovascular risks. In those with ≥2 of prior HF/MI/stroke/arrhythmia, the number of cardiovascular comorbidities may be prognostically more important than the type of comorbidities present.
AUTHOR CONTRIBUTIONS
Jeffrey Shi Kai Chan: Conceptualization, data curation, methodology, formal analysis, visualization, project administration, writing – original draft, writing – review and editing. Yan Hiu Athena Lee: Investigation, writing – review and editing. Jeremy Man Ho Hui: Investigation, writing – review and editing. Kang Liu: Investigation, writing – review and editing. Edward Christopher Dee: Supervision, writing – review and editing. Kenrick Ng: Supervision, writing – review and editing. Tong Liu: Supervision, funding acquisition, writing – review and editing. Gary Tse: Supervision, funding acquisition, resources, project administration, writing – review and editing. Chi Fai Ng: Supervision, funding acquisition, resources, project administration, writing – review and editing.
The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
This work was partly supported by the Research Matching Grant (reference number 8601454), the Tianjin Key Medical Discipline (Specialty) Construction Project (Project number: TJYXZDXK-029A) and a grant from Hong Kong Metropolitan University (Project Reference No. RIF/2022/2.2). ECD is funded in part through the Cancer Center Support Grant from the National Cancer Institute (P30 CA008748). The funders played no role in any part of our study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Our study was approved by Joint Chinese University of Hong Kong- New Territories East Cluster Clinical Research Ethics Committee (reference number 2202.051) and was conducted in accordance with the Declaration of Helsinki. As only retrospective, deidentified data were used, the requirement for individual consent was waived.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.