Interplay between m6A epitranscriptome and epigenome in cancer: current knowledge and therapeutic perspectives
Abstract
Chromatin has an extremely flexible structure that allows the fine regulation of gene expression. To orchestrate this process, small chemical modifications are dynamically added or removed on DNA, RNA and histone substrates. Epigenetic modifications govern a plethora of key cellular functions, whose dysregulation contributes to oncogenesis. The interrelationship between (irreversible) genetic mutations and (reversible) epigenetic alterations and how this crosstalk regulates gene expression has long been a major area of interest. Marks modulating the RNA code (epitranscriptome), such as the well-studied N6-methyladenosine (m6A), are known to influence stability, metabolism and life cycle of many mRNAs, including cancer-associated transcripts. Together, epigenetic and epitranscriptomic pathways therefore control the entire cellular expression profile and, eventually, cell fate. Recently, previously undescribed crosstalk between these two pathways has started to be unrevealed. For example, m6A and its effectors cooperate with histone modifications to localize chromatin-modifying complexes to their target regions. Epigenetic marks governing the expression of m6A factors can also be found at specific genetic loci. m6A itself can mark noncoding RNAs (including lncRNAs, circRNAs and miRNAs), influencing their structure, maturation and function. These interactions affect both cell physiology and pathology. Clear evidence that dysregulation of this network plays a role in cancer has emerged, suggesting a new layer of complexity in the landscape of gene expression. Here, we summarize current knowledge on the interplay between m6A epitranscriptome and epigenome, focusing on cancer processes. We also discuss strategies to target m6A machinery for future therapeutic intervention.
Graphical Abstract
Abbreviations
-
- ALKBH5
-
- ALKB homolog 5
-
- AML
-
- acute myeloid leukemia
-
- BC
-
- breast cancer
-
- BLC
-
- bladder cancer
-
- BRD4
-
- bromodomain containing 4
-
- carRNAs
-
- chromatin-associated RNAs
-
- circRNAs
-
- circular RNAs
-
- CRC
-
- colorectal cancer
-
- DGCR8
-
- DiGeorge syndrome critical region 8
-
- eEF2K
-
- eukaryotic elongation factor 2 kinase
-
- EMT
-
- epithelial-to-mesenchymal transition
-
- ENC
-
- endometrial cancer
-
- eRNA
-
- enhancer RNA
-
- ESCC
-
- esophageal squamous cell carcinoma
-
- EZH2
-
- enhancer of zeste homolog 2
-
- FOXM1
-
- Forkhead box M1
-
- FTO
-
- fat mass and obesity associated
-
- GBM
-
- glioblastoma
-
- GC
-
- gastric cancer
-
- H3K4me3
-
- histone 3 lysine 4 tri-methylation
-
- HAKAI
-
- E3 ubiquitin-protein ligase
-
- HCC
-
- hepatocellular carcinoma
-
- HNRNPA2B1
-
- heterogeneous nuclear ribonucleoprotein A2/B1
-
- hnRNPs
-
- heterogeneous nuclear ribonucleoproteins
-
- HNSCC
-
- head and neck squamous cell carcinomas
-
- IGF2BPs
-
- insulin-like growth factor 2 mRNA-binding proteins
-
- KD
-
- knockdown
-
- KDM3B
-
- lysine demethylase 3B
-
- KO
-
- knockout
-
- LC
-
- lung cancer
-
- lncRNAs
-
- long noncoding RNAs
-
- m6A
-
- N6-methyladenosine
-
- MA
-
- meclofenamic acid
-
- MAC
-
- m6A methyltransferase complex
-
- MACOM m6A
-
- methyltransferase-associated complex
-
- MeRIP-seq
-
- methylated RNA immunoprecipitation sequencing
-
- METTL
-
- methyltransferase-like
-
- miRNAs
-
- microRNA
-
- MM
-
- multiple myeloma
-
- ncRNAs
-
- noncoding RNAs
-
- NSCLC
-
- nonsmall cell lung carcinoma
-
- OC
-
- ovarian cancer
-
- OS
-
- osteosarcoma
-
- PAAD
-
- pancreatic adenocarcinoma
-
- PRAD
-
- prostate adenocarcinoma
-
- PTMs
-
- posttranslational modifications
-
- RBM15/15B
-
- RNA binding motif protein 15
-
- RCC
-
- renal cell carcinoma
-
- SASP
-
- senescence-associated secretory phenotype
-
- SOX4
-
- SRY-related high-mobility-group box 4
-
- TET2
-
- Ten-Eleven translocation 2
-
- VIRMA
-
- Vir-like m6A methyltransferase associated
-
- WDR5
-
- WD repeat domain 5
-
- WTAP
-
- WT1-associated protein
-
- YTH
-
- YT521-B homology
-
- YY1BM
-
- Yin Yang 1-binding anticancer micro-peptide
-
- ZC3H13
-
- Zinc finger CCCH-type containing 13
1 INTRODUCTION
The Greek prefix “epi” means on top of and is used to convey the idea that epi-modifications guide gene expression without altering underlying DNA and RNA sequences. Currently, the epi-signature comprises more than 170 RNA modifications discovered in eukaryotes,1 46 in vivo-verified DNA marks2 and 114 histone posttranslational modifications as reported in the HISTome2 database.3 These chemical tags can be reversibly added or removed at either pretranscriptional, co-transcriptional or posttranscriptional level. The presence of enzymes that deposit (writers), remove (erasers) or decode (readers) these modifications is a common feature in epi-dynamics4 (ie, the mechanisms involving epigenetic factors that impact on RNA synthesis, processing and degradation influencing gene expression). Intriguingly, despite the huge number of marks known, only for a small minority has the complete enzymatic machinery been fully described. Very few DNA/histone modifications have been shown to play a role in transcription nal processes. Major efforts have therefore long been focused on studying such modifications and epigenetic mechanisms that impact gene expression, such as long noncoding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs; reviewed in Allis and Jenuwein5). Conversely, the existence of epitranscriptomic marks has been known since 1960, when pseudouridine was first described.6 However, investigation into these marks lagged for decades, until the discovery of the m6A demethylase fat mass and obesity-associated gene (FTO).7 This finding demonstrated the reversibility of m6A, increasing interest in the field and leading to the breakthrough development of methylated RNA immunoprecipitation sequencing (MeRIP-seq).8, 9 m6A sequencing approaches are currently able to detect m6A profiles of RNAs.10 Emerging evidence suggests that m6A regulates many steps of mRNA biology, including stability, degradation, processing and translation (reviewed in Zaccara et al11). Interestingly, aberrant distribution of m6A is reported in several cancer types, and many m6A effectors are described as playing an oncogenic or oncosuppressor role depending on the tumor-specific context (reviewed in Gu et al12). Thus, the involvement of m6A in oncogenesis and the reversibility of the m6A mark make this modification attractive for therapeutic investigations. In addition, increasing studies connect m6A and epigenetic mechanisms.13 Notably, m6A methylation was found on transcripts coding for chromatin modifiers, possibly altering their translation and, eventually, the cellular epi-signature.14 Similarly, factors associated with enzymes involved in the deposition of the m6A mark support the activity of epigenetic modifying enzymes at chromatin level. Epigenetic marks can also drive the methylation of specific RNAs by recruiting the m6A machinery complex.15 Importantly, both a direct interaction between m6A-related effectors and histone modifications and the presence of the m6A mark on various classes of ncRNAs have been reported.13 Here, we provide an overview of the current knowledge of m6A biology and discuss recent findings illustrating the role of m6A in cancer. We also describe the crosstalk between m6A and epigenetic regulatory pathways, looking at how this interplay affects the cellular transcriptome and its involvement in oncogenic processes. Lastly, we highlight the crucial role of the m6A modification in simultaneously linking multiple and diverse gene regulatory networks and its potential use in novel therapeutic applications.
2 THE m6A MODIFICATION
2.1 Insights into m6A biology
The m6A modification is dynamically regulated by a known set of proteins that act in synergy to deposit (writers), remove (erasers) or decode (readers) the mark. The deposition of the m6A modification in eukaryotic RNAs mainly relies on the activity of methyltransferase-like (METTL) proteins, of which more than 30 different types are encoded by the human genome (HGNC database). However, not all METTL proteins have been characterized or have enzymatic activity. To date, METTL3 is the most studied. METTL3 forms a complex with METTL14 called m6A methyltransferase complex (MAC),16 where METTL3 is the catalytic component, and which co-transcriptionally methylates the vast majority of RNA substrates (Figure 1).17 Conversely, METTL16 preferentially catalyzes the addition of m6A to small nuclear RNAs (snRNAs) and a few mRNAs.18 In addition, METTL5, in association with its allosteric activator TRMT112, and ZCCHC4 were found to methylate ribosomal RNAs (rRNAs).19, 20 Despite the variety of catalytically active METTL proteins, the silencing or genetic deletion of METTL3 and/or METTL14 abolishes up to 99% of m6A peaks in mRNA,21 suggesting that m6A deposition almost completely depends on the activity of METTL3. However, knockout or knockdown (KD) of METTL3-associated proteins reveals various degrees of m6A reduction in different contexts,22 pointing to the role of these proteins in driving deposition of m6A.
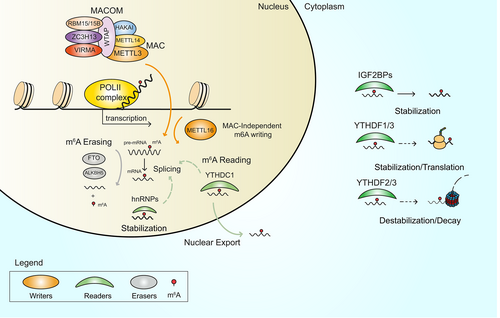
2.2 Dynamics of the m6A modification
MAC activity is supported by the m6A methyltransferase-associated complex (MACOM),16 which includes the accessory proteins: vir like m6A methyltransferase associated protein (VIRMA, also known as KIAA1429), WT1 associated protein (WTAP), zinc finger CCCH-type containing 13 (ZC3H13), RNA binding motif protein 15 (RBM15/15B) and the E3 ubiquitin-protein ligase (HAKAI). ZC3H13 regulates the nuclear localization of the complex,23 while VIRMA and RBM15 are involved in recruitment of MAC to transcriptionally active sites on chromatin.24, 25 WTAP is thought to facilitate the interaction of RBM15 and METTL3,25 while binding of HAKAI to MAC increases its stability.26 Two erasers, FTO and ALKBH5, are currently known to catalyze removal of the m6A mark from target transcripts7, 27 (Figure 1). The m6A modification pattern is mainly interpreted by members of the YT521-B homology (YTH) family of reader proteins, including the nuclear YTHDC1 as well as the cytosolic YTHDF1, YTHDF2, YTHDF3 and YTHDC2 readers.28 Other protein families act as m6A readers, such as cytosolic insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs)29 and heterogeneous nuclear ribonucleoproteins (hnRNPs).30 The readers' interpretation of the m6A mark recruits or activates downstream binding partners and influences the stability of mRNAs, causing either stabilization or destabilization of target transcripts (Figure 1), which strongly impacts RNA metabolism and translation, affecting the entire cellular output. Intriguingly, recent evidence suggests that the three cytoplasmic YTHDF readers can bind the same m6A-modified mRNAs and work cooperatively in an interactive network to influence mRNA stability31, 32 and possibly translation33, 34 (Figure 1). Specifically, YTHDF1 has been reported to increase mRNA stability32 and potentially promote translation.35 While m6A interpretation by YTHDF2 has been correlated with mRNA destabilization and transcript decay.31 For YTHDF3 a dual role in inducing and suppressing mRNA translation has been described.34 However, since other works have also reported no function for YTHDF factors in regulating protein synthesis, their implication in the translation process remains still controversial.35, 36 Moreover, selective KO of one of the YTHDF proteins highlighted that might exist dose-dependent cell context-specific compensatory mechanisms between YTHDFs supporting the hypothesis of functional redundancy for these proteins.36, 37 This implies an additional layer of complexity for the interpretation of m6A modification, however, further investigations are required to deeper elucidate this point.
2.3 Distribution, conservation and proposed mechanisms of m6A deposition
m6A is the most frequent and conserved internal RNA modification.38 Unlike other epitranscriptomic marks that target one or few classes of RNAs, the m6A modification appears widespread on many RNA types, including mRNAs,7 rRNAs,39 lncRNAs,40 pri-miRNAs,41 snRNAs18 and circRNAs.42 Of note, transcripts produced by genetic regions proximal to enhancers elements [enhancer RNAs (eRNAs)] can also be m6A-modified.43 Specifically, pervasive distribution of m6A is found on mRNA molecules, where the modification is particularly enriched in 3′-UTR regions.11 Consensus RRACH (R = A/G, H = A/C/U) motifs are benchmarks for m6A deposition. However, only a fraction of sites is methylated. Although the precise molecular mechanism of m6A deposition remains elusive, two models have been proposed in which (i) RNA binding proteins such as RBM15 and/or (ii) the pausing of Pol II direct the selection of specific RRACH motifs for addition of the modification on nascent transcripts.11 Interestingly, the two models are not mutually exclusive and may co-occur, regulating m6A deposition. A novel machine learning method recently identified regulatory regions flanking RRACH sites that can positively or negatively control addition of the m6A mark.44
3 THE m6A MODIFICATION IN CANCER
Several independent studies have investigated the role of m6A and its biological determinants as a widespread regulatory mechanism that controls abnormal gene expression via different pathological pathways, leading to cancer development. In this section, we describe the role of the m6A mark and its effectors in tumorigenesis.
3.1 Writers
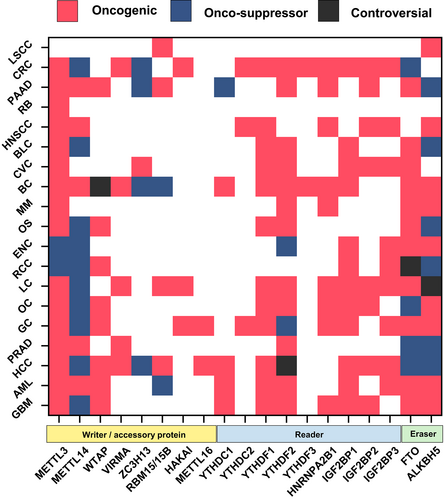
MAC plays a dual role, either promoting or suppressing cancer progression depending on the cellular context, type and origin of the tumor. The catalytic component of MAC, METTL3, is overexpressed in cancer, including hepatocellular carcinoma (HCC),45 prostate adenocarcinoma (PRAD),46 gastric carcinoma (GC),47 colorectal cancer (CRC),48 pancreatic adenocarcinoma (PAAD),49 ovarian cancer (OC),50 breast cancer (BC),51 acute myeloid leukemia (AML),52 bladder cancer (BLC),53 osteosarcoma (OS)54 and others. The enzymatic activity of METTL3 is described as driving different hallmarks of cancer.55 Mechanistically, METTL3-mediated methylation can be directed to oncogenic or oncosuppressor mRNAs, either stabilizing or destabilizing target transcripts and affecting expression of onco- and oncosuppressor genes. In most cancers, METTL3 acts as an oncogene (reviewed in Zeng et al56). However, a tumor suppressor role for METTL3 is described in renal cell carcinoma (RCC)57 and endometrial cancer (ENC).58 Surprisingly, METTL14, the co-partner of METTL3, is reported to have an oncosuppressor function in cancers, including HCC, CRC and GC (reviewed in Liu et al59). Therefore, the opposite role of METTL3 and METTL14 in the same cancer type suggests that these proteins may regulate oncogenesis not only via m6A, but possibly also in an m6A-independent manner. For example, has been recently shown that in senescent cells METTL3 and METTL14 individually localize at distinct genomic regions to regulate the expression of senescence-associated secretory phenotype (SASP) genes and drive tumor progression, independently of m6A.60 However, the role of METTL3/METTL14 in supporting tumor progression besides m6A remains still unclear and may possibly be cancer context-dependent. Further investigations are needed to shed light on these new mechanisms. The METTL3's other co-partner, WTAP, also plays a major role as an oncogene, mediating tumor progression (Figure 2). Ancillary MAC subunits (VIRMA, ZC3H13, RBM15/B, HAKAI) are also involved in promoting or arresting cancer growth by acting as either tumor driver or tumor suppressor genes, depending on the context. VIRMA is reported with an oncogenic role in PRAD,61 HCC,62 BC,63 GC64 and lung cancer (LC),65 whereas ZC3H13 exerts oncosuppressor functions in PAAD,66 BC67 and CRC.68
3.2 Readers
Aberrant expression of m6A readers leads to an altered interpretation of the m6A mark on modified RNAs and is correlated with the development and progression of cancer.12 YTHDF1 and YTHDF2 are the most studied members of the YTHDF protein family in cancer. YTHDF1 is reported to have an oncogenic function in LC, OC, CRC and head and neck squamous cell carcinoma (HNSCC), where it recognizes the m6A mark and increases the stability of tumor transcripts, thus enhancing the translation of oncogenes.69-72 Similarly, YTHDF2 was shown to support tumor progression in glioblastoma (GBM),73 BC,74 AML,75 PRAD,76 OC77 and CRC,78 while its role in HCC remains controversial.79, 80 Interestingly, YTHDF2 acts via a different mechanism promoting the degradation of oncosuppressor mRNAs, thus stimulating cancer growth. To date, the involvement of YTHDF3 in cancer has only been studied in CRC and BC, where it acts as an oncogene.81, 82 Similarly, very few studies have investigated the role of the YTHDC family of m6A nuclear readers in cancer, although YTHDC1 and YTHDC2 are described as having both oncogenic and oncosuppressor functions (Figure 2).83-86 A role in tumor progression is also reported for IGF2BP1 (BC, OC, AML, LC, CRC, BLC), IGF2BP2 (OC, LC, CRC, PAAD), and IGF2BP3 (RCC, LC, CRC; Figure 2; reviewed in Deng et al87), which mainly act by stabilizing m6A-modified oncogenic mRNAs. Lastly, heterogeneous Nuclear Ribonucleoprotein A2/B1 (HNRNPA2B1), a member of the hnRNP family, is also overexpressed in HNSCC, LC, BC and multiple myeloma (MM), where it promotes cancer progression.88-91
3.3 Erasers
Eraser proteins function by removing the m6A mark from RNA molecules (reviewed in Meyer92). Mounting evidence reveals that FTO and ALKBH5 either support or inhibit tumor progression in cancer-dependent manner. ALKBH5 is overexpressed in several tumors, including GBM, GC and OC, where it promotes tumor growth through m6A demethylation and consequent stabilization of oncogenic transcripts.93-95 Conversely, a tumor suppressor role for ALKBH5 is described in PRAD, HCC and OS.96-98 In these tumors, ALKBH5 mediates the destabilization of oncogenic transcripts, impairing cancer development. The involvement of FTO in tumor settings has been widely studied. Similarly to ALKBH5, mechanisms involving either stabilization or destabilization of tumor transcripts inhibiting or stimulating cancer progression are reported for FTO.87 Several other cancers including GBM,99 RCC,100 AML,101 GC,102 LC,103 BC104 and MM105 exhibit overexpression of FTO, highlighting its oncogenic role, while downregulation of FTO and its oncosuppressor function is described in HCC,106 OC107 and PRAD.108
4 INTERPLAY BETWEEN m6A FACTORS AND EPIGENETIC PROCESSES
The complex landscape of gene expression involves an ever-growing number of cellular players and mechanisms. Genetic mutation is the major cause of transcriptional alterations and plays a pivotal role in physio-pathological contexts. Epigenetic mechanisms are now widely accepted as a further layer controlling transcriptional regulation, and the importance of crosstalk between epigenetic and genomic pathways in driving disease has been extensively documented, especially in cancer. An additional level of gene regulation involving RNA modifications has recently become an exciting area of study, and the newly discovered relationship between genome, epigenome and epitranscriptome is proving to be crucial in depicting a comprehensive scenario of gene regulation mechanisms that drive disease. In the following subsections, we describe the latest advances in the interplay between epigenetic mechanisms and m6A effectors, focusing on its implication in cancer.
4.1 Interplay m6A writers and epigenetic processes
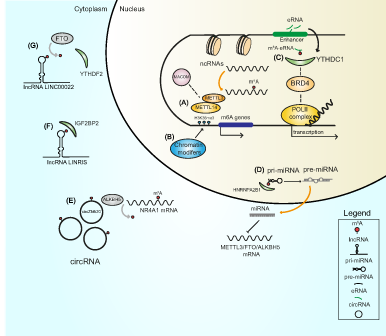
Emerging evidence suggests that the deposition of the m6A mark on RNAs and the activity of MAC influence numerous epigenetic mechanisms. For example, METTL3 and its associated proteins can directly recognize and bind chromatin remodeling factors and histone modifications, while the presence of m6A on transcripts coding for epigenetic modifiers can affect mRNA stability, (directly) influencing its expression and (indirectly) governing the deposition of histone marks that in turn shape the chromatin state. Intriguingly, disruption of m6A installation by METTL3 or METTL14 KD reduced methylation of CBP/p300 and EZH2 mRNAs, increasing levels of H3K27ac (marker of transcriptional activation) and decreasing levels of H3K27me3 (marker of transcriptional repression), respectively.14, 109 METTL3 silencing also reduced levels of H3K4me3, resulting in increased chromatin accessibility and production of chromatin-associated RNAs (carRNAs).110 Additionally, depletion of METTL3 significantly increased H3K9me2 levels, whereas its overexpression rescued the aberrant upregulation of H3K9me2.111 This finding suggests that the broad catalytic activity of METTL3 regulates the deposition of numerous histone marks, possibly playing a further role in determining chromatin conformation. Interestingly, METTL14 was shown to directly bind the H3K36me3 modification at histone level.15 Such binding stimulates the recruitment of MAC/MACOM complex to open chromatin regions and the co-transcriptional addition of the m6A mark, demonstrating that histone modifications also influence m6A dynamics (Figure 3).15 The deposition of epigenetic marks on the promoter of m6A genes and the addition of m6A to ncRNAs are further examples of epigenetic/epitranscriptomic interplay (Figure 3). Specifically, insertion of the H3K27ac mark in the promoter of METTL3 enhanced its expression,112 while removal of H3K4me3 from the promoter of METTL14 caused the opposite effect.113 These findings highlight the importance of histone marks in the expression of m6A factors. Notably, hypomethylation at the METTL3 promoter not only decreases METTL3 transcription but also, in turn, reduces m6A levels on pri-miR-25, impairing its maturation.114 Lastly, ncRNAs play a role in regulating m6A. For example, lncRNAs can exploit m6A machinery to acquire the m6A modification. Interpretation of the m6A mark on modified lncRNAs by reader proteins leads to an increase in their stability,115 while miR-186 reduces the expression of METTL3 and disrupts the deposition of m6A.116
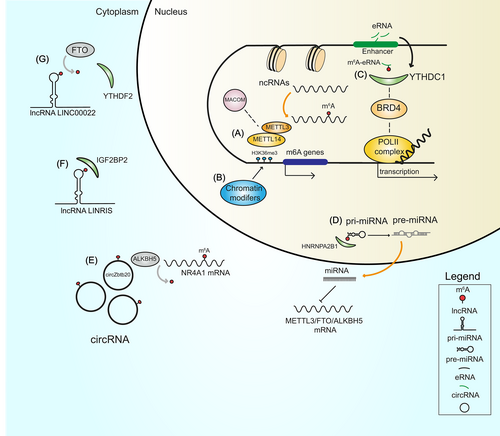
4.2 Interplay between m6A readers and epigenetic processes
Readers of the m6A system not only determine the fate of m6A-marked RNA molecules but play a central role in connecting multiple biological networks, including epigenetic pathways. The nuclear m6A reader YTHDC1 was shown to be involved in the formation of heterochromatin by cooperating with Setdb1/Trim28 for the deposition of the H3K9me3 mark in mouse embryonic stem cells.117 Another study demonstrated that the binding of YTHDC1 to m6A-modified carRNAs stimulates their degradation and its KO results in increased chromatin accessibility and transcription.110 Furthermore, the presence of the m6A mark on eRNAs stimulates the interaction of YTHDC1 with both the m6A mark and Bromodomain Containing 4 (BRD4) protein in the nucleus, inducing the formation of transcriptional condensates, and increasing gene expression (Figure 3).43 This result demonstrates the role of YTHDC1 in regulating the chromatin state, depending on its molecular partner and cellular context. YTHDC1 also drives the deletion of H3K9me2 by interacting with lysine demethylase 3B (KDM3B) on nascent transcripts.111 In the cytosol, the m6A reader YTHDF2 was found to increase H3K27me3 levels by destabilizing methylated lysine demethylase 6B (KDM6B) mRNA.118 Several studies have also explored the interplay between lncRNAs and m6A readers. The lncRNA LINRIS promotes the stability of the m6A reader IGFBP2 by binding to its K139 ubiquitination site and rescuing it from autophagic degradation119 (Figure 3). Similarly, the lncRNA LINC00266-1 produces a small peptide that increases the activity of IGFBP1 through direct binding to the m6A reader.120 Another example of interplay between m6A readers and lncRNAs is represented by circYTHDC2, a novel circRNA produced from the YTHDC2 transcript in vascular smooth muscle cells under high glucose conditions.121 circYTHDC2 is stabilized by the installation of the m6A mark and functions by targeting the 3′-UTR region of the TET2 mRNA, reducing TET2 levels and altering the DNA methylation pattern.121 The interpretation of the m6A mark by the HNRNPA2B1 reader has also been reported to accelerate the maturation of pri-miRNA.30 Lastly, miR-145 was shown to target the 3′-UTR region of the YTHDF2 mRNA, downregulating its expression.122
4.3 Interplay between m6A erasers and epigenetic processes
Selective removal of m6A from mRNAs impairs detection of the mark by epitranscriptomic readers and affects several features of RNA transcripts, including the formation of secondary structures and the stability, biogenesis and function of RNAs. The m6A demethylase FTO is reported to remove m6A from the lncRNA LINC00022, which alters the recognition activity of the m6A reader YTHDF2 and enhances the stability of LINC00022 (Figure 3).123 In contrast, overexpression of ALKBH5 via hypomethylation in CpG islands at its promoter region drives the removal of m6A from the lncRNA LINC00278, impairing binding of YTHDF1 to the m6A mark and decreasing LINC00278 stability.124 These findings indicate that ncRNAs can hijack m6A to perform their functions. The maturation of miRNAs also depends on the deposition of m6A on pri-miRNAs (Figure 3), and selective removal of m6A by eraser proteins can negatively affect this process.41 Specifically, in lung fibroblasts, ALKBH5 KD decreased pri-miR-320a-3p levels, halting the maturation activity of the microprocessor complex subunit DGCR8 and resulting in reduced targeting of the FOXM1 mRNA via mature miR-320a-3p.125 The interaction between novel ncRNA species, such as circRNAs, and m6A erasers was also recently reported. circZbtb20 was shown to direct ALKBH5 to the nuclear receptor subfamily 4 group A member 1 (NR4A1) mRNA, causing its demethylation and increasing its stability (Figure 3).126 The removal of m6A is reported to influence the deposition of histone marks via different mechanisms. For example, demethylation of the lncRNA TRERNA1 by ALKBH5 increased expression of TRERNA1, stimulating recruitment of EZH2 to chromatin and increasing deposition of the H3K27me3 mark.127 Similarly, ALKBH5 KD was found to increase H3K9me3 levels in mouse embryonic stem cells, although the exact biological mechanism has not yet been described.117 This finding indicates a strong interrelationship between the removal of m6A and epigenetic pathways in driving gene expression.
5 INTERPLAY BETWEEN CANCER m6A EPITRANSCRIPTOME AND EPIGENOME
Onset of tumorigenesis is due to the accumulation over time of genetic mutations and epigenetic changes that interplay altering correct gene expression patterns. The RNA modification m6A has a role in tumorigenesis and is involved in a third level of gene regulation. Studying the intersection of genetic, epigenetic and epitranscriptomic pathways in tumor settings is therefore crucial, and a growing body of compelling evidence points to an unexpected complexity. Examples of crosstalk between all classes of m6A effectors and epigenetic regulation in cancer are reported.13 In CRC, downregulation of METTL14 via removal of the H3K4me3 mark from its promoter reduced the deposition of m6A on SOX4 mRNA, rescuing it from YTHDF2-mediated degradation.113 This resulted in enhanced migration and SOX4-mediated epithelial-to-mesenchymal transition of CRC cells, both in vitro and in vivo.113 Similarly, overexpression of METTL3, though hypomethylation of its promoter, increased m6A deposition on miR-25-3p and stimulated its maturation, eventually driving the proliferation of PAAD cells via the AKT-p70S6K pathway.114 Further, MeRIP-seq analysis of GC cells depleted for METTL14 showed decreased m6A levels on the circRNA circORC5.128 Absence of the m6A mark on circORC5 increased its expression by directing its activity toward the oncosuppressor miR-30c-2-3p, leading to GC progression.128 These results indicate that METTL3/14 influences epigenetic pathways and could be a promising axis to target in cancer. The IGFBP protein family of m6A readers has also been shown to dialog with epigenetic pathways in cancer settings. Specifically, stabilization of the lncRNA LINRIS by the m6A-reading activity of IGF2BP1 or the interaction of IGF2BP2 with an oncopeptide produced by the lncRNA LINC00266-1 supported the growth of CRC cells by increasing expression of MYC and cellular glycolysis.119, 120 In this context, the use of inhibitors against IGF2BP1/2 could be a novel strategy to target CRC. Downregulation of YTHDF2 by miR-145 also suppressed the proliferation of HCC cells, although the exact mechanism of action has not yet been elucidated.122 m6A erasers also have a broad effect in crosstalk with epigenetic regulation in cancer. In esophageal squamous cell carcinoma (ESCC), FTO KD increased levels of the m6A-modified lncRNA LINC00022, altering its stability and impacting the proliferation of ESCC cells.123 Similarly, overexpression of ALKBH5 via hypomethylation of CpG islands at its promoter stimulated the removal of m6A from the lncRNA LINC00278 and decreased both its stability and the production of the anticancer Yin Yang 1 (YY1)-binding micropeptide (YY1BM), eventually promoting the expression of eukaryotic elongation factor 2 kinase (eEF2K) and the proliferation of ESCC cells.124 In addition, ALKBH5-mediated m6A demethylation of the lncRNA PVT1 supported OS progression,129 suggesting a role for chromatin modifiers in regulating the expression of m6A genes. Lastly, overexpression of ALKBH5, caused by an increase in the deposition of H3K4me3 at its promoter, mediated by WDR5 in hepatitis B virus-infected cells, promoted the development of HCC.130 Mechanistically, the removal of m6A from the viral mRNA of HBx protein increases its stability and stimulates the recruitment of methylation complexes at the ALKBH5 locus, creating a positive feedback loop.130
6 CONCLUSIONS AND THERAPEUTIC PERSPECTIVES
Despite the increasing number of studies describing the crosstalk between the epigenome and epitranscriptome, how all the actors interact to finely regulate gene expression and shape the overall cellular transcriptional output remains largely unknown. DNA methylation, ncRNAs, histone marks and RNA modifications including m6A are only a small part of the transcriptional landscape.131 The interplay between different levels of regulation involved in transcription makes the scenario even more complex. However, since the ever-growing burden of cancer worldwide urgently requires the identification of novel therapeutic targets and prognostic biomarkers, elucidating the mechanisms of crosstalk among different networks of gene expression regulation is fundamental for achieving new milestones in drug discovery. The reversible m6A mark that contributes to several cancer hallmarks may offer new therapeutic opportunities, and rapid advancements are being made in this direction. In 2014, meclofenamic acid (MA) was identified as FTO inhibitor,132 and the anticancer activity of MA and its derivative133, 134 was successfully tested in preclinical studies.133-137 Since MA is already used in the clinic, a drug repurposing approach may represent a promising strategy in cancer. Several molecules targeting m6A effectors, mainly writers and erasers, have been developed133, 138-151 (Table 1). However, most of these m6A-targeting drugs have low target selectivity and poor pharmacokinetic properties which hinder clinical applications. To date, the METTL3 inhibitor STC-15,152 developed by Storm Therapeutics, is the only epitranscriptomic compound that has entered clinical trials (NCT05584111). Interestingly, natural compounds153 and AI-based approaches have also been used to speed up the lengthy process of drug discovery, but with mixed results.87 Since m6A writer and eraser proteins possess a broad range of substrates, it would also be worthwhile to develop molecules specifically targeting m6A readers. A better understanding of the three layers of gene regulation and the critical involvement of m6A in cancer could provide a platform to design new drugs co-targeting multiple pathways and to identify new druggable targets. For example, competitive inhibitors of IGFBPs may affect the interaction between m6A readers and their lncRNA targets, exerting a therapeutic effect in CRC.119 In addition, the combinatory use of drugs targeting METTL3 or ALKBH5 and epigenetic regulators of m6A players might revert the oncogenic effects of m6A in PAAD and ESCC.114, 124 In the future, combination therapies using drugs simultaneously targeting the epitranscriptome and epigenome might open up new therapeutic avenues, especially in patients experiencing poor efficacy of epigenetic drugs or drug resistance. In sum, the complex world of transcription regulation has been dramatically changed by the discovery of the interplay between genetic, epigenetic and epitranscriptomic mechanisms. Dissecting this interplay will be fundamental for therapeutic intervention.
| Compound name | Target | Cancer type | Status | References |
|---|---|---|---|---|
| STC-15 (STORM Therapeutics) | METTL3 | AML | Phase I | 152 |
| n.a. (Accent Therapeutics) | METTL3 | AML, NSLCL | Preclinical | 139 |
| n.a. (Gotham Therapeutics) | METTL3 | AML | Preclinical | 139 |
| Quercetin | METTL3 | PRC, HCC | Preclinical | 153 |
| UZH1a | METTL3 | AML, OS | Preclinical | 140 |
| UZH2 | METTL3 | AML, PRC | Preclinical | 141 |
| SB-497115-GR | METTL3/14 | AML | Preclinical | 142 |
| Compound 2/7 adenosine-derivative | METTL3 | — | Enzymatically tested | 143 |
| Rhein | FTO | AML, neuroblastoma | Preclinical | 144, 145 |
| Meclofenamic acid (MA) | FTO | GBM, CRC | Preclinical | 132, 134 |
| MA2 | FTO | GBM | Preclinical | 133, 134 |
| FB23/FB23-2 | FTO | AML | Preclinical | 152 |
| CS1/CS2 | FTO | AML, GBM, BC, PRAD | Preclinical | 146 |
| Fluorometric aptamers | FTO | — | Preclinical | 147 |
| R2HG | FTO | AML | Preclinical | 148, 149 |
| HUHS015 | PCA-1/ALKBH3 | PRAD | Preclinical | 150 |
| 20m | ALKBH5 | HCC | Preclinical | 151 |
AUTHOR CONTRIBUTIONS
Nunzio Del Gaudio and Lucia Altucci: Supervision and Writing. Nunzio Del Gaudio and Guglielmo Bove: Conceptualization and Writing. Sajid Amin and Mehrad Babaei: Contributed to conceptualization. Angela Nebbioso and Rosaria Benedetti: Review and Editing. All authors read and approved the final manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGEMENTS
This work was supported by the Campania Regional Government Lotta alle Patologie Oncologiche iCURE (CUP B21C17000030007); MIUR Proof of Concept (POC01_00043); MISE: Nabucco Project; VALERE: Vanvitelli per la Ricerca Program: EPInhibitDRUGre (CUP B66J20000680005). Bando di Ateneo per il finanziamento di progetti di ricerca fondamentale ed applicata dedicato ai giovani Ricercatori: IDEA (CUP: B63C22001470005). Nunzio Del Gaudio was supported by PON Ricerca e Innovazione 2014-2020 Linea 1, AIM (AIM1859703). We thank C. Fisher for English language editing.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.