Temporal patterns of childhood cancer survival 1991 to 2016: A nationwide register-study based on data from the German Childhood Cancer Registry
Daniel Wollschläger and Friederike Erdmann contributed equally to this work.
Abstract
Childhood cancer is the leading disease-related cause of death among under 15 year olds in Europe. Since primary preventive measures are lacking, improving survival probabilities and long-term well-being remain primary goals. With this report, we provide the first long-term assessment and interpretation of patterns in childhood cancer survival in Germany, covering a period of 30 years. Using data from the German Childhood Cancer Registry, we assessed temporal patterns of cancer survival among children (0-14 years) diagnosed in Germany from 1991 to 2016, by cancer type, age at diagnosis and sex. We calculated overall survival (OS) and average annual percentage changes of the respective 5-year OS estimates. OS improved across all cancer types, age groups as well as for boys and girls over time. Five-year OS for all childhood cancers combined increased from 77.8% in 1991-1995 to 86.5% in 2011-2016, with stronger improvements during the early 1990s. The most pronounced survival improvement was seen for acute myeloid leukaemia, at 2% annually and 5-year OS recently reaching 81.5%. Survival improvements for some diagnoses such as neuroblastoma, renal tumours and bone tumours have flattened out. Tremendous enhancements in diagnostics, treatment and supportive care have affected average survival improvements for most cancer types. Recently, survival improvements have decelerated overall and for some cancer types, it plateaued at an unsatisfactory level. As not all children benefited equally from the survival improvements, personal factors (eg, socioeconomic circumstances, health literacy, access to care) likely affect individual prognosis and warrant further investigation.
Graphical Abstract
What's new?
Aetiology and risk factors for many childhood cancers remain unknown. Thus, primary preventive measures for childhood cancer are lacking and improving survival probabilities and long-term well-being remain primary goals. In this investigation of childhood cancer patients in Germany, the authors examined relationships between survival patterns and diagnostic and therapeutic factors. Improvements in overall survival for all cancer types, across all age groups and sexes, were observed over time. For some cancer types, the rate of improvement has decelerated in recent years. The findings emphasise the impact of diagnostic and therapeutic enhancements on childhood cancer survival, but also revealed persistent inequalities.
Abbreviations
-
- AAPC
-
- average annual percentage change
-
- AML
-
- acute myeloid leukaemia
-
- BFM
-
- Berlin-Frankfurt-Münster study group
-
- CI
-
- confidence interval
-
- CNS
-
- central nervous system
-
- CoALL
-
- Cooperative Study Group for Childhood Acute Lymphoblastic Leukaemia
-
- GCCR
-
- German Childhood Cancer Registry
-
- HIC
-
- high-income countries
-
- ICCC-3
-
- International Classification of Childhood Cancer-third edition
-
- LL
-
- lymphoblastic leukaemia
-
- MRD
-
- minimal residual disease
-
- NHL
-
- non-Hodgkin lymphoma
-
- OS
-
- overall survival
-
- SCT
-
- stem cell transplantation
-
- SPN
-
- subsequent primary neoplasm
1 INTRODUCTION
Cancer is the leading disease-related cause of death among children in Europe and other high-income countries (HICs).1-3 Even though its annual incidence is comparatively low, with about 170 cases per million children in Germany (ages 0-14 years),4, 5 it is associated with a high burden of disease for patients, their relatives and for the public health. In addition to the risk of death, intensive treatment at a young age and the patients' long remaining life spans put them at risk of somatic late effects as well as adverse psychosocial and socioeconomic consequences later in life.6-9 Childhood cancer represents a heterogeneous group of diseases, including malignancies and nonmalignant tumours in the central nervous system (CNS), showing a wide variety of patterns regarding incidence,5, 10, 11 aetiology,12-15 treatment8, 16, 17 and clinical outcomes.5, 18-21 In HICs, leukaemias, tumours of the CNS and lymphomas are the most frequent cancers in children.1, 5, 11
The aetiology of most childhood cancers remains poorly understood, with established risk factors explaining only about 10% of all incident cases.13, 14 Consequently, approaches for primary preventive measures are lacking, rendering treatment effectiveness to improve survival especially important in reducing the overall burden of disease. Over the past five decades, advances in the understanding of tumour biology, diagnostics, pharmacology, adapted treatment combinations and risk grouping and supportive care have indeed led to remarkable enhancements in the treatment of and survival from childhood cancer, with 5-year survival exceeding 80% in most HICs today.8, 16
Notably, however, not all children benefit equally from these recent diagnostic and therapeutic improvements. Disparities in childhood cancer survival are particularly evident according to cancer type and age at diagnosis.1, 5, 8, 19, 22, 23 Moreover, large survival disparities have been observed between countries, with a general pattern of higher survival in countries ranking higher on the Human Development Index.24 Within Europe, about 10% poorer outcome overall is reported for Eastern nations vs Northern or Western Europe.8, 18, 19, 22, 25, 26 Given the heterogeneity across cancer types and the lack of primary preventive measures, further advances in childhood cancer survival remain the primary goal, enabling a higher life expectancy for every affected child.
With this report, we sought to provide the first long-term assessment and interpretation of patterns in childhood cancer survival in Germany using data from the national German Childhood Cancer Registry (GCCR) covering the diagnostic years 1991 to 2016. The nationwide population-based data offer a high granularity for evaluating persisting survival disparities, relating survival patterns to changes in diagnostics and therapeutic regimes, and thus contributing to a better understanding of long-term temporal trends and persistent disparities in childhood cancer survival.
2 MATERIALS AND METHODS
2.1 Study population and data source
The study population comprised all incident cancer diagnoses reported to the GCCR from 1991 to 2016 (including subsequent primary neoplasms [SPNs]) among children aged 0 to 14 years residing in Germany at time of diagnosis. SPNs were defined as any new primary tumours diagnosed in a child (0-14 years of age) with a previous cancer diagnosis, regardless of the time between the two diagnoses.5
The GCCR was established in 1980 and has been collecting and monitoring data on incident diagnoses of cancers in children aged 0 to 14 years (since 2009 expanded to 0-17 years) with a completeness of incident cases exceeding 95%.5 The cancer diagnoses are classified according to the International Classification of Childhood Cancer-third edition (ICCC-3).27 Following the definition of ICCC-3, intracranial and intraspinal tumours of nonmalignant behaviour are included in the present study. On average, approximately 1850 incident cases are reported to the GCCR each year from a population of around 11 million children aged 0 to 14 years in Germany.
The GCCR collects information on vital status regularly (at least every 2 years) using information from relevant therapy trials, paediatric haematology-oncology units, and local population registries. For this study, we used vital status information from the GCCR database as of March 15, 2022. Of the identified 46 989 incident cancer diagnoses (including SPNs), we excluded those diagnoses with no follow-up information (N = 501; 1.1%). The analytical sample thus comprised 46 488 cancer diagnoses.
2.2 Statistical analysis
We defined 5-year overall survival (OS) as our main outcome of interest and censored follow-up at 5 years after diagnosis. Patients were followed from date of cancer diagnosis until death from any cause, emigration, end of the 5-year follow-up, or March 15, 2022, whichever came first.
We analysed descriptively the observed number of microscopically verified cases, cases with unspecified morphology codes and cases with complete 5-year follow-up, as well as the total number of person-years of follow-up since diagnosis, stratified by ICCC-3 (sub-)group, sex (female/ male), age group at diagnosis (<1, 1-4, 5-9 and 10-14 years), and diagnostic period (1991-1995, 1996-2000, 2001-2005, 2006-2010 and 2011-2016).
Using the Kaplan-Meier method, we calculated 1-, 3- and 5-year OS estimates with corresponding 95% confidence intervals (95% CIs) for all cancers combined, as well as stratified by diagnostic group, sex and age group at diagnosis per diagnostic period. We also calculated the sex ratio of the 5-year OS estimates (male OS/female OS) and corresponding pointwise 95% CI, based on Katz' method and adapted by using the Kaplan-Meier survival estimates and Greenwood's variance estimate.28, 29
Since the age distribution of Germany's child population has changed over the past decades, all OS estimates were adjusted for age using the method established in the EUROCARE-6 project.23 We thus calculated age-adjusted OS by applying weights according to the age distribution of the respective diagnostic group in Germany during 1991 to 2016 (Table S4). In additional analyses, we calculated age-adjusted OS using the weights from the EUROCARE-6 project (considering only tumours with malignant behaviour, since EUROCARE included solely malignant tumours) to enable a direct comparison to the European survival estimates of the EUROCARE-6 study.23 To assess the temporal changes, Joinpoint regression models30 were applied using the German age-weighted 5-year OS estimates and corresponding standard errors to derive average annual percentage changes (AAPC) and corresponding 95% CI for 5-year OS estimates. We allowed up to five joinpoints.
Statistical analyses were performed using SAS Statistical Software 9.431 and Joinpoint Regression Program, version 4.9.0.1, National Cancer Institute.30
3 RESULTS
We identified 46 488 childhood cancer diagnoses at ages 0 to 14 years between 1991 and 2016 in Germany with follow-up information. An overview of the age distribution by diagnostic group is given in Table S1. Of those, 95.6% (N = 44 442) had complete follow-up information for at least 5 years after diagnosis (199 596 person-years of follow-up) (Table 1). Except for malignant CNS tumours and retinoblastoma, more than 90% of the diseases were reported to be microscopically verified. Few diagnoses were registered with an unspecific morphology (N = 546, 1.2%). In total, 605 (1.3%) registered diagnoses were SPNs, with the highest proportions seen for acute myeloid leukaemia (AML) and epithelial tumours and melanomas (Table 1).
| N cases/deaths (%)a,b | SPN (%)a | Microscopically verified (%)a | NOS morphology (%)a,c | Person-years of follow-upb | Complete 5-year follow-up (%)a | |
|---|---|---|---|---|---|---|
| Diagnosisd | ||||||
| All cancer types | 46 488/8055 (17.3) | 605 (1.3) | 44 076 (94.8) | 546 (1.2) | 199 596.3 | 44 442 (95.6) |
| Leukaemias | 15 567/2284 (14.7) | 193 (1.2) | 15.541 (99.8) | 142 (0.9) | 68 984.0 | 15 165 (97.4) |
| Lymphoblastic leukaemia | 12 278/1286 (10.5) | 37 (0.3) | 12 269 (99.9) | 0 | 56 557.9 | 11 956 (97.4) |
| Acute myeloid leukaemia | 2121/722 (34.0) | 102 (4.8) | 2113 (99.6) | 0 | 7665.6 | 2074 (97.8) |
| Lymphomas | 5337/390 (7.3) | 62 (1.2) | 5318 (99.6) | 12 (0.2) | 24 769.4 | 5160 (96.7) |
| Hodgkin lymphoma | 2248/55 (2.5) | 12 (0.5) | 2239 (99.6) | 0 | 10 907.9 | 2180 (97.0) |
| Non-Hodgkin lymphoma | 1951/241 (12.4) | 41 (2.1) | 1943 (99.6) | 0 | 8692.0 | 1885 (96.6) |
| CNS tumours | 10 711/2581 (24.1) | 141 (1.3) | 8923 (83.3) | 192 (1.8) | 42 449.3 | 10 091 (94.2) |
| Malignant | 6454/2430 (37.7) | 103 (1.6) | 5083 (78.8) | 25 (0.4) | 22 585.9 | 6148 (95.3) |
| Nonmalignant | 4257/151 (3.6) | 38 (0.9) | 3840 (90.2) | 167 (3.9) | 19 863.4 | 3943 (92.6) |
| Non-CNS solid tumours | 14 873 /2800 (18.8) | 209 (1.4) | 14 294 (96.1) | 200 (1.3) | 63 393.6 | 14 026 (94.3) |
| Neuroblastoma | 3434/827 (24.1) | 16 (0.5) | 3422 (99.7) | 0 | 14 237.6 | 3299 (96.1) |
| Retinoblastoma | 1027/18 (1.8) | 2 (0.2) | 652 (63.5) | 0 | 4892.9 | 968 (94.3) |
| Renal tumours | 2645/231 (8.7) | 16 (0.6) | 2606 (98.5) | 2 (0.1) | 12 250.8 | 2533 (95.8) |
| Hepatic tumours | 532/134 (25.2) | 3 (0.6) | 498 (93.6) | 0 | 1990.2 | 482 (90.6) |
| Bone tumours | 2083/585 (28.1) | 49 (2.4) | 2067 (99.2) | 8 (0.4) | 8574.6 | 2008 (96.4) |
| Soft tissue sarcomas | 2775/787 (28.4) | 37 (1.3) | 2745 (98.9) | 177 (6.4) | 10 878.4 | 2541 (91.6) |
| Germ cell tumours | 1524/100 (6.6) | 4 (0.3) | 1507 (98.9) | 0 | 6961.0 | 1420 (93.2) |
| Epithelial tumours and melanomas | 791/98 (12.4) | 79 (10.0) | 739 (93.4) | 0 | 3387.9 | 721 (91.2) |
| Other malignant neoplasms | 62/20 (32.3) | 3 (4.8) | 58 (93.6) | 13 (21.0) | 220.2 | 54 (87.1) |
| Sex | ||||||
| Female | 20 672/3489 (16.9) | 278 (1.3) | 19 479 (94.2) | 245 (1.2) | 88 760.9 | 19 687 (95.2) |
| Male | 25 816/4566 (17.7) | 327 (1.3) | 24 597 (95.3) | 301 (1.2) | 110 835.4 | 24 755 (95.9) |
| Age at diagnosis | ||||||
| <1 year | 4740/887 (18.7) | 10 (0.2) | 4396 (92.7) | 87 (1.8) | 19 508.2 | 4483 (94.6) |
| 1-4 years | 16 239/2595 (16.0) | 81 (0.5) | 15 431 (95.0) | 107 (0.7) | 70 669.9 | 15 536 (95.7) |
| 5-9 years | 12 540/2140 (17.1) | 199 (1.6) | 11 818 (94.2) | 146 (1.2) | 54 304.8 | 12 116 (96.6) |
| 10-14 years | 12 969/2433 (18.8) | 315 (2.4) | 12 431 (95.9) | 206 (1.6) | 55 113.4 | 12 307 (94.9) |
| Diagnostic period | ||||||
| 1991-1995 | 8674/1929 (22.2) | 92 (1.1) | 8268 (95.3) | 94 (1.1) | 35 834.6 | 8430 (97.2) |
| 1996-2000 | 9323/1826 (19.6) | 118 (1.3) | 8959 (96.1) | 91 (1.0) | 39 528.5 | 9049 (97.1) |
| 2001-2005 | 9138/1536 (16.8) | 120 (1.3) | 8711 (95.3) | 97 (1.1) | 39 637.4 | 8940 (97.8) |
| 2006-2010 | 8893/1373 (15.4) | 141 (1.6) | 8313 (93.5) | 131 (1.5) | 39 227.4 | 8724 (98.1) |
| 2011-2016 | 10 460/1391 (13.3) | 134 (1.3) | 9825 (93.9) | 133 (1.3) | 45 368.5 | 9299 (88.9) |
| Subsequent primary neoplasmse | 605/277 (45.8) | 540 (89.3) | 12 (2.0) | 1951.5 | 589 (97.4) |
- Abbreviations: N, number; SPN, subsequent primary neoplasm; CNS, central nervous system.
- a Refers to the proportion of the row.
- b Censored at 5 years after diagnosis; database as of March 15, 2022.
- c Not otherwise specified (NOS) ICCC-3: I(e) Unspecified and other specified leukaemias; II(e) Unspecified lymphomas; III(f) Unspecified intracranial and intraspinal neoplasms; VI(c) Unspecified malignant renal tumours; VII(c) Unspecified malignant hepatic tumours; VIII(e) Unspecified malignant bone tumours; IX(e) Unspecified soft tissue sarcomas and XII(b) Other unspecified malignant tumours.
- d Defined according to the International Classification of Childhood Cancer-third edition (ICCC-3).27
- e Including basal cell carcinoma of the skin and benign meningioma.
Within 5 years of follow-up after diagnosis, 8055 (17.3%) deaths were reported. The highest proportions of deaths were seen in AML and malignant CNS tumour patients (34.0% and 37.7%, respectively). The proportion of deaths for all cancer types combined decreased steadily over time from 22.2% in 1991-1995 to 13.3% in 2011-2016. Of the children affected by SPN by age 15 years, nearly 50% died within 5 years after the SPN diagnosis (Table 1).
3.1 Overall survival by cancer type
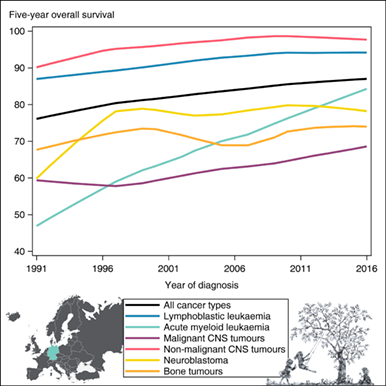
Five-year OS for all childhood cancers combined improved from 77.8% in 1991-1995 to 86.5% in 2011-2016 (Table 2). This pattern was evident across all diagnoses and all age groups. In the most recent diagnostic period studied, the highest survival probabilities were observed in children diagnosed with Hodgkin lymphoma, nonmalignant CNS tumours, and retinoblastoma, with 5-year OS exceeding 98%. Children with lymphoblastic leukaemia (LL), lymphoma, renal tumours, germ cell tumours and epithelial tumours and melanomas had 5-year OS exceeding 90%. The lowest survival probabilities were seen in children with malignant CNS tumours (66.4%), bone tumours (74.5%) and soft tissue sarcomas (77.3%) (Table 2). The decline from 1- to 5-year OS for all cancers combined was eight percentage points in 2011 to 2016 (Figures 1A and 2). This decline was most pronounced for malignant CNS tumours and bone tumours (around 20 percentage points). An overview of tumour-type-specific 1-, 3- and 5-year OS estimates for the most recent diagnostic period (2011-2016) is presented in Figure 2. Crude 5-year OS estimates and diagnosis-specific 5year OS estimates based on age weights from the EUROCARE-6 study are given in Tables S2 and S3, respectively. Survival estimates were similar to those of the main analysis after applying the EUROCARE-6 study age weights.
| Diagnostic period | 1991-1995 | 1996-2000 | 2001-2005 | 2006-2010 | 2011-2016 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5-year OS (95% CI)a | Sex ratiob,c (95% CI) | 5-year OS (95% CI)a | Sex ratiob,c (95% CI) | 5-year OS (95% CI)a | Sex ratiob,c (95% CI) | 5-year OS (95% CI)a | Sex ratiob,c (95% CI) | 5-year OS (95% CI)a | Sex ratiob,c (95% CI) | |
| Diagnosisd | ||||||||||
| All cancer types | 77.78% (76.86-78.67) | 1.00 (0.99-1.02) | 80.41% (79.55-81.23) | 0.97 (0.96-0.99) | 83.25% (82.44-84.03) | 0.99 (0.98-1.01) | 84.61% (83.82-85.37) | 0.99 (0.97-1.01) | 86.51% (85.81-87.18) | 0.99 (0.98-1.01) |
| Leukaemias | 81.73% (80.19-83.15) | 0.95 (0.93-0.98) | 84.06% (82.64-85.37) | 0.97 (0.94-1.00) | 88.71% (87.46-89.85) | 1.01 (0.98-1.03) | 90.34% (89.15-91.41) | 0.99 (0.97-1.01) | 92.34% (91.31-93.25) | 1.00 (0.98-1.02) |
| Lymphoblastic leukaemia | 87.94% (86.47-89.25) | 0.94 (0.91-0.96) | 89.24% (87.86-90.47) | 0.98 (0.95-1.00) | 92.30% (91.08-93.35) | 1.00 (0.98-1.02) | 93.42% (92.25-94.42) | 0.99 (0.97-1.01) | 94.26% (93.21-95.16) | 0.99 (0.97-1.01) |
| Acute myeloid leukaemia | 51.28% (46.36-55.99) | 1.00 (0.91-1.11) | 58.18% (53.44-62.61) | 0.89 (0.82-0.98) | 68.39% (63.50-72.77) | 0.93 (0.85-1.02) | 73.43% (68.52-77.70) | 1.03 (0.94-1.13) | 81.49% (77.24-85.02) | 1.04 (0.96-1.12) |
| Lymphomas | 92.59% (90.76-94.07) | 1.00 (0.97-1.04) | 91.43% (89.49-93.03) | 0.99 (0.95-1.03) | 94.03% (92.31-95.37) | 1.02 (0.99-1.05) | 93.76% (91.93-95.19) | 1.00 (0.96-1.03) | 94.98% (93.37-96.21) | 1.02 (0.99-1.06) |
| Hodgkin Lymphoma | 96.23% (93.07-97.76) | 1.02 (0.98-1.06) | 96.30% (93.89-97.77) | 0.99 (0.96-1.03) | 98.24% (96.36-99.16) | 1.02 (0.99-1.05) | 98.91% (96.98-99.61) | 1.00 (0.98-1.02) | 98.53% (96.76-99.34) | 1.01 (0.99-1.04) |
| Non-Hodgkin Lymphoma | 88.21% (84.51-91.07) | 1.00 (0.93-1.07) | 84.18% (80.05-87.52) | 1.01 (0.94-1.09) | 87.75% (83.89-90.75) | 1.09 (1.01-1.18) | 88.26% (84.18-91.34) | 1.04 (0.96-1.12) | 92.18% (88.90-94.52) | 1.08 (1.01-1.17) |
| CNS tumours | 70.65% (68.32-72.84) | 1.02 (0.98-1.07) | 71.50% (69.37-73.50) | 0.94 (0.90-0.98) | 75.99% (74.03-77.82) | 0.95 (0.91-0.98) | 79.03% (77.20-80.74) | 0.99 (0.96-1.03) | 79.65% (78.03-81.16) | 1.00 (0.97-1.03) |
| Malignant | 59.47% (56.41-62.39) | 0.98 (0.92-1.04) | 56.03% (53.06-58.88) | 0.94 (0.89-1.00) | 62.23% (59.38-64.94) | 0.95 (0.90-1.01) | 64.37% (61.53-67.05) | 1.00 (0.95-1.06) | 66.41% (63.94-68.76) | 1.02 (0.97-1.07) |
| Nonmalignant | 93.18% (90.64-95.05) | 1.09 (1.04-1.14) | 95.40% (93.57-96.72) | 0.98 (0.95-1.01) | 96.99% (95.52-97.99) | 1.00 (0.98; 1.03) | 98.60% (97.56-99.20) | 0.99 (0.98-1.01) | 98.13% (97.11-98.80) | 1.00 (0.98-1.01) |
| Non-CNS solid tumours | 74.64% (72.95-76.24) | 1.01 (0.97-1.04) | 81.69% (80.22-83.01) | 0.99 (0.96-1.01) | 80.42% (78.87-81.88) | 0.98 (0.95-1.01) | 81.59% (80.02-83.04) | 0.96 (0.94-0.99) | 84.14% (82.78-85.40) | 0.95 (0.93-0.98) |
| Neuroblastoma | 66.10% (62.20-69.70) | 0.91 (0.85-0.98) | 80.35% (77.34-83.01) | 0.99 (0.93-1.04) | 77.51% (73.86-80.72) | 1.01 (0.95-1.09) | 78.03% (73.37-81.24) | 0.98 (0.92-1.05) | 79.83% (76.45-82.79) | 0.95 (0.89-1.02) |
| Retinoblastoma | 98.40% (95.15-99.48) | 1.01 (0.97-1.04) | 97.86% (95.52-99.18) | 1.00 (0.96-1.04) | 98.45% (95.13-99.51) | 0.99 (0.96-1.03) | 96.77% (92.65-98.59) | 1.00 (0.94-1.05) | 98.73% (95.83-99.61) | 1.03 (0.99-1.06) |
| Renal tumours | 90.02% (86.89-92.44) | 0.95 (0.90-1.01) | 91.89% (88.90-94.11) | 1.02 (0.97-1.07) | 93.24% (90.28-95.32) | 0.97 (0.93-1.02) | 94.39% (91.60-96.27) | 0.96 (0.92-1.01) | 92.73% (89.82-94.83) | 0.98 (0.94-1.04) |
| Hepatic tumours | 65.34% (52.62-75.43) | 1.10 (0.87-1.40) | 74.82% (62.36-83.68) | 1.44 (1.10-1.87) | 69.26% (58.57-77.71) | 0.98 (0.80-1.19) | 82.08% (72.50-88.58) | 1.02 (0.87-1.20) | 87.58% (80.34-92.28) | 0.98 (0.87-1.10) |
| Bone tumours | 67.72% (62.47-72.40) | 1.05 (0.95-1.15) | 74.87% (69.87-79.17) | 1.00 (0.91-1.09) | 71.09% (66.00-75.56) | 1.00 (0.91-1.10) | 68.88% (63.63-73.53) | 0.98 (0.89-1.09) | 74.49% (69.85-78.52) | 1.01 (0.93-1.10) |
| Soft tissue sarcomas | 66.25% (62.02-70.13) | 0.90 (0.83-0.98) | 71.58% (67.52-75.22) | 1.05 (0.98-1.13) | 70.57% (66.55-74.20) | 1.04 (0.96-1.12) | 70.82% (66.45-74.73) | 0.97 (0.89-1.06) | 77.34% (73.47-80.71) | 0.93 (0.86-1.00) |
| Germ cell tumours | 87.35% (82.65-90.84) | 1.03 (0.96-1.12) | 94.00% (90.50-96.23) | 1.00 (0.95-1.06) | 96.04% (92.78-97.85) | 0.97 (0.92-1.02) | 92.31% (87.91-95.15) | 0.93 (0.87-1.01) | 93.18% (89.68-95.52) | 0.89 (0.83-0.94) |
| Epithelial tumours and melanomas | 72.77% (60.03-82.04) | 0.83 (0.67-1.03) | 83.97% (74.80-90.03) | 0.79 (0.63-0.93) | 84.48% (76.11-90.11) | 0.87 (0.75-1.00) | 91.52% (85.43-95.14) | 0.97 (0.88-1.06) | 94.09% (90.08-96.51) | 0.97 (0.91-1.04) |
| Other malignant neoplasms | 47.04% (10.59-77.62) | 0.95 (0.42-2.15) | 63.33% (20.82-87.55) | 0.52 (0.28-0.95) | 93.60% (35.68-99.58) | 1.21 (0.85-1.72) | 60.78% (27.36-82.59) | 1.43 (0.82-2.50) | 77.22% (46.79-91.58) | 0.87 (0.55-1.39) |
| Sex | ||||||||||
| Female | 77.65% (76.23-79.00) | 81.59% (80.33-82.79) | 83.66% (82.44-84.80) | 85.10% (83.91-86.21) | 86.86% (85.81-87.84) | |||||
| Male | 77.90% (76.67-79.07) | 79.45% (78.28-80.56) | 82.91% (81.81-83.95) | 84.24% (83.15-85.26) | 86.25% (85.29-87.14) | |||||
| Age group at diagnosis | ||||||||||
| <1 years | 74.94% (71.89-77.71) | 0.96 (0.91-1.02) | 79.75% (77.01-82.20) | 0.94 (0.90-0.99) | 80.34% (77.62-82.76) | 1.03 (0.98-1.08) | 83.75% (81.21-85.98) | 0.98 (0.93-1.03) | 84.35% (81.94-86.47) | 1.01 (0.97-1.06) |
| 1-4 years | 78.32% (76.85-79.71) | 0.99 (0.96-1.02) | 82.57% (81.23-83.83) | 0.99 (0.96-1.01) | 84.18% (82.85-85.42) | 0.99 (0.97-1.02) | 86.46% (85.17-87.64) | 0.98 (0.95-1.00) | 86.83% (85.64-87.93) | 0.98 (0.96-1.00) |
| 5-9 years | 77.79% (76.08-79.39) | 1.02 (0.99-1.05) | 79.34% (77.71-80.86) | 0.98 (0.95-1.01) | 84.93% (83.44-86.29) | 1.00 (0.97-1.03) | 85.37% (83.89-86.73) | 1.01 (0.98-1.04) | 85.80% (84.38-87.10) | 1.01 (0.98-1.04) |
| 10-14 years | 76.74% (74.88-78.49) | 1.02 (0.99-1.06) | 77.78% (76.08-79.38) | 0.95 (0.92-0.98) | 80.76% (79.19-82.26) | 0.98 (0.95-1.01) | 81.33% (79.75-82.80) | 0.99 (0.96-1.02) | 85.52% (84.20-86.73) | 1.00 (0.97-1.02) |
- Abbreviations: CI, confidence interval; CNS, central nervous system; OS, overall survival.
- a Five-year overall survival estimates and corresponding 95% CI are weighted according to the age distribution of the respective diagnostic group in 1991 to 2016.
- b Sex ratio = 5-year overall survival for males divided by 5-year overall survival for females.
- c 95% CIs for the sex ratios were calculated applying the approximation by Katz et al (1978).28, 29
- d Defined according to the International Classification of Childhood Cancer-third edition (ICCC-3).27
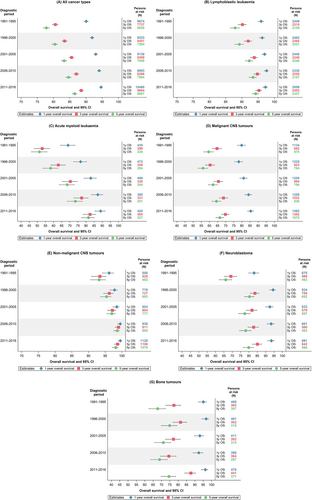
3.2 Overall survival by age and sex
Across age groups, 5-year OS has increased similarly since 1991, by about 10 percentage points. During the entire study period, survival probabilities were higher in children aged 1 to 4 years at diagnosis than in older or younger children (Table 2, Figure 3A), primarily driven by the superior prognosis of the large group of 1- to 4-year olds with LL (Figure 3B,C). Children aged <1 year at diagnosis showed lowest survival for LL and CNS tumours, yet highest survival for neuroblastoma (Figure 3B-F). We also observed sex-specific survival differences over time for some diagnostic groups. Survival for LL and malignant CNS tumours appeared to have been slightly higher in girls during the 1990s and rather similar to boys in more recent years. Sex ratios for survival from hepatic tumours indicated somewhat better outcomes for boys compared to girls during the 1990s. While recent sex ratios for germ cell tumour survival indicate superior survival for girls, we observed indications of superior survival in boys with AML and non-Hodgkin lymphoma (NHL) (Table 2).
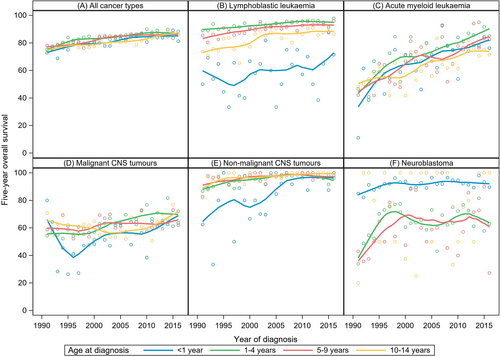
3.3 Temporal trend analysis
For all cancers combined, results from the Joinpoint regression analysis revealed more pronounced survival improvements during the early 1990s (AAPC = 1.6%, 95% CI: 0.3-3.1 for 1991-1995) compared to later years (AAPC = 0.4%, 95% CI: 0.3-0.5 for 1995-2016). Notably, although the average estimated slope decreased from 1.6% to 0.4% per year, the latter point estimate and its CI do represent an ongoing improvement of survival probabilities. This trend was mostly driven by girls (Table 3). Accordingly, the difference between 1- and 5-year OS decreased over time from around 13 to 8 percentage points (Figure 1A). OS survival improved across all age groups. The Joinpoint regression revealed one joinpoint for the age group 5 to 9 years at diagnosis indicating a stronger increase in OS from 1991 to 2003 followed by a period of overall unchanged survival probabilities (Tables 2 and 3).
| Slope 1 | Slope 2 | |||
|---|---|---|---|---|
| Period | AAPC (95% CI)a,b | Period | AAPC (95% CI)a,b | |
| Diagnosisc | ||||
| All cancer types | 1991-1995 | 1.64% (0.25-3.06) | 1995-2016 | 0.42% (0.33-0.51) |
| Leukaemias | 1991-2016 | 0.58% (0.48-0.68) | ||
| Lymphoblastic leukaemia | 1991-2016 | 0.35% (0.25-0.45) | ||
| Acute myeloid leukaemia | 1991-2016 | 1.98% (1.46-2.50) | ||
| Lymphomas | 1991-2013 | 0.10% (−0.01-0.22) | 2013-2016 | 1.60% (0.19-3.04) |
| Hodgkin Lymphoma | n.a. | |||
| Non-Hodgkin Lymphoma | 1991-2016 | 0.40% (0.21-0.59) | ||
| CNS tumours | 1991-2016 | 0.63% (0.47-0.79) | ||
| Malignant | 1991-2016 | 0.73% (0.47-1.00) | ||
| Nonmalignant | 1991-2009 | 0.31% (0.17-0.45) | 2009-2016 | −0.26% (−0.64-0.11) |
| Non-CNS solid tumours | 1991-1996 | 2.64% (0.23-5.10) | 1996-2016 | 0.21% (−0.03-0.45) |
| Neuroblastoma | 1991-1996 | 6.81% (1.15-12.79) | 1996-2016 | 0.04% (−0.43-0.51) |
| Retinoblastoma | n.a. | |||
| Renal tumours | 1991-2016 | 0.18% (−0.06-0.43) | ||
| Hepatic tumours | n.a. | |||
| Bone tumours | 1991-2016 | 0.17% (−0.28-0.62) | ||
| Soft tissue sarcomas | 1991-2016 | 0.58% (0.24-0.39) | ||
| Germ cell tumours | n.a. | |||
| Epithelial tumours and melanomas | n.a. | |||
| Other malignant neoplasms | n.a. | |||
| Sex | ||||
| Female | 1991-1995 | 2.26% (0.33-4.22) | 1995-2016 | 0.36% (0.25-0.48) |
| Male | 1991-2016 | 0.50% (0.41-0.59) | ||
| Age group at diagnosis | ||||
| <1 years | 1991-2016 | 0.56% (0.37-0.74) | ||
| 1-4 years | 1991-2016 | 0.48% (0.34-0.63) | ||
| 5-9 years | 1991-2003 | 0.90% (0.53-1.27) | 2003-2016 | 0.17% (−0.12-0.45) |
| 10-14 years | 1991-2016 | 0.55% (0.42-0.68) | ||
- Abbreviations: AAPC, average annual percentage change; CI, confidence interval; CNS, central nervous system; n.a., not applicable (due to low incidence and mortality).
- a Joinpoint regression analysis was based on 5-year overall survival estimates and corresponding standard errors weighted according to the age distribution of the respective diagnostic group in 1991 to 2016. The analysis allows for detection of statistically significant changes in slope throughout a period of time. For childhood cancer types where a change was observed, two separate slopes are reported. The overlapping upper (slope 1) and lower (slope 2) calendar year represents the so-called joinpoint identified by the analysis technique. For others, no change in slope was identified; a single slope is reported. By default, up to five joinpoints were allowed.
- b A positive slope denotes improvement of survival rates. A confidence interval including the value 0 is considered not statistically significantly different from a flat slope (on average, no change in survival rates in the period of study).
- c Defined according to the International Classification of Childhood Cancer-third edition (ICCC-3).27
Analyses by cancer type demonstrated some differences in the temporal survival patterns. On average, 5-year OS for leukaemia improved by 0.6% (95% CI: 0.5-0.7) annually. The increase was largely due to the survival improvements in AML patients with an increase of 30 percentage points from 51.3% to 81.5% (AAPC = 2.0%, 95% CI: 1.5-2.5), which was the strongest enhancement across all cancer types (Tables 2 and 3). Notably, this trend was least pronounced in infants (Figure 3C). The gap between LL and AML 5-year OS narrowed over time from almost 37 in 1991 to 1995 to 13 percentage points in 2011 to 2016 (Tables 2 and 3). Overall, the difference between 1- and 5-year OS in leukaemia narrowed considerably during the study period. This pattern was evident for both LL and AML (Figure 1B,C). The 5-year OS estimates for NHL improved unevenly over time and reached 92.2% in 2011 to 2016 (Table 2). Nonetheless, the Joinpoint regression did not identify any statistically significant change in time trend (AAPC = 0.4%, 95% CI: 0.2-0.6) (Table 3). Five-year OS of malignant CNS tumours improved steadily from 59.5% in 1991-1995 to 66.4% in 2011-2016 (AAPC = 0.7%, 95% CI: 0.5-1.0 for 1991-2016; Tables 2 and 3). The decline from 1- to 3-year OS was not reduced over time, as observed for other tumour types (Figure 1D). Survival for nonmalignant CNS tumours has improved slightly and levelled off at around 98% in the most recent years studied (Figure 1E). Five-year OS of neuroblastoma only increased during the early 1990s (AAPC = 6.8%, 95% CI: 1.2-12.8 in 1991-1996) and remained largely unchanged at around 80% after the mid-1990s, the AAPC for bone tumour survival indicated plateauing survival probabilities (Tables 2 and 3, Figure 1F,G). For soft tissue sarcomas, we observed a steady 0.6% annual improvement in 5-year OS over the entire study period (Table 3).
Due to low incidence and mortality, the Joinpoint regression method was not suitable for generating AAPCs for Hodgkin lymphoma, retinoblastoma, germ cell tumours, epithelial tumours and melanomas and for the group of “other malignant neoplasms.” However, the period-specific 5-year OS estimates also indicated survival improvements for these cancer types (Table 2).
4 DISCUSSION
This comprehensive register-based assessment using high-quality national population-based data demonstrated clearly improved survival for all childhood cancer types over a 30-year period in Germany. Five-year OS reached 86.5% for all cancers combined. Moreover, the decline from 1- to 5-year OS was reduced for nearly all cancer types over time. The most pronounced survival improvement was observed for AML, at 2% annually (from 51.3% in 1991-1995 to 81.5% in 2011-2016). Although LL survival also showed steady improvement, the survival gap between these two leukaemia types narrowed during the study period. The observed survival improvements were evident for all age groups as well as for boys and girls.
4.1 Survival time trends related to development in treatment regimes
We observed that childhood cancer survival has improved considerably in Germany over time. Contributing factors for this pattern include improved treatment regimes, advances in diagnostics and supportive care in long-running (inter)national paediatric oncology collaborations. Particularly, the development of nation-wide, highly standardised treatment protocols enabled a complete registration, standardised diagnostics, stratification and risk-adapted therapy allocation.8 The success of childhood leukaemia treatment during the 20th century, which resulted from collaborating multidisciplinary study groups, comprehensive genetic testing, and the development of minimal residual disease (MRD)-guided risk grouping in prospective randomised clinical trials was the impetus for treating other paediatric malignancies more effectively.8, 16, 32 Standardised treatment protocols have been regularly adapted following the results of clinical trials.33 Finding more pronounced survival improvements in the early 1990s compared to the later years (observed in the Joinpoint analysis) is likely attributable to these consecutive therapy optimisation efforts, which had more success during the early 90s than thereafter.
In Germany, virtually all patients diagnosed with LL have been treated according to standardised protocols, namely BFM (Berlin-Frankfurt-Münster study group) and CoALL (Cooperative Study Group for Childhood Acute Lymphoblastic Leukemia).34, 35 The Joinpoint regression revealed continuously improving outcomes since 1991. We observed tendencies of lower OS for LL in boys compared to girls during the 1990s (as seen in the sex ratios). Notably, in the ALL-BFM 95 protocol, a treatment optimisation study that started in 1995, the duration of maintenance therapy for the standard-risk group differed between boys and girls (36 vs 24 months, respectively).34 Results from the ALL-BFM 95 study revealed no significantly improved event-free survival compared to the previous study (ALL-BFM 90) with equal duration of maintenance therapy for boys and girls.36 Nevertheless, as it is known that boys have a higher underlying risk of relapse,36 the results of our study indicate that this, a risk stratification and treatment adaptation, has affected OS probabilities and may suggest that relapse treatment became more effective after we recently observed sex ratios around 1. After having achieved high survival probabilities, current treatment protocols focus on the balance between maintaining or improving survival probabilities and reducing, where feasible, treatment-related late effects and negative impacts on quality of life.35 The AIEOP-BFM ALL 2009 study showed that 5-year event-free survival had plateaued at 80%. The current AIEOP-BFM ALL 2017 study seeks to examine whether immunotherapy may further improve prognosis of acute lymphoblastic leukaemia.37, 38
The Joinpoint regression analysis indicated the strongest improvements were in AML survival. Indeed, the treatment protocols for AML have been constantly revised, including tailoring risk stratification and improving second-line treatment.16, 39, 40 During the 1990s, AML relapse was a highly fatal event and, as such, a major obstacle for improving the overall prognosis of patients affected by AML. Since 1997, AML relapse treatment has been increasingly standardised, including safer conditioning regimes for stem cell transplantation (SCT).41 Finally, the implementation of the AML SCT-BFM 2007 trial was likely the main contributor to the tremendous survival improvements for AML and offered a realistic chance for cure for more than half of the children with AML relapse.41, 42
Adapted from BFM studies, NHL treatments showed only moderate efficacy during the 1990s.16 Treatment of mature B-ALL or B-NHL according to the BFM trail group was and still is very toxic. Nevertheless, the high-dose chemotherapy is highly effective against rapidly proliferating B-cells. Better supportive care and clinical guidelines may have resulted in less treatment-associated deaths and improved survival during our study period.43 Our results support the reported success considering the slight but steady increase in NHL survival probabilities, averaging 0.4% per year.
The HIT treatment network (brain tumours in childhood and adolescence) aimed to implement nationwide multimodal treatment protocols for childhood CNS tumours in Germany. During the 1990s and 2000s, this network considered new prognostic factors and treatment options depending on risk stratification.44, 45 The slight but constant survival improvements in malignant CNS tumours, with an AAPC of 0.7% over time, reflect the success of clinical trials. Also, during the past decade, many advances have been made in diagnostics as well as surgical and nonsurgical treatment methods for CNS tumours while considering on the child's age and other risk factors.46 The limited therapeutic options and the higher risk of relapse for infants may have contributed to comparatively inferior survival in the past.47 Nevertheless, infants seem to have benefited most from the latest treatment advances, since we observed increasing survival estimates.
Similarly, for neuroblastoma, the NB trials have successively introduced multiple biological and clinical risk stratification markers since the 1980s in Germany.16, 48-50 This effort may be reflected in our results, which show strong survival improvements during the 1990s (AAPC = 6.8%). The subsequent plateau in survival is in line with the results from the 1997 to 2004 NB clinical trials.50 Notably, the implementation of a neuroblastoma screening at 1 year of age (conducted from 1995 to 2000), with the aim to reduce the incidence of advanced disease and thus mortality, did not achieve the desired outcome.51
The introduction of multiagent chemotherapy for Ewing sarcomas and osteosarcomas during the 1980s led to remarkable survival improvements in both tumour types. Notably, 25% of the affected children are diagnosed with advanced-stage metastatic disease, which worsens prognosis dramatically.52 This may explain, at least to some extent, the relatively wide gap between 1-, 3- and 5-year OS for bone tumours observed in our study. More recently, innovative treatment techniques such as intensity-modulated radiotherapy or proton therapy have been implemented.52 We did not observe any corresponding survival improvements; further follow-up is necessary to document future survival trends.
More precise risk adaptations for clinical factors of rhabdomyosarcoma—the most common soft tissue sarcoma in childhood—within collaborative European clinical trials have led to improvements in both survival and quality of life over time.53 Our results showing recently increasing survival probabilities support the findings of those clinical trials. Nevertheless, considering the 5-year OS lower than 80% as well as the risk of relapse, new approaches such as proton therapy have been introduced to further improve outcome.54
Improved treatment for relapses may have contributed to rising survival probabilities in many diagnostic groups, but this effect is likely to be most pronounced for AML. Nevertheless, as has been described in the context of LL, among others, the obvious survival improvements have now been made, and it will take considerable effort to identify new targets for risk stratification in order to improve outcomes for children affected by malignancies that have proven to be more resistant to classic therapeutic approaches.55
4.2 International comparison
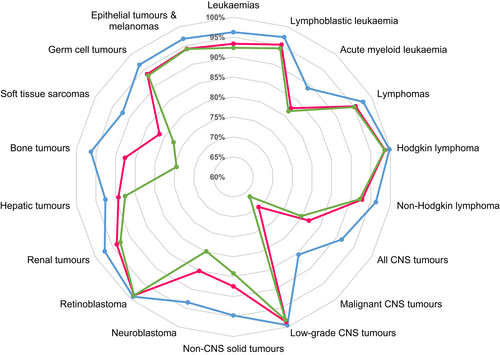
We observed German 5-year OS for all cancers combined to be comparable to, for example, Austria, Belgium and the Nordic countries. However, survival estimates appeared to be somewhat higher in Germany compared to some Eastern European countries, including Bulgaria, Estonia, Lithuania and Slovakia (all <80% in 2000-2013) as reported from EUROCARE-6.23 The EUROCARE-6 authors found rather small improvements in childhood cancer survival (with the exception of AML) in Europe from 2004 to 2014,23 which our results confirmed only to some extent, since we observed continuing increases in survival in recent years.
As in other studies on temporal survival patterns, improvements in childhood cancer survival were also evident in other European countries such as Finland, Austria and Switzerland,22, 26, 56 where universal access to first-line diagnostics and treatment and enrolment in clinical trials are available. Today, many European countries treat childhood cancer patients following similar standardised treatment protocols. However, European findings suggested that survival in Germany has been among the highest over time compared to some other regions, including France.19, 47, 57, 58 During the past decade, there have been concerns in HICs that clinical trials may have almost reached the limits of treatment efficacy.59 Recent protocols increasingly attempt to balance improvements in short-term survival against ensuing side and late effects. Indeed, survival probabilities of paediatric cancer patients in the Nordic countries and some other parts of Europe appear to have plateaued in recent years.19, 26, 60, 61 Besides the steady increase in 5-year OS for all cancers combined revealed by the Joinpoint regression, we also observed some indications of recently plateauing OS estimates for CNS tumours, neuroblastoma, renal tumours and bone tumours.
The remarkable increase in AML survival is in line with findings from international observations.22, 25, 26, 47, 62-65 Within Europe, the increase in AML survival was observed much earlier in Finland and Northern England than in Switzerland and Estonia where treatment to standardised protocols has been implemented several years later.22, 65 Similar to our study of Germany, the findings from Northern England and EUROCARE indicated that the previously lower LL survival in boys has caught up to the girls' level,19, 23, 25 while reports from France and Switzerland still observed this sex gap in more recent years.22, 47
CNS tumour survival showed rather inconsistent patterns across Europe. Indeed, our survival estimates for all CNS tumours combined are in line with those reported from Denmark, where recent improvements have also been observed.66 In Switzerland, however, survival for all CNS tumours appears to have plateaued since the 2000s.22 Regarding survival for malignant CNS tumours, our results suggested a steadily increasing tendency in Germany. By contrast, no survival increase was observed in Finland and Estonia during the past two decades.26, 65 As it did in Germany, the registration of CNS tumours in other European countries may have improved unevenly over time, which is likely to have influenced both incidence and survival—especially for nonmalignant CNS tumours.67 Thus, survival estimates should be compared with caution.
We know that clinical factors for risk stratification have a major impact on treatment and prognosis, but so do socioeconomic and cultural factors, which differ between and within countries, and may influence both healthcare provision and individual resources—even within Europe.18, 64, 68 Apparently, not all affected children benefit equally from the remarkable advances in diagnostics and treatment, since children with cancer across Europe continue to experience inequalities in accessing the best available diagnostics, treatments (including clinical trial participation) and supportive care. These disparities contribute to differences in survival and are of greater concern in Eastern European countries than in Northern and Western Europe.18 Nevertheless, childhood cancer survival in Eastern European countries has improved remarkably throughout the past decades. For Germany in particular, socioeconomic circumstances in Eastern federal states were quite similar to those in other Eastern European countries before German reunification (1990). Indeed, a previous study from the GCCR showed that 5-year OS for LL was lower in Eastern Germany during the 1990s compared to Western Germany and was followed by strong improvements.64
An overview of the international comparison of patterns in childhood cancer survival is given in Table S5.
4.3 Strengths and limitations
First, this present study is strengthened by the high-quality data from the GCCR, with a high degree of completeness of cases (>95%)5 and follow-up information (98.9%). Germany's sizable population permitted stratified analyses with high statistical power. This resource offers a solid basis for future investigations on selected disease groups, allowing researchers to zoom in on characteristics specific to certain childhood cancer subtypes and other clinical details. Moreover, the age-adjusted survival estimates following the methodology of the EUROCARE-6 project and OS estimates based on EUROCARE-6 age weights enabled international comparisons across Europe for the first time.
As the data availability of EUROCARE-6 depended on many European cancer registries, the data presentation differed to some extent from our study. Although we applied the same methodology for age standardisation, the comparison of survival estimates should be interpreted with caution, as the age distribution used for the survival analysis was data driven in both studies. For future international investigations on childhood cancer survival, we would recommend conducting survival analyses while applying an external standard population, as has been recently described by Miranda-Filho et al.69 Since other international investigations on childhood cancer may have been influenced by respective population size, organisation of healthcare, cancer registration and possible differences in data quality, some points for discussion are speculative and comparisons should also be made with caution.
5 CONCLUSIONS
The tremendous improvements observed in childhood cancer survival are likely due to advances in diagnostics and risk-adapted treatment allocation. Nevertheless, not all affected children benefited equally. Some childhood cancer types continue to be associated with poor outcomes or survival recently plateauing at an unsatisfactory level. Social and socioeconomic inequalities seem to influence survival probabilities even in Europe. Therefore, besides clinical studies, further research on socioeconomic determinants and their possible impact on childhood cancer survival are warranted, since these inequalities persist both between countries and within countries.
AUTHOR CONTRIBUTIONS
Maike Wellbrock: Conceptualization; Methodology; Formal analysis; Writing – Original Draft; Writing – Review & Editing; Visualisation; Project administration. Claudia Spix: Data curation; Writing – Review & Editing. Cécile M Ronckers: Writing – Review & Editing. Desiree Grabow: Data curation; Writing – Review & Editing. Anna-Liesa Filbert: Writing – Review & Editing. Arndt Borkhardt: Writing – Review & Editing. Daniel Wollschläger: Conceptualization; Methodology; Writing – Review & Editing; Supervision; Project administration. Friederike Erdmann: Conceptualization; Methodology; Data curation; Writing – Review & Editing; Supervision; Project administration. The work reported in the paper has been performed by the authors, unless clearly specified in the text. All authors contributed to the data interpretation, critically reviewed the manuscript for important intellectual content, and revised the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
ACKNOWLEDGEMENTS
We wish to thank Claudia Trübenbach for her technical support with data preparation for this article. Furthermore, the authors are grateful to the EUROCARE-6 project team for sharing weights of the age distribution per cancer type from the EUROCARE-6 study on childhood cancer survival in Europe. The authors are grateful to the German Society for Paediatric Oncology and Haematology (GPOH) and the Paediatric Haematology-Oncology units for their data contributions to the German Childhood Cancer Registry. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This research was partly funded by a grant from the “Tour der Hoffnung” foundation. Furthermore, the work of the authors was supported by core funds from their respective institutions, namely the Institute of Medical Biostatistics, Epidemiology and Informatics at the University Medical Center of the Johannes Gutenberg University and the Heinrich Heine University, Medical Faculty, Düsseldorf. The German Childhood Cancer Registry is part of the Division of Childhood Cancer Epidemiology at the Institute of Medical Biostatistics, Epidemiology and Informatics and is funded by the Federal Ministry of Health and the Health Ministries of the 16 federal states of Germany. The funding sources were not involved in the conceptualisation, design, content or preparation of the manuscript, or the decision to submit for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial, personal or financial relationships with other people or organisations that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the German Childhood Cancer Registry (currently only as aggregated data) upon reasonable request and in compliance with national data protection regulations.