Treadmill training improves cognitive function by increasing IGF2 targeted downregulation of miRNA-483
Abstract
Optimal exercise can promote the development of cognitive functions. Nevertheless, mechanisms that elicit these positive effects of exercise still need to be elucidated. Insulin-like growth factor 2 (IGF2) is known to act as a potent enhancer of memory and cognitive functions, whereas the mechanism by which IGF2 regulates cognitive functions in terms of moderate treadmill exercise remains largely vague. In the study, rats were subjected to low-, moderate-, and high-intensity treadmill training for 6 weeks. Then, the Morris water maze test was used to investigate spatial learning and memory ability in rats subjected to treadmill exercises of different intensities. Subsequently, gene chip and bioinformatics analyses were used to explore IGF2 and predict target microRNAs (miRNAs). Quantitative real-time polymerase chain reaction, western blot, and immunofluorescence analysis were performed to detect the levels of IGF2. Furthermore, IGF2-small interfering RNA, the miRNA-483-mimic, and the miRNA-483-inhibitor were transfected to determine the role of IGF2 and miRNA-483 in the growth of hippocampal neurons. The results of the Morris water maze test showed that moderate-intensity treadmill training enhanced cognitive functions; meanwhile, the expression of IGF2 was significantly upregulated in the hippocampus after moderate-intensity treadmill exercise. From databases, miRNA-483 was screened and predicted as the target gene of IGF2. Moreover, silencing IGF2 inhibited neurite growth in the hippocampus of rats, the miRNA-483-inhibitor ameliorated silencing IGF2 induced impairment of hippocampal neurons. These findings suggested that treadmill training could enhance cognitive functions, wherein the underlying mechanism involved an increase in the expression of IGF2 and downregulation of miRNA-483.
1 INTRODUCTION
Physical activity is beneficial to brain function.1 Physical exercise strengthens the body, but it also enhances the cognitive functions of the brain.2 Exercise of optimal intensity can promote the development of cognitive function and personality by enhancing the proliferation, survival, and differentiation of neurons and synapse plasticity, improve information transmission and learning and memory ability of the hippocampus, and enhance cerebral blood flow function;1, 3, 4 however, excessive exercise load causes ischemia and hypoxia in the brain and damages the cells in the hippocampus. Therefore, high-intensity exercise could disrupt the balance of the body and worsen cognitive impairment. Comparatively, moderate-intensity exercise has been found to be associated with memory and hippocampal plasticity,5 while the mechanisms of these positive effects of exercise are yet to be elucidated.
The insulin-like growth factor (IGF) axis plays an essential role in normal growth and development.6 IGF2 is now known as a multifunctional growth regulator in the insulin-like growth family that promotes cell differentiation and proliferation and is associated with the regulation of physical activity.7 Increasing evidence has demonstrated the functional significance of IGF2 in hippocampal-dependent learning and memory of rats.8 Researchers recently revealed that increased IGF2 could improve learning and memory and activate the neurons in the hippocampus,9, 10 suggesting that IGF2 exerts a crucial role in cogniti and memory function. Furthermore, the expression of IGF2 was found to improve cognitive functions in mouse models, indicating that IGF2 could represent a target for regulating cognitive functions.11 Exercise of optimal intensity can promote the development of cognitive functions. Thus, analysis of IGF2 may broaden our understanding of the molecular mechanisms of learning and memory ability induced by moderate-intensity treadmill training.
In this study, we used the Morris water maze test to evaluate the learning and memory ability of rats who were subjected to low-, moderate-, and high-intensity treadmill training for 6 weeks. Subsequently, gene chip and Gene Ontology (GO) analyses on the hippocampus of rats subjected to exercise were performed and it was found that IGF2 plays a crucial role in the regulation of learning and memory ability after treadmill training. From TargetScan_7.1 and miRDB databases and quantitative real-time polymerase chain reaction (qRT-PCR) verification, microRNA-483 (miRNA-483) was screened out as a target of IGF2. Moreover, to better understand the potential roles of IGF2 and miRNA-483 in the growth of hippocampal neurons, IGF2-small interfering RNA (siRNA) and miRNA-483 (mimic-483, inhibitor-483) were transfected into neurons reflected by immunofluorescence double staining of Tuj1 and IGF2. Taken together, our findings could enable the identification of an appropriate exercise program and provide a potential theoretical explanation for improving cognitive functions after moderate-intensity exercise, which might be associated with the regulation of IGF2 and miRNA-483.
2 MATERIALS AND METHODS
2.1 Animals/subjects
Thirty-two 2-month-old male (200 ± 20 g) and timed pregnant female Sprague–Dawley rats were purchased from the Center of Experimental Animals, Kunming Medical University. Animal care and all experimental protocols were approved by the guidelines of the Institutional Medical Experimental Animal Care Committee of Kunming Medical University with the approval number SYXK 2015-0002. Guidelines for laboratory animal care and safety from NIH have been followed. The animals in each group (n = 8 per group) were kept in a separate container (containing two 57 × 39 × 20 cm3 laboratory cages with 4 rats per cage)12 under controlled laboratory conditions in a temperature (22 ± 2°C)- and humidity (45 ± 10%)-controlled room under a 12 h (h) light/dark cycle with food and water available ad libitum throughout the study.
2.2 Treadmill exercise protocol and animal grouping
Briefly, rats were trained for 2 days by adaptive treadmill training (running at a speed of 8.2 m/min for 30 min) to enable them to adapt to the new environment. Then, normal treadmill training was conducted, and the strength and speed of the treadmill were adjusted for the rats. Rats were randomly divided into four groups: control group, low-intensity group (SI group, ran at a speed of 18 m/min for 30 min), moderate-intensity group (MI group, ran at a speed of 24 m/min for 30 min), and high-intensity group (HI group, ran at a speed of 30 m/min for 30 min). Additionally, training was conducted once daily for 6 weeks (6 days a week, Sunday off). According to Bedford's methods,13 a rough estimate of the treadmill load intensity was made as follows: (1) less than 200 days: VO2max = 0.19 × weighting (g) + 91.16 (male); VO2max = 0.20 × weighting (g) + 95.58 (female). (2) More than 200 days: VO2max = 0.35 × weighting (g).
2.3 Morris water maze test
The Morris water maze test was carried out to examine spatial learning and memory at 24 h after treadmill exercise as described previously.14 A circular container (50 cm deep, 160 cm wide) was filled with black opaque water, and the temperature was maintained at 20–24°C. Various prominent visual cues were placed on the walls around the pool. A camera connected to a computer equipped with Tracking System SMART 3.0 was installed over the pool to automatically record the swimming trace of rats. The rats were put back in their cages and placed in a standard animal room, with food and water available during the training sessions. The pool was divided into four equal quadrants (target quadrant, adjacent right, adjacent left, and opposite quadrant). An escape platform was submerged 2 cm beneath the water surface in the target quadrant. The test was conducted, including training and probe trials, for 4 consecutive days. For each training trial, the rats were placed in the water facing the wall at one of the four starting points. Before the training was initiated, rats were allowed to swim freely in the water for 120 s with the platform to enable them to adapt to the new environment. Each rat received trials of four quadrants per day for three consecutive days, with an interval between each of the trials of 15–20 min. The rats were given 120 s to find the platform and were permitted to stay on the platform for 5 s before being removed, while rats that were unable to find the platform within 120 s were placed on the platform for 10 s before being removed; the escape latency was recorded using a video camera. On the fourth day, the platform was removed and the number of crossings over the previous platform location was recorded over one 120 s trial. Tracking System SMART 3.0 automatically recorded the time spent on the target quadrant and the distance in the target quatrant.
2.4 Sample collection
Twenty-four hours after the Morris water maze test, gene analysis and qRT-PCR as well as western blot were performed to predict and verify the expression of IGF2 in hippocampus tissue after exercise of different intensities. The rats were anesthetized with sodium pentobarbitone (10 ml/kg, i.p.) before an incision was made in the middle of the skull to expose the brain. Then, the hippocampal part was removed and stored at −80°C.
2.5 Differential gene expression profiles
The differentially expressed genes in hippocampus tissue from rats of the control, low-intensity, moderate-intensity, and high-intensity groups were screened using a gene chip. In brief, the RNA was extracted from the frozen hippocampus within a week using the RNesay Mini Kit according to the procedure recommended by the supplier (Qiagen). The quality and integrity of RNA were measured using a 2% Agilent Bioanalyzer 2100 System (Agilent Technologies). Then, the RNA was sent to KangChen Biotech for sequencing. Gene sequence were used to generate a heat map and intersection of differential gene expression to detect the upregulated and downregulated genes, which were conducted to screen the multiple variational genes. GO analysis is an international standardized gene functional classification system associated with differentially expressed genes. GO analysis comprises three structured networks that describe the properties of gene products (molecular functions, cellular components, and biological processes). p Values indicate the significant differences in the GO term in differentially expressed gene lists. Additionally, the smaller the p value, the greater the significance of the gene. The purpose of this experiment is to find the highest enriched genes by the four groups’ GO analysis of memory function, and use TargetScan (http://www.targetscan.org/mamm_31/) and miRDB (http://www.mirdb.org/) to predict possible miRNAs regulating IGF2.
2.6 Primary culture for hippocampal neurons
Hippocampal neurons were obtained from rat pups according to the method detailed in previous reports.15 Briefly, animals were culled, the brain and meninges were extracted, and then the hippocampus was isolated and cut into the smallest possible pieces, mixed with Dulbecco's modified Eagle's medium, and transferred to sterile tubes. The tissue was allowed to digest with papain and DNase I at 50 μg/ml. The enzymes were neutralized by adding 10× the original volume of the Neurobasal medium with 10% fetal bovine serum (FBS). Afterwards, the cells were collected by centrifugation at 200g for 5 min. Neurons were incubated in the neurobasal medium at a density of 5 × 105 cells/ml supplemented with 2 mM l-glutamine, 1 μg/ml gentamicin, 2% B27, and 10% FBS at 37°C with 5% CO2. The medium was changed every third day and neurons were cultured for 10 days.
2.7 Screening for the effective fragment of IGF2-siRNA
Three siRNAs with suppressed IGF2 were designed and purchased from RuiBo Company. The target mRNA sequence of Fragment 1 was GTCGCATGCTTGCCAAAGA, Fragment 2 was GCAAGTTCTTCAAATTCGA, and Fragment 3 was AGAGCTCGAAGCGTTCAGA. PC12 cell lines were used for screening the effective siRNA. The cell line was transfected with 100 nM siRNA separately. Then, 100 ng/μl siRNA reagent was added and the media were replaced after 15 min. Afterwards, qRT-PCR was used to evaluate the effects of siRNA on the expression of IGF2 mRNA. The most effective one was selected for subsequent experiment.
2.8 Transfection of si-IGF2 and miRNA-483
To determine the role of IGF2 and miRNA-483 in the cell growth, hippocampal neurons were cultured and randomly divided into the following groups: normal, reagent (only added transfection reagents), si-negative control (random transfected garbled fragment), si-IGF, miRNA-483-mimic-nc, miRNA-483-inhibitor-nc, miRNA-483-mimic, and miRNA-483-inhibitor groups. Subsequently, si-IGF2 was transfected to the cells harboring miRNA-483 and IGF2; we added the miRNA-483-inhibitor to the cells. Then, the cell growth, viability, and apoptosis were detected at 48 h post transfection using a high-content imaging system (EVOS, Thermo Fisher Scientific).
2.9 Quantitative real-rime PCR (qRT-PCR)
RNA from the hippocampus was used as a template to synthesise complementary DNA (cDNA) using the SuperScript™ First-Strand Synthesis System (Invitrogen). The primers were as follows: IGF2 sense, 5′-CAAGTCCGAGAGGGACGTGT-3′; antisense, 5′-CACGCAGGAGGGCAGGCAG-3′ and miRNA-483 primer sequence from GeneCopoeia: GAGGGGGAAGACGGGAGAAGAGAAGGGAGUGGUUUUUGGGUGCCUCACUCCUCCCCUCCCGUUUGUUCUCUC. The β-actin gene was used as a housekeeping gene using the specific primers 5′-GTAAAGACCTCTATGCCAACA-3′ and 5′-GGACTCATCGTACTCCTGCT-3′. The PCR reaction was prepared by mixing 12.5 µl of IQ SYBR Green Supermix (Bio-Rad), 10.5 µl of nuclear-free PCR water, and 0.5 µl of each primer stocked at 5 nM and 1 µl cDNA template. The PCR reaction set for 35 cycles was as follows: 94°C for 10s, 53°C (IGF2)/58°C (microRNA) for 10 s, and 72°C for 20 s. Relative expressions were calculated by normalization to β-actin values using the 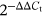 method.
method.
2.10 Western blot
To determine the protein expression of IGF2, the protein was extracted from hippocampus tissues from each group using RIPA lysis buffer (Beyotime) containing 2% of a cocktail pill. Afterwards, the protein concentration was quantified using the BCA protein assay kit (Beyotime Institute). Then, the protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 60 V for 30 min and then at 120 V for 1.2 h. Afterwards, the protein bands were transferred to polyvinylidene fluoride membranes (Millipore). After blocking by TBST consisting of 5% evaporated milk at room temperature for 2 h, the membrane was rinsed and incubated with the IGF2 antibody (1:500, rabbit; BIOSS) and β-actin antibodies (1:1000, mouse; Abbkine) for 18h at 4°C. Thereafter, the membrane was incubated with the secondary antibody (1:5000, goat anti-rabbit IgG and goat anti-mouse IgG; ZSGB-BIO) for 2h. Finally, after rinsing in TBST, the immunoreactive protein was visualized using Alpha Innotech (Bio-Rad Laboratories) with ECL and quantified by densitometry.
2.11 Immunofluorescence staining
To determine the effect of silencing IGF2 and overexpression/down-expression of miRNA-483 on the growth of hippocampal neurons in vitro, immunofluorescence analysis was performed. Briefly, the hippocampal neurons were grown on glass coverslips; thereafter, the slices were directly washed with phosphate-buffered saline (PBS) and blocked with 5% goat serum for 30 min at 37°C. Then, the slices were incubated with primary antibodies of Neuronal Class III β-Tubulin (Tuj1) (1:200, rabbit; Abclonal), IGF2 (1:200, mouse; Abclonal), and 2% goat serum as a negative control at 4°C overnight and rinsed with PBS three times. Subsequently, the slices were incubated with a species-specific secondary antibody (DyLight 488-labeled goat-anti-mouse, 1:200 [Abbkine]; DyLight 594-labeled goat-anti-rabbit, 1:200 [Abbkine]) for 1 h at 37°C. Finally, the slices were observed and obtained using a high-content cell imaging system (Leica). The cell numbers and average length of neuron axons were calculated using Image-Pro Plus 6.0 software (MediaCybernetics).
2.12 Statistical analysis
All values were expressed as the mean ± standard deviation (SD) and analyzed using SPSS19.0. Statistical significance of the data was calculated by one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. In the Morris water maze test, measures of learning during the training trial were averaged for each day; the data were analyzed using repeated-measures two-way ANOVA using Dunnett's multiple comparisons test. Differences were considered significant at p < 0.05.
All abbreviations used in this paper are shown in Table 1.
| Full | Abbreviations |
|---|---|
| Insulin-like growth factor | IGF |
| Insulin-like growth factor 2 | IGF2 |
| Gene Ontology | GO |
| Quantitative real-time polymerase chain reaction | qRT-PCR |
| Sprague–Dawley | SD |
| Small interfering RNAs | siRNAs |
3 RESULTS
3.1 Moderate-intensity treadmill training could enhance cognitive function
The Morris water maze test was used to investigate hippocampus-dependent spatial learning and memory ability of rats at 24 h after treadmill exercise. The training trials for spatial learning were conducted for three consecutive days. The results showed a significant decrease in the time of latency to target among the control, low-intensity, and moderate-intensity groups for Days 2 and 3 compared to Day 1 (Figure 1A, p < 0.05). Analyses of escape latency for slight intensity training revealed no significant difference compared to the control group on Days 1, 2, and 3 (Figure 1A, p > 0.05). Besides, the results revealed a significantly shorter time to find the platform for high-intensity training group than that of the control group on Day 3 (Figure 1A, p < 0.05). The rats in the moderate-intensity group performed better than those in the control group in finding the target platform, with a better learning performance on Days 1, 2, and 3 (Figure 1A, p < 0.05), indicating that moderate-intensity treadmill training improved the spatial learning ability of rats. In addition, to assess whether the rats had learned the position of the escape platform, a probe trial for spatial memory was conducted on the fourth day. The time taken for crossing over the original location of the quadrant in rats receiving moderate-intensity training was longer compared with the opposite quadrant and control rats with no treadmill training (Figure 1B, p < 0.05). Similarly, the rats subjected to moderate-intensity training also exhibited more distance in the target quatrant, indicating better spatial memory (Figure 1C, p < 0.05). Meanwhile, the time taken for crossing over the target platform location in the moderate-intensity group was also remarkably increased compared with the opposite quadrant and the control group (Figure 1D, p < 0.05), indicating that moderate-intensity treadmill exercise can improve spatial learning and memory in rats.
3.2 IGF2 expression was increased following exercise-induced enhancement of spatial learning and memory
To investigate the underlying molecular mechanism linked to improved learning and memory ability after treadmill training, gene chip analysis was performed and heatmaps were obtained of the low-, moderate-, and high-intensity groups in the hippocampus compared with the control group of rats; there was little difference in the different intensities of exercise (Figure 2A). After performing the intersection analysis of these differentially expressed genes, we found that 587 genes were upregulated in the hippocampus (Figure 2B). In addition, the intersection of differently expressed genes in the four groups by GO analysis revealed that 431 genes are involved in biological processes (Figure 2C). In particular, there were six differently expressed genes for the critical biological process involved in learning and memory among the control, low-, moderate-, and high-intensity groups (Figure 2D). Finally, combined with gene chip and GO analyses, six genes were screened out, which were associated with memory function, including IGF2, Ptgs2, Grin2b, Grin2a, Cyp7b1, and Slc24a2, and we found that the fold change of IGF2 was the highest (Figure 2E).

3.3 The expression of IGF2 mRNA and protein was significantly increased in the hippocampus
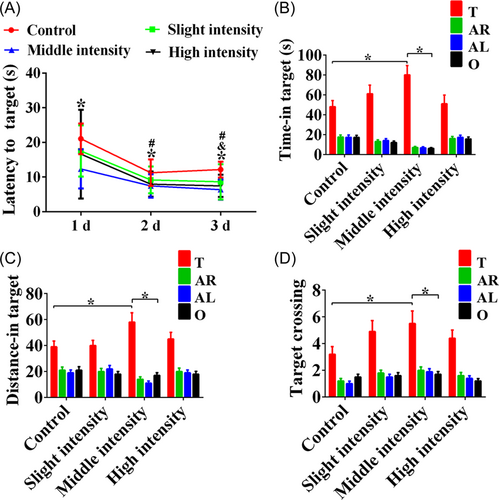
To confirm the expression of IGF2 after moderate-intensity treadmill training, qRT-PCR was performed and it showed that the expression of the IGF2 was notably increased in the rats that received moderate-intensity training compared with the rats in the control and low-intensity training groups (Figure 3A, p < 0.05). Additionally, western blot results revealed a similar increase in the expression of IGF2 protein in the moderate-intensity training group compared with the control group (Figure 3B, p < 0.05).
3.4 miRNA-483 was screened out and showed low expression in moderate-intensity treadmill exercise
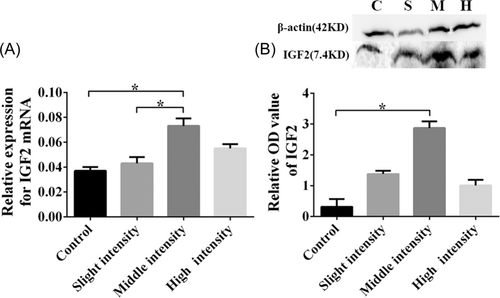
To further detect the regulatory mechanisms of IGF2, 45 miRNAs were predicted as the targets of IGF2 by TargetScan_7.1 and 22 miRNAs were predicted as the targets of IGF2 by miRDB. Two miRNAs (miRNA-483 and miRNA-665) were found from two databases (Figure 4A). Moreover, according to the prediction information of the TargetScan database, the context++ score of miR-483 was lower and the context++ score percentile was higher, which indicated that the probability of it being a real target was higher (Figure 4A). Therefore, we selected microRNA-483 as a regulatory mechanism molecule of IGF2. Consequently, the expression of miRNA-483 was further validated by qRT-PCR, and we found that the expression of miRNA-483 mRNA was obviously lower in rats subjected to moderate- and high-intensity training (Figure 4B, p < 0.05) as compared to the control group, especially in the moderate-intensity training group (Figure 4B, p < 0.01).
3.5 miRNA-483 and IGF2-siRNA were successfully transfected
To further verify the effect of IGF2 and miRNA-483 on hippocampal neurons after treadmill training, we estimated the effects of the candidate sequences F1-F3 on the silencing production of IGF2. The results indicated that the neurons adhered after 1 day and matured after 7 days (Figure 5A). At the same time, it was observed under an inverted fluorescence microscope that the red fluorescent marker was expressed in the neurons of IGF2, and expressed in the cytoplasm and neurites of miRNA-483 (Figure 5B), indicating that the production of IGF2-siRNA and miRNA-483 was efficiently transfected into the hippocampal neurons. Remarkably, a significant decrease in the expression of IGF2 was observed in siRNA-F1, F2, and F3 compared with the NC group, especially in siRNA-F3, by qRT-PCR (Figure 5C, p < 0.05).

3.6 IGF2 could promote the growth of hippocampal neurons and may be involved in miRNA-483 inhibition
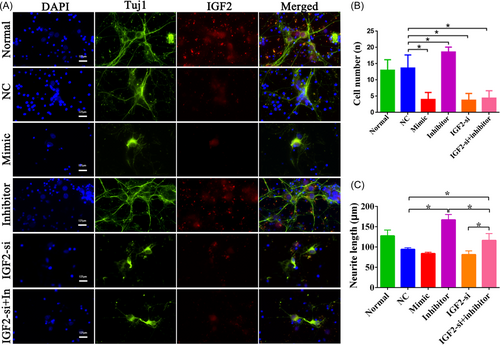
Immunofluorescence double staining of Tuj1 and IGF2 was used to determine the growth of hippocampal neurons after treatment with NC, the miRNA-483-mimic, the miRNA-483-inhibitor, and IGF2-si (Figure 6A). Additionally, for the rescue experiment, we transfected the miRNA-483-inhibitor-treated cells with IGF2-si (Figure 6A) to investigate whether IGF2 regulates cell growth by modulating miRNA-483. As a result, the miRNA-483-inhibitor improved neuron growth, whereas the miRNA-483-mimic and IGF2-si caused neuronal damage. Moreover, the miRNA-483-inhibitor could markedly counteract with IGF2-si and induce neuron deterioration (Figure 6A). Quantitative analysis revealed that the number and length of cells in the miRNA-483-inhibitor were notably increased compared with the NC groups (Figure 6B,C, p < 0.05). Conversely, in the miRNA-483-mimic and IGF2-si as well as IGF2-si + inhibitor groups, cell numbers were lower compared with the NC group (Figure 6B, p < 0.05). Furthermore, we also found that the damaged neurons induced by IGF2-si were obviously rescued by the miRNA-483-inhibitor (Figure 6C, p < 0.05).
4 DISCUSSION
In this study, we found that moderate-intensity treadmill training could obviously enhance learning and memory function, as shown by the Morris water maze test. Subsequently, we have revealed that the expression of IGF2 was remarkably upregulated in the hippocampus after moderate-intensity training. Through TargetScan_7.1 and miRDB databases, miRNA-483 was screened as the target gene of IGF2 and was found to have low expression in the moderate-intensity treadmill exercise group compared with the control group. Furthermore, in vitro studies indicated that the damaged hippocampal neurons induced by silencing of IGF2 were obviously rescued with the miRNA-483-inhibitor, and promoted neurite outgrowth. These results revealed that treadmill training increased the learning and memory ability in rats, which may be associated with an increase in IGF2 and downregulation of miRNA-483.
4.1 Treadmill training enhances cognitive functions by upregulating IGF2
Our data revealed that following moderate-intensity treadmill training, rats performed remarkably better in terms of learning and memory ability. Previous studies suggested that exercise increases neurogenesis and neurotrophic factors, and ameliorates hippocampal neuronal activities.16-18 Additionally, moderate-intensity exercise could regulate the brain functions for learning and memory ability by modulating central and peripheral factors.19 It is reported that moderate-intensity aerobic exercise could promote the vitality of hippocampal neurons20, 21 and attenuate the increase in the nitric oxide (NO) concentration. It has been proven that NO is involved in many stress responses in the hippocampus, such as neuronal injury in the hippocampal CA1 region,22 whereas over-load exercise can lead to cell apoptosis and even necrosis, resulting in toxicity. Besides, improper load management is an important risk factor for acute illness and overtraining syndrome.23 Therefore, in the present study, moderate-intensity training (24 m/min for 30 min) was conducted to explore the neuroprotective effects of treadmill exercise on the hippocampus in rats. Moreover, through conjoint analysis of gene chip, GO prediction, and qRT-PCR verification, we have found that IGF2 was evidently upregulated in the hippocampus after moderate-intensity exercise, which is consistent with studies indicating that IGF2 levels are extensively elevated in the central nervous system and are particularly concentrated in the hippocampus.9, 24 Moreover, it was proven that IGF2 improved the cognitive functions by inducing synapse formation and maturation, and improving hippocampal-dependent memory ability.25, 26 Although animal disease models were not applied in the study, our data pointed out that treadmill training significantly increased learning and memory abilities by IGF2 upregulation.
4.2 IGF2 enhancement of cognitive functions after moderate-intensity treadmill training is associated with inhibition of miRNA-483
To investigate the interaction between brain function, exercise, and IGF2 upregulation, TargetScan_7.1, exploration of miRDB databases, and qRT-PCR verification were performed. We found that miRNA-483 was a target of IGF2 in the hippocampus and showed low expression in rats that received moderate-intensity training. Accumulating evidence showed that microRNAs could dynamically regulate synaptic plasticity, function, and morphology,27 resulting in lower learning and memory abilities in the aging process, while aerobic exercise could reverse micro-RNA-induced cognitive impairment.28, 29 It is known that in the second intron of the IGF2 gene on chromosome 11p15, human miRNA-483 is considered to show co-expression with its IGF2 host gene.30 miRNA-483 plays different roles in a variety of cancer types. Recent studies have shown that miRNA-483 is involved in the proliferation of tumors by upregulating the expression of IGF2 in nephroblastoma and colon cancer,31, 32 whereas miRNA-483 was not reported in the IGF2-related cognitive function. Our data revealed that silencing IGF2 inhibited the neurite growth in the hippocampus of rats. In contrast, the miRNA-483-inhibitor ameliorated the silencing of IGF2-induced hippocampal neuron impairment and promoted neurite outgrowth.
5 CONCLUSION
Moderate-intensity treadmill training could enhance learning and memory ability. The underlying mechanism might be associated with the upregulation of IGF2 and the downregulation of miRNA-483. These results provide an understanding of exercise-induced regulatory factors and their effect on neuron activity and present a reliable theoretical basis for improving cognitive function after moderate-intensity exercise.
AUTHORS CONTRIBUTIONS
All authors are responsible for the accuracy of data in this study and approved the final version of the manuscript. Xiu-Juan Dong designed and supervised the whole study. Lu-Lu Xue and Jun-Jie Chen performed all the experiments and the data collection and analysis. Mohammed Al-hawwas provided guidance on experiments and revision of the manuscript.
ACKNOWLEDGMENT
This study was supported by the Program Innovative Research Team in Science and Technology in Yunnan province (2017HC007).
CONFLICT OF INTEREST
Professor Mohammed Al-hawwas is a member of the Ibrain Journal editorial board and is not involved in the peer-review process of this article. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Animal care and all experimental protocols were carried out according to the guidelines of the Institutional Medical Experimental Animal Care Committee of Kunming Medical University with the approval number: SYXK 2015-0002.
Open Research
DATA AVAILABILITY STATEMENT
The data generated and analyzed in the present study can be obtained from the corresponding author.




