Dendritic cell immunoreceptor 2 (DCIR2) deficiency decreases hepatic conventional dendritic cell content but not the progression of diet-induced obesity
Abstract
Aims
Inflammatory pathways and immune system dysregulation participate in the onset and progression of cardiometabolic diseases. The dendritic cell immunoreceptor 2 (DCIR2) is a C-type lectin receptor mainly expressed by conventional type 2 dendritic cells, involved in antigen recognition and in the modulation of T cell response. Here, we investigated the effect of DCIR2 deficiency during the development of obesity.
Methods
DCIR2 KO mice and the WT counterpart were fed with high-fat diet (HFD) for 20 weeks. Weight gain, glucose and insulin tolerance were assessed, parallel to immune cell subset profiling and histological analysis.
Results
After HFD feeding, DCIR2 KO mice presented altered conventional dendritic cell distribution within the liver without affecting markers of hepatic inflammation. These observations were liver restricted, since immune profile of metabolic and lymphoid organs-namely adipose tissue, spleen and mesenteric lymph nodes-did not show differences between the two groups. This reflected in a similar metabolic profile of DCIR2 KO compared to WT mice, characterized by comparable body weight gain as well as adipose tissues, spleen, Peyer's patches and mesenteric lymph nodes weight at sacrifice. Also, insulin response was similar in both groups.
Conclusion
Our data show that DCIR2 has a redundant role in the progression of diet-induced obesity and inflammation.
Ectopic fat accumulation is the hallmark of obesity and promotes moderate chronic inflammation, which accelerates the development of cardiovascular disease, diabetes, and cancer.1 Lipid deposition triggers the activation of an immune-inflammatory response that prompts the proinflammatory activation and migration of immune cells in the adipose tissue and in the liver.2 In this setting, the role of dendritic cells (DCs), which act as a bridge between innate and adaptive immunity,2 is still poorly explored.
DCs are bone marrow-derived cells, categorized into classical or conventional DCs (cDCs), plasmacytoid DCs (pDCs), monocyte-derived DCs (MoDCs), and Langerhans cells (LCs). They reside in different tissues where they sense stimuli that impact to their activation, polarization, and maturation.3 While it has been shown that adipose tissue DCs are central players in the initiation and progression of obesity-induced inflammation and insulin resistance,3 the contribution of DCs to metabolic failure of other organs, such as the liver, is less clear. Here, DCs mainly reside within the periportal and pericentral space4 and, although it is known that they contribute to tissue homeostasis as well as obesity-associated immune response,3 their role is still controversial. The infiltration of CX3CR1+ MoDCs in injured liver driven by increased CX3CL1 expression, supports local inflammatory response5 and, accordingly, blocking CX3CR1 was shown to limit hepatic inflammation and damage.4 Instead, recent publications have proposed different functions of DCs according to their lipid content: while lipid-enriched liver-derived DCs show a proinflammatory role that supports the activation of the effector arm of the adaptive immune response, lipid-poor DCs promote T-cell mediated tolerance.6 In addition, DCs also contribute to liver fibrosis7 and, particularly, liver type 2 conventional DCs (cDC2) positively correlate with the progression of metabolic steatohepatitis.4
A peculiar characteristic of murine cDC2 is the expression of the dendritic cell immunoreceptor 2 (DCIR2), whose role—beyond being a cDC2 marker—has not been fully elucidated. Indeed, DCIR2 has been involved in the induction of the tolerogenic CD4+ T-cell response in NOD mice,8 while its deficiency enhanced cytokine production and T-cell priming following Toll-like receptor (TLR)-mediated activation in vivo9 but was shown to limit atherogenesis in hypercholesterolemic mice.10
Thus, this work aimed to investigate the impact of DCIR2 deficiency during obesity and metabolic syndrome development in high-fat diet (HFD)-fed mice.
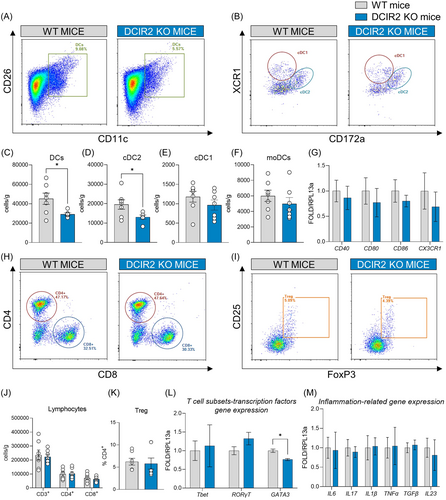
First, we evaluated the impact of DCIR2 deficiency on immune cell distribution after 20 weeks of HFD feeding. Total DCs and cDC2 cells were decreased in the liver of DCIR2 KO mice, while the levels of cDC1 and MoDCs remained unchanged compared to matched controls (Figure 1A–F). Although the number of cDC2 was reduced, the expression of costimulatory molecules such as CD40, CD80, CD86, and the chemokine receptor CX3CR1, which contribute to the activation and recruitment of DCs, was similar in the two groups (Figure 1G), thus excluding differences in the activation status of immune cells in the liver. In line with these results, similar levels of CD3+ T lymphocytes within the liver, both CD4+ and CD8+ cells, as well as the percentage of T regulatory cells (Figure 1H–K) were reported. Further characterization of T lymphocyte polarization was performed through the profiling of transcriptional factors Tbet, RORγT, and GATA3, which are master regulators of T helper (Th)1, Th17, and Th2 response, respectively. This analysis showed decreased GATA3 expression (Figure 1L), supporting the observation that cDC2 are mainly involved in the maintenance of Th2 immunity. Together, these results indicate that DCIR2 deficiency impacts cDC2 resulting in reduced Th2 cell polarization within the liver, without directly affecting the gene expression of IL6, IL17, IL1β, TNF-α, TGFβ, and IL23 as parameters of hepatic inflammation (Figure 1M).
These differences were however restricted to the liver since visceral adipose tissue (Supporting Information: Figure S2A), and lymphoid organs, such as spleen, mesenteric lymph nodes (mLNs), and Peyer's patches showed a similar distribution of DCs between DCIR2 KO and WT mice (Supporting Information: Figure S2B–D), thus suggesting that the impact of DCIR2 deficiency is mainly affecting the liver.
To next explore whether these changes might have resulted in a different metabolic response of DCIR2 KO mice under high caloric intake, we investigated the effect of HFD feeding on metabolic phenotype. Body weight (Supporting Information: Figure S3A) and weight gain (Figure 2A) were similar in DCIR2 KO and WT mice, and the same was true for plasma lipid levels (Figure 2B,C). Moreover, the percentage of liver, pancreas, visceral adipose tissue (VAT), subcutaneous adipose tissue (SCAT), and brown adipose tissue (BAT) weight on body weight was similar among the animal models (Figure 2D,E). Also, the weight of the lymphoid tissues—thymus and spleen—was not different (Figure 2F). Interestingly, glycemia after overnight fasting was slightly elevated in DCIR2 KO compared to WT mice (Figure 2G), and DCIR2 KO mice were less glucose tolerant compared to WT mice (Figure 2H and Supporting Information: Figure S3B), showing a preserved insulin tolerance (Supporting Information: Figure S3C). Despite this result would be possibly explained by a decreased trend of circulating insulin levels of KO mice compared to WT observed at two timepoints of GTT (Supporting Information: Figure S3D), lipid deposition in the liver parenchyma was similar between WT and KO mouse (Figure 2I,J and Supporting Information: Figure S4A), casting for a redundant role of DCIR2 in the metabolic response.
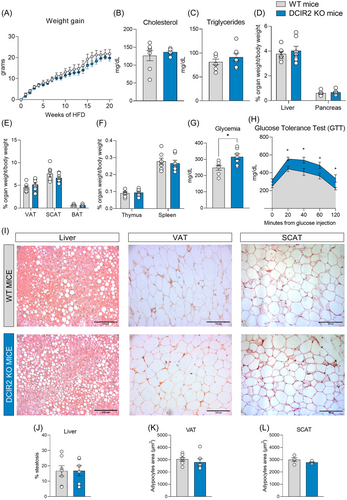
This extended also to adipose tissue, where adipocyte area was similar in both visceral and subcutaneous adipose tissue (VAT and SCAT, respectively) (Figure 2I,K,L). Similarly, the expression of markers of adipose tissue biology, such as PPARγ, and adiponectin (Supporting Information: Figure 4B) was similar in WT and KO adipose tissue. We then considered the expression of dipeptidyl peptidase-4 (CD26), an amino peptidase that reduces the activity of incretin peptides (glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide) resulting in reduced insulin secretion and, even, insulin resistance.11 However, although our flow cytometry analysis showed a similar expression of CD26 in visceral DCs of WT and DCIR2 KO (Supporting Information: Figure 4C), we cannot exclude that DCIR2 deficiency could have been compensated by other mechanisms, thus masking its contribution to obesity-associated inflammation. Indeed, given its role in the maintenance of immune tolerance, it is plausible that its function is redundant with other similar pathways.
In conclusion, the profiling of DCIR2 KO fed on HFD for 20 weeks indicates that DCIR2 has a redundant role in obesity and obese-related inflammation. Indeed, despite the absence of DCIR2 impacted DC distribution and Th2 polarization within the liver, this did not affect liver steatosis or adipose tissue inflammation and lipid accumulation.
Details on material and methods are presented as supplemental material.
AUTHOR CONTRIBUTIONS
Rossella Bellini: Formal analysis; investigation; validation; visualization; writing—original draft. Annalisa Moregola: Investigation; methodology; validation. Jasmine Nour: Investigation. Patrizia Uboldi: Investigation. Fabrizia Bonacina: Conceptualization; formal analysis; funding acquisition; methodology; project administration; resources; supervision; validation; visualization; writing—original draft; writing—review and editing. Giuseppe D. Norata: Conceptualization; funding acquisition; project administration; resources; supervision; writing—original draft; writing—review and editing.
ACKNOWLEDGMENTS
We are grateful to Y. Rombouts and O. Neyrolles—Institut de Pharmacologie et de Biologie Structurale, Universitè de Toulouse, CNRS, UPS, France which provided us the DCIR2 KO mice10 and we thank the Genotoul Anexplo-IPBS platform (Toulouse) for help with animal husbandry. GDN is supported by Telethon Foundation [GGP19146], Progetti di Rilevante Interesse Nazionale [PRIN 2017 K55HLC], Ricerca Finalizzata, Ministry of Health [RF-2019-12370896], PNRR Missione 4, [Progetto CN3-National Center for Gene Therapy and Drugs based on RNA Technology], PNRR Missione 4 [Progetto MUSA-Multilayered Urban Sustainability Action], PNRR Missione 6 [PNRR-MAD-2022-12375913], Nanokos [EUROPEAID/173691/DD/ACT/XK]. Cariplo Foundation [1560-2019] and Roche Foundation to FB. Graphical abstract figure was created with BioRender.com. The authors acknowledge the support of the APC central fund of the University of Milan.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author, G.D.N., upon reasonable request.