Sampling soil water along the pF curve for δ2H and δ18O analysis
Abstract
Soil water stable isotopes are widely used across disciplines (e.g., hydrology, ecology, soil science, and biogeochemistry). However, the full potential of stables isotopes as a tool for characterizing the origin, flow path, transport processes and residence times of water in different eco-, hydro-, and geological compartments has not yet been exploited. This is mainly due to the large variety of different methods for pore water extraction. While recent work has shown that matric potential affects the equilibrium fractionation, little work has examined how different water retention characteristics might affect the sampled water isotopic composition. Here, we present a simple laboratory experiment with two well-studied standard soils differing in their physico-chemical properties (e.g., clayey loam and silty sand). Samples were sieved, oven-dried and spiked with water of known isotopic composition to full saturation. For investigating the effect of water retention characteristics on the extracted water isotopic composition, we used pressure extractors to sample isotopically labelled soil water along the pF curve. After pressure extraction, we further extracted the soil samples via cryogenic vacuum extraction. The null hypothesis guiding our work was that water held at different tensions shows the same isotopic composition. Our results showed that the sampled soil water differed isotopically from the introduced isotopic label over time and sequentially along the pF curve. Our and previous studies suggest caution in interpreting isotope results of extracted soil water and a need to better characterize processes that govern isotope fractionation with respect to soil water retention characteristics. In the future, knowledge about soil water retention characteristics with respect to soil water isotopic composition could be applied to predict soil water fractionation effects under natural and non-stationary conditions. In this regard, isotope retention characteristics as an analog to water retention characteristics have been proposed as a way forward since matric potential affects the equilibrium fractionation between the bound water and the water vapour.
1 INTRODUCTION
Stable isotopes of water (2H and 18O) are valuable natural tracers to study soil water movement and mixing processes in the vadose zone. Precise measurements of soil water content and its isotopic composition at different depths are key to reliably quantify plant water uptake as well as the partitioning of evapotranspiration into evaporation and transpiration (e.g., Mahindawansha et al., 2018; Rothfuss et al., 2010). The storage and interaction of different soil water compartments (mobile vs. tightly bound water) in the vadose zone is affected by a variety of soil properties (Gaj et al., 2019); for instance, the interactions between minerals, organic matter, and microorganisms (Pronk et al., 2017) or the presence of macropores (Sprenger et al., 2019). For isotopic applications, these different properties can affect the reliable determination of the isotopic composition of soil waters.
It has been known for many years that bound soil water has a distinct isotopic identity compared to that of mobile water (Araguás-Araguás, Froehlich, & Rozanski, 2000). Many studies have compared the various techniques available for sampling soil water held at different tensions. For example, mobile water sampled by suction cups, has a different isotopic composition than soil water extracted by cryogenic vacuum extraction, which is known to be a “brute force technique” (Brooks, Barnard, Coulombe, & McDonnell, 2010; Figueroa-Johnson, Tindall, & Friedel, 2007; Orlowski, Breuer, & McDonnell, 2016; Zhao, Tang, Zhao, Wang, & Tang, 2013). Further, the various existing soil water extraction methods for isotope analysis can be affected by soil water content (Hendry, Schmeling, Wassenaar, Barbour, & Pratt, 2015; Newberry, Prechsl, Pace, & Kahmen, 2017; Wassenaar, Hendry, Chostner, & Lis, 2008), texture (Koeniger, Marshall, Link, & Mulch, 2011; Orlowski, Pratt, & McDonnell, 2016; West, Bowen, Cerling, & Ehleringer, 2006), clay mineral composition (Adams et al., 2019; Gaj, Kaufhold, Koeniger, et al., 2017; Oerter et al., 2014), carbonate content (Meißner, Köhler, Schwendenmann, Hölscher, & Dyckmans, 2014), organic matter (Orlowski, Breuer, et al., 2016) and the different pore spaces that may or may not be extracted via the different approaches (Kübert et al., 2020; Orlowski, Pratt, et al., 2019). Recently, Bowers, Mercer, Pleasants, and Williams (2020) stressed the fact that mechanisms controlling the isotopic composition and exchange between the mobile and more tightly bound soil water pools in natural ecosystems are largely unexplored. This incomplete understanding leads to complications when interpreting soil water contributions to plant water uptake under different moisture conditions as well as an accurate partitioning of evapotranspiration.
1.1 Why are water retention characteristics important?
The soil water retention curve (SWRC) describes the relationship between soil water content and soil water potential (Vogel & Cislerova, 1988). The SWRC not only provides detailed knowledge on the physical and hydraulic properties of soil, but also affects soil infiltration, redistribution, root water uptake, evaporation and microbial activity (Ciocca, Lunati, & Parlange, 2014; Pan, Hou, Liu, & Tan, 2019; Quade et al., 2018; Solone, Bittelli, Tomei, & Morari, 2012). The SWRC is considered one of the most important soil hydraulic properties (Hillel, 2004; Rawls & Brakensiek, 1989).
We know that soil physical properties are closely linked to the pore size distribution expressed by soil texture. However, we do not know much about how interactions between the soil matrix, water vapour and liquid water exchange within the subsurface are affecting the soil water isotopic composition. It is not necessarily correct to assume that soil water and vapour in vadose zones have the same isotopic compositions as equilibrated bulk water and vapour, respectively, which is underlined by a study of Lin, Horita, and Abe (2018). Evaporation from the soil surface, and thus the underlying soil water vapour, play an important role in the hydrologic cycle and affect the soil water (vapour) isotopic composition (Brooks, 2015; Soderberg, Good, Wang, & Caylor, 2012). Most often, the Craig-Gordon model is applied to estimate equilibrium and kinetic isotopic fractionation during evaporation (Craig & Gordon, 1965; Horita, Rozanski, & Cohen, 2008). However, significant deviations between measured and modelled values (from Craig-Gordon) of soil evaporate isotopic composition can occur (Braud, Bariac, & Vauclin, 2009; Haverd et al., 2011; Rothfuss et al., 2010). Soderberg et al. (2012) recommended to include the soil water potential effect on kinetic fractionation during soil water evaporation in the Craig-Gordon model and Quade et al. (2018) call for further investigations of the temporal dynamics of kinetic fractionation factors. This parameter can also be calculated from soil water content using an appropriate SWRC. It is however difficult to determine the exact behaviour of the SWRC above (or below) the residual water content, as measurements are time consuming and data are scarce (Ciocca et al., 2014). Moreover, for dry conditions occurring preferentially at shallow soil depths where evaporation into the atmosphere takes place, the application of stable water isotope techniques with regard to the dry end of the water retention curve (especially around the wilting point) is largely unknown but nevertheless important (Gaj et al., 2019). Particularly under unsaturated conditions and when clay contents are high, the tightly bound soil water pool becomes more relevant, especially in the context of plant water uptake (Adams et al., 2019; Bowers et al., 2020). Gaj et al. (2019) pointed out that not only the water content but the soil tension is the dominant controlling factor on the isotopic equilibrium fractionation factor. Hence, a texture with high clay fraction and a water content of 10% will show a similar effect as a sandy texture at 1% water content. The authors further showed that the wettability of soil grains expressed by the contact angle between the water drop and the soil grain affects the equilibrium condition of bound water and water vapour.
Few studies have looked into the relevance of soil water held across different sized pores and water adsorbed on various soil materials with respect to their isotopic composition. Thus, our current knowledge on potential isotope fractionation effects is very limited and inconclusive as highlighted by Lin et al. (2018). This is problematic since much research is linking the soil water isotopic composition to that of plant water sources or atmospheric water vapour, which is further used for modelling processes within the plant-water-atmosphere continuum. An assessment of soil water isotopes across various pore sizes and tensions is therefore needed to explore whether plants take up matrix water that is incompletely mixed with isotopically distinct mobile soil water. Thus, the objective of our lab experiments was to investigate the effect of water retention characteristics on the water isotopic composition of soil pore water. The null hypotheses guiding our work was that soil water sampled along the pF curve (experiment 1) and sequentially over a period of 7 days under a 15 bar pressure (experiment 2) shows the same isotopic composition and does not differ isotopically from the introduced isotopic label. Additionally, we checked whether an isotopic exchange between the ceramic plate water of the pressure extractors and the sequentially extracted soil water occurred (experiment 2) since the ceramic plate could potentially absorb and release water.
2 METHODOLOGY
2.1 Experimental design
For our experiment, we chose two well-studied (see e.g., Orlowski, Breuer, et al., 2018; Orlowski, Pratt, et al., 2016) physico-chemically different soil types – a clayey loam (LUFA 2.4) and a silty sand (LUFA 2.1) – from the German State Research Institute for Agriculture (LUFA Speyer, 2015). For a detailed description of the soil properties, the reader is referred to Orlowski et al. (2018) and Wilske et al. (2014). Soils were sieved (2 mm) and oven-dried (48 hr, 200°C). We chose two experimental approaches to test the effect of different pressure levels on the extracted soil water isotopic composition. Experiment 1 aimed at sampling soil water along the pF curve (at different pressure levels). During experiment 2, soil water was sampled sequentially over 7 days only at the highest pressure level (15 bar), which was kept constant throughout the experiment. We further tested whether there is an isotopic exchange between the ceramic plate water of the extractor and the water to be extracted from the soil samples sitting on these ceramic plates during extraction. Both experiments are based on spiking approaches with two different waters of known isotopic composition (see e.g., Orlowski, Breuer, et al., 2018; Orlowski, Pratt, et al., 2016). During both experiments, the lab temperature was controlled at 20°C with no diurnal fluctuations to avoid potential impacts of evaporation and condensation.
For the soil water extractions and the determination of water retention curves, we used pressure extractors (Soilmoisture Equipment Corp., USA; Figure 1). For errors associated with this method, the reader is referred to Solone et al. (2012). Each pressure extractor cell contains a porous ceramic plate covered on one side by a thin Neoprene diaphragm sealed to the edges of the plate (Figure 1). Soil water is extracted via air pressure under controlled conditions. Once air pressure inside the pressure extractor cell is raised above atmospheric pressure, the higher pressure inside the extractor forces excess water through the microscopic pores in the ceramic plate. An internal screen between the ceramic plate and a diaphragm further allows the extracted water to exit the pressure plate cell via an outlet tube running through the plate, which connects this water passage. However, the high pressure air will not flow through the pores in the ceramic plate since the pores are filled with water and the surface tension of the water, at the gas–liquid interface at each of the pores, supports the pressure much the same as a flexible rubber diaphragm (Soilmoisture, 2008). Thus, the pressurized air will not isotopically interact with the soil water. However, the high pressure air intrudes into the soil pores and expels water first from the large pores, and with increasing pressure gradually from the fine pores (see Table A1). In a pre-test, we determined the equilibrium time for each pressure level at which the mass of the soil sample remains unchanged (state of equilibrium) and the next pressure level can be applied. In other words, when soil water is in equilibrium with the applied pressure, flow ceases, and the applied pressure is equal to the soil matric suction (Gee, Ward, Zhang, Campbell, & Mathison, 2002). Nevertheless, there is little guidance for determining when equilibrium is actually achieved for a given soil type (Gee et al., 2002). As known for this method, the time required to reach a state of equilibrium increases with increasing pressure and is different for different soil types (e.g., Wang, Kong, and Zang (2015). Low ceramic plate conductance in combination with declining soil hydraulic conductivities at high pressure levels strongly affects equilibrium times, which theoretically may extend to months or years (Gee et al., 2002). We applied equilibrium times of up to 16 days for the highest pressure level (pF 4.2) for experiment 1. For experiment 2, a constant pressure of 15 bar was applied for 7 days.
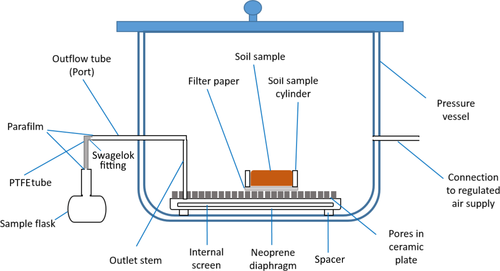
2.1.1 Experiment 1
The null hypothesis guiding experiment 1 was that soil water sampled along the pF curve has the same isotopic composition over different pF values, which also does not differ from the isotopic label used for spiking the soils.
For rehydration, disturbed oven-dried soil was packed (silty sand: 105 g; clayey loam 121 g) into 100 ml open-bottom, stainless steel cylinders (N = 4 for each pF level). The bottom of the cylinders were covered with a sterile polypropylene mesh to allow for water uptake but to prevent a loss of soil material. Cylinders were placed in a water bath, filled with distilled water (DIW) of known isotopic composition (δ2H: −58.4 ± 0.2‰, δ18O: −8.6 ± 0.1‰). The water bath was covered and sealed with a gas-tight lid to prevent evaporation. Soils were left to saturate for two days. The ceramic plates of the pressure extractors were likewise placed in a water bath containing the same type of water and were also left to saturate for two days (following the technical description by Soilmoisture, 2008). Thielemann, Gerjets, and Dyckmans (2019) showed that for spiking experiments with water of known isotopic composition, 94% of the isotopic change is already manifested after 1 day of equilibration. Afterwards, cylinders including the saturated soils were placed inside the pressure extractors and increasing pressure levels were applied (pF: 1.4–4.2) (Table A1). The water being extracted from the soil samples at each pressure level was directed via an outlet tube (consisting of Swagelok® fittings; Swagelok Company, Solon, OH, US) into a sampling flask (Figure 1). Before applying the next pressure level, ports and tubing were dried with compressed air and a new sampling flask was attached to the outlet tube of the extractor. After each pressure level, the amount of sampled water was determined and soil samples were weighed. After two pressure stages (pF: 3 and 4.2), eight samples of each soil type were transferred into glass vials for cryogenic vacuum extraction to remove any remaining water. Cryogenic vacuum extraction was performed using the facility described in Orlowski, Frede, Brüggemann, and Breuer (2013). Following Orlowski et al. (2018), clayey loam samples were extracted for 240 min and silty sand soils for 45 min at a temperature of 98°C and a baseline pressure of 0.1 Pa. After cryogenic extraction, soils were oven-dried (24 hr, 105°C) and weighed again with no significant additional weight loss indicating that the water extraction process was complete. For temperature-related isotope effects with regard to cryogenic vacuum extraction the reader is referred to Gaj, Kaufhold, Koeniger, et al. (2017); Gaj, Kaufhold, and McDonnell (2017); Orlowski et al., 2013. A critical assessment about cryogenically related isotope effects is given in Orlowski et al. (2013, 2016).
For isotope analysis, the extracted soil waters were filtered on 0.45 μm disk filters, transferred to 2 mL amber glass vials covered by solid silicone septa, and tightly sealed with Parafilm®.
2.1.2 Experiment 2
With experiment 2, we tested whether soil water collected sequentially over a period of 7 days under the highest pressure level (15 bar, kept constant over the duration of the experiment) would differ isotopically from the water used for spiking the soil samples. Additionally, we checked whether there is an isotopic exchange between the ceramic plate water and the water to be extracted from the saturated soil samples. Thus, a different rehydration and extraction approach was used. This time, soil samples (N = 30 per soil type, 2 per time step) were rehydrated in the same manner as for experiment 1 but with “Lauretana” water (LW, commercial sparkling water; δ2H: −64.6 ± 0.6‰, δ18O: −9.8 ± 0.1‰) and the ceramic plates of the pressure extractors were rehydrated with the same DIW as in experiment 1. Both waters differ isotopically. Mean water content of the clayey loam samples after rehydration was 44.2 ± 1.2 Vol% and for the silty sand samples 25.5 ± 0.6 Vol%. For water extraction, the highest pressure level (15 bar) was directly applied to the pressure extractors and a sequential soil water extraction was performed. Therefore, water was sampled after 10–80 min (every 10 min), 105, 115, 125, 150, 180, 240 min, 1, 2, 5 and 7 days. After pressure extraction, cryogenic vacuum extraction was performed on all soil samples (as in experiment 1). We further crushed the ceramic plates used for this experiment and cryogenically extracted the bound water (in the same manner as the clayey loam). Pre- and post-extraction (pressure extractor and cryogenic vacuum extraction) weights and oven-drying weights of soil samples were determined. There was no significant additional weight loss after oven-drying of the cryogenically extracted soil samples (mean ± SD weight loss for the clayey loam and silty sand samples, respectively: 0.07 ± 0.03 g, 0.03 ± 0.02 g).
2.2 Isotope analysis
δ2H and δ18O compositions of extracted soil water samples were measured at the Institute for Landscape Ecology and Resources Management (Justus Liebig University Giessen, DE) on a L2130-i isotope analyzer (Picarro Inc., US). The accuracy of the isotope analyses was ±0.2/±0.8‰ for δ18O/δ2H (determined via repeated measurements of the same sample). All isotope ratios are reported in per mil (‰) relative to Vienna Standard Mean OceanWater (VSMOW) (δ2H or δ18O = (Rsample/Rstandard−1) × 1000 ‰), where R is the isotope ratio of the sample and the known reference (i.e., VSMOW) (Craig, 1961). In-house standards, were run as samples to allow the results to be reported against VSMOW (Nelson, 2000). Isotope data of water extracts were checked for spectral interferences (caused by potentially co-extracted organics such as methanol or ethanol) using ChemCorrect™. This software attempts to identify contaminations in water samples both through fitting to a known library of spectral features, and by examining changes in baseline, slope, line-broadening and residual noise of the spectra (A. G. West, Goldsmith, Matimati, & Dawson, 2011). Further information about this approach is available from the manufacturer (Picarro, 2010). No sample was found to be contaminated with organics.
2.3 Statistical analysis and evaluation
We used R for statistical analyses (R version 3.6.3; R Core Team (2014)). All data were tested for normality using the Shapiro–Wilk test. Homoscedasticity was tested using either the Levene's test for normally distributed data or the Fligner–Killeen test for non-normally distributed data. Cluster analysis based on the furthest neighbour approach using the Euclidean distance as measure was performed in order to identify outliers. This method is more robust for non-normally distributed data. Depending on the type of data (normally distributed and homoscedastic), either Kruskal–Wallis rank sum tests or Analyses of Variances (ANOVAs) were applied and posthoc tests (e.g., Tukey-HSD tests (equality of variances) or Dunnett-T3 tests (non-equality of variances)) were run to determine which groups were significantly different (p ≤ .05). Dual isotope (δ18O vs. δ2H) graphical representation was used to compare water extracts from different pF levels (experiment 1) and times of extraction (experiment 2). Statistically significant (p ≤ .05) linear regressions were added to dual isotope plots as well as the Global Meteoric Water Line (GMWL: δ2H = 8.2 × δ18O + 11.3‰, as defined by Rozanski et al., 1993).
3 RESULTS AND DISCUSSION
3.1 SWRC
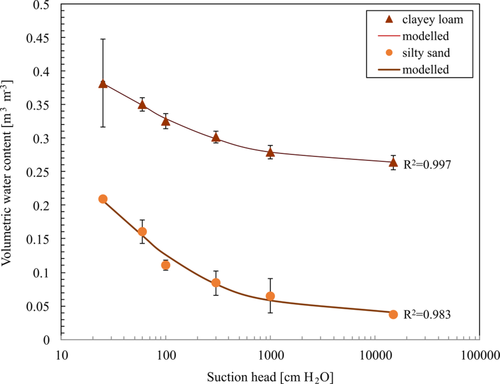
Figure 2 shows the modelled SWRCs for the measured means of the silty sand and clayey loam used in our study. The agreement between the fitted curves and the measured data was very good (R2 = 1.0 for the clayey loam and 0.98 for the silty sand, respectively). The silty sand's SWRC was steeper and declined faster than the SWRC of the clayey loam. A sudden steepening of the slope indicates a distinct air-entry tension value, common for coarse soils (Wassar, Gandolfi, Rienzner, Chiaradia, & Bernardoni, 2016). The SWRC for the clayey loam showed a very high water retention at a suction head of 15,000 cm H2O. This water, held in the smallest pore spaces, is considered immobile or residual water. Soils with a high clay content have a larger bound/residual water pool (Adams et al., 2019). The residual water content for the clayey loam was 26.4 ± 1.1 Vol%, whereas the silty sand's residual water content was 3.7 ± 0.4 Vol%. Differences in SWRCs are dependent on various soil properties such as bulk density, organic carbon, soil texture and aggregate size (Lipiec et al., 2007). Sandy soils involve mainly capillary binding, and therefore release most of the water at higher potentials, while clayey soils, with adhesive and osmotic binding, release water at lower (more negative) potentials (Binkley & Fisher, 2012). Cryogenic water extraction following pF 3 resulted in a mean water content for the silty sand of 4.8 ± 2.6 Vol% and for the clayey loam of 28.7 ± 0.9 Vol% indicating that water extraction was not fully complete when compared to the values at the end of the pressure extraction. Cryogenic water extraction following pF 4.2 led to mean volumetric water contents of 3.8 ± 1. Vol% and 26.1 ± 1.3%, respectively, which is comparable to the residual water content obtained via pressure plate extraction.
3.2 pF effects on isotopic composition
When now comparing the stable isotope values in dual isotope space from the sampling along the pF curve, most of the soil water isotope values plotted slightly to the right of the Global Meteoric Water Line (GMWL) (Figure 3), whereas the introduced isotopic label (DIW) plotted on the GMWL. In general, the pF curve extracts from the silty sand and the clayey loam showed a similar isotopic composition and extraction behaviour. With increasing pF values, the δ2H and δ18O composition tended to get heavier and moved up and slightly to the right of the GMWL (apart from pF 4.2). Only extracts from pF 4.2 showed a depletion in heavy isotopes for both soil types in comparison to the introduced label (DIW). This depletion was more pronounced for the silty sand extracts. When looking at the cryogenic soil extracts, the clayey loam cryo extracts taken after pF 4.2 did not show significant differences to the silty sand's extracts at pF 4.2, the silty sand's cryo extracts taken after pF 4.2 and the clayey loam cryo extracts taken after pF 3. However, they differed statistically significantly from all other pressure extracts. The silty sand's cryo extracts taken after pF 3 showed the largest mean difference to the DIW in 18O-direction (3.3‰) and + 12.4‰ in 2H-direction, whereas the cryo soil extracts taken after pF 4.2 and the pressure extracts from pF 4.2 differed from the DIW mainly in negative 2H-direction by −9.35 and −9.84‰, respectively. For the clayey loam, the pF 1.8 pressure extract showed the largest mean difference to the DIW (+1.19‰ for δ18O and + 3.62‰ for δ2H). For both soil types, the extracts from pF 1.4 showed the smallest mean difference to the DIW in 18O-direction (−0.03‰ for the silty sand and +0.06‰ for the clayey loam). For δ2H, the mean difference to the DIW was within the range of the measurement accuracy of the isotope analysis (−0.12‰ for the silty sand and +0.40‰ for the clayey loam, respectively).
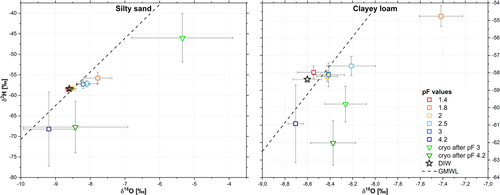
In general, the isotope values for the silty sand showed a much larger SD than the values of the clayey loam extracts (Figure 3, note the different x- and y-axis scales). For the silty sand, the cryo soil extracts from pF 3 were enriched in comparison to the DIW, whereas, the cryo soil extracts from pF 4.2 were depleted in heavy isotopes. Both differed significantly between each other (p = .006). Such depletion could be an artefact of the cryogenic extraction and has been observed during water recovery tests of the same soil types in a dataset by Orlowski et al. (2013) and by others (e.g., Adams et al., 2019). However, in the study by Orlowski et al. (2013), the clayey loam cryo soil extracts showed a much larger depletion than the silty sand extracts, which was further dependent on water extraction times. The SDs of the δ18O values of the silty sand ranged between 0.02 and 0.73‰ and for the clayey loam between 0.07 and 0.22‰ (pF 1.4–4.2). Whereas for δ2H, the SDs of the silty sand varied between 0.10 and 9.03 and for the clayey loam between 0.30 and 2.21. Except for the SD of 9.03 (pF 4.2, silty sand), the SDs of the δ2H and δ18O values were either within the range of the measurement accuracy for our isotope analyses (±0.2/±0.8‰ for δ18O/δ2H) or only slightly higher.
Lin et al. (2018) and Lin and Horita (2016) showed that the equilibrium fractionation factor changes if the vapour pressure controls the quantity of water adsorbed on a surface. These surface isotope effects are much stronger for 2H than for 18O (Chen, Auerswald, & Schnyder, 2016; Lin et al., 2018). As such, we found a much higher variation in 2H direction. However, SDs for both isotopes were much larger for the silty sand than for the clayey loam pressure extracts. This is surprising since clay soils usually have a higher surface area (which varies depending on the clay mineral composition) than sandy soils (Marshall, Holmes, & Rose, 1996a) and thus tend to adsorb more water (Marshall, Holmes, & Rose, 1996b). Gaj and McDonnell (2019) found out that soil tension affects the equilibrium fractionation factor for soil tensions above pF 3.1 (1,260 hPa). Their study included the exact same soil types as used in our study. In their study, the tension effect on the equilibrium fractionation factor increased linearly with increasing soil tension. The higher the soil tension, the farther away the isotope values plot from the introduced isotope label in their water recovery study using the water-vapour equilibrium method by Wassenaar et al. (2008). The authors hypothesized that adhesion is the cause of the additional fractionation on the water vapour isotopic composition. In our study, liquid water extracts were compared and not vapour samples. Nevertheless, our work showed that under dry conditions, soil tension is the main driver for isotope fractionation leading to isotopically enriched water extracts (see Figure 3). According to Gaj and McDonnell (2019) immobile water at high soil tension would be depleted in the heavy isotopic species, which was only the case for the pF 4.2 extracts in our study but we did not take samples between pF 3 and 4.2, which could have further underlined this hypothesis. Nevertheless, this does not explain the isotopically enriched values of the silty sand's cryo extracts taken after pF 3 (Figure 3). We therefore suggest that future studies should consider testing soil tension effects for a variety of different soil types and at much higher resolution than in our study. This could be done via a community approach since pF curve experiments are extremely time-consuming. However, if our findings are supported by others in future experiments, this would have important consequences for recent research on plant water uptake studies and interpreting the water isotopic composition of mobile and bulk water in soils (with respect to the tension water is held in the soil). We know that plants can apply high tensions to withdraw soil water, especially under dry conditions and that responses to water stress are species-specifically different (Fotelli, Radoglou, & Constantinidou, 2000). Under dryer conditions the soil water isotopic composition is affected by evaporation causing enriched isotope values. Given our findings, this would imply that isotopically more enriched water would be taken up by plants during dryer conditions (at higher tensions). However, soil properties and the soil's water retention characteristics affect the isotopic composition of this tightly bound water pool. Further, water uptake strategies of plants are highly species-specific and are not only influenced by soil water availability (Larcher, 2003). In contrast, Vargas, Schaffer, Yuhong, and Sternberg (2017) showed that avocado plants might preferentially take up 1H and 16O, leaving the remaining pool of water in the soil enriched. This discrimination was a function of the soil water loss and soil type. Barbeta et al. (2020) recently conducted a drought experiment with Fagus sylvatica where they compared the soil and stem water isotopic compositions. Under drier conditions, the authors observed soil-stem isotopic offsets. They hypothesized that the fraction of adsorbed water increases relative to the total water content under dry conditions. Depending on the balance between the isotopic enrichment caused by evaporation and the depletion caused by the higher fraction of adsorbed water, the isotopic composition of bulk soil water may therefore exhibit different trends with regard to its 2H and 18O composition. This further affects the observed soil-stem isotopic offset (Barbeta et al., 2020). Lu (2016) demonstrated that the fraction of adsorbed water varies highly depending on the soil type and may range from 1.7% VWC in sandy soils to 12.8% VWC in silty clay soils. In the experiment by Barbeta et al. (2020), soil types showed a significant effect on the drying rate. Thus, exploring soil water retention characteristics with regard to plant water availabilities is important when comparing soil and plant water isotopic compositions and drawing assumptions with respect to plant water uptake depths, times and water stress responses.
With regard to estimating plant water uptake depths, mixing models (e.g., the multi-source mixing model SIAR (Parnell et al., 2013) following Rothfuss and Javaux (2017)) are commonly applied to determine the sources' contributions to plant root water uptake. Recently, soil water content was included as an additional factor to improve mixing model results in a study by Mahindawansha et al. (2018). Further including soil water retention characteristics into such mixing models would additionally improve modelling accuracy since plants take up water that is differently bound in the soil. A measurement of soil water retention characteristics in combination with soil water isotopic composition could therefore improve the estimation of plant water uptake depths.
3.3 Isotopic variation over time
With experiment 2, we tested whether soil water collected sequentially over a period of 7 days under the highest pressure level (15 bar, kept constant over the duration of the experiment) would differ isotopically from the water used for spiking the soil samples (LW). We further investigated whether there is an isotopic exchange between the water used for rewetting the ceramic plates (DIW) and the water to be extracted from the saturated soil samples (LW). In theory, the high pressure air, which is applied during the pressure extraction, will not flow through the pores in the ceramic plate and thus, no ceramic plate water should technically be present in the outflow samples of the pressure extractor.
Figure 4 depicts the δ2H and δ18O variation over 7 days for the two soil types when permanently exposed to a constant pressure of 15 bar in the pressure extractor (experiment 2).

In general, the δ2H and δ18O values showed a similar trend. The silty sand extracts' isotope values started off within the range of the DIW values, approached LW values over time and then got progressively heavier again. More specifically, the δ-values started to decrease after 30 min of extraction. From 150 to 7,200 min the δ-values increased until the final sample reached approximately the starting value of −58.7‰ for δ2H. For silty sand's δ18O values, the last sample's δ18O value was by 0.5‰ higher than the starting value of −8.5‰. For silty sand's δ2H values, the point of greatest difference to the starting value was reached after 60 min and it seemed like a plateau was reached which remained constant (within the range of measurement inaccuracy) for the next five values. The greatest SDs for the silty sand isotope values were observed at 7,200 min extraction time for both isotopes. Interestingly, for the silty sand, the heavier isotopes were more dominant in the earlier outflow samples (until 60 min extraction time) and then showed a similar isotopic composition than the LW, which was used for spiking the soil samples.
For the clayey loam, the isotopic variation over time was generally smaller. Interestingly, the δ18O values remained close to the DIW value (−8.6 ± 0.2‰) almost over the entire 7 day extraction period, which was not the case for the silty sand extracts. This is surprising since LW was used for spiking the soil samples and DIW for wetting the ceramic plates of the extractors. The δ2H values remained within the range of the starting value (−58.7‰) and the DIW (−58.4‰), respectively, until they slightly decreased from 60 min onwards. The difference to the LW was smallest after 150 min extraction time. Surprisingly, the δ-values never reached the LW isotope values used for spiking the soil samples, neither for δ2H nor for δ18O. The last sample's δ-values (at 10,080 min) were even more positive than the DIW signature (used for wetting the ceramic plates) and showed the greatest difference to both the DIW and LW.
Figure 5 shows the mean differences over time between the clayey loam and silty sand extracts compared to the DIW's and LW's δ-values. Statistically, mean δ2H and δ18O differences to the LW were significantly different from zero for both soil types (p = .00). However, the mean δ2H and δ18O differences to the DIW were only significantly different for the silty sand (p = .00 for δ18O and δ2H) but nor for the clayey loam (p > .05 for both isotopes).
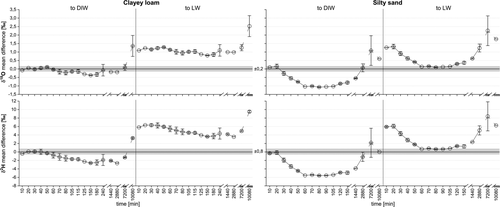
Mean differences for the clayey loam extracts to the DIW ranged from −2.7 to 0.1‰ for δ2H and from −0.4 to 0.1‰ for δ18O, respectively. Mean differences to the LW showed a larger variation: from 3.6 to 6.3‰ for δ2H and from 0.8 to 1.3‰ for δ18O. For the δ2H values of the silty sand extracts, mean differences to the DIW ranged from −5.6 to 0.0‰ and to the LW from 0.7 to 6.3‰. For δ18O, mean differences to the DIW ranged from −1.1 to 0.6‰ and to the LW from 0.1 to 1.8‰. For the δ18O values of the clayey loam extracts, largest differences occurred at extraction times of 80 min (to DIW) and 10,080 min (to LW). Generally, δ-value differences were smaller to DIW than to LW for the clayey loam. This was not the case for the silty sand extracts. Interestingly, silty sand extracts from 60 to 105 min showed the smallest isotopic difference to the introduced LW, which was used for spiking the soil samples.
Thus, our null hypothesis that soil water collected sequentially over a period of 7 days under a 15 bar pressure would not differ isotopically from the introduced isotopic label did not hold true. The time at which the water draining from the pressure extractor did play a crucial role for the recovery of the introduced isotopic label. We further observed that the clayey loam extracts seemed to have interacted with water from the ceramic plates, which was imprinted in the extracted isotopic composition (Figures 4 and 5). However, the spiked label for the ceramic plates could still be recovered via cryogenic vacuum extraction and did not differ statistically significantly from the DIW (see Figure 5). Thus, not as expected, we observed a temporal change (over 7 days) of the isotopic composition of the water extracted at a constant pressure of 15 bar. Our findings suggest that isotopically different fractions of water in the two soil types were released over time. Since the silty sand releases water much faster than the clayey loam (Figure 2), the introduced isotopic label was visible in the extracts after 60 min. However, for the clayey loam, astonishingly the isotopic composition remained almost constant over the 7 days extraction time under 15 bar pressure (Figure 4) and the introduced isotopic label could not be recovered even when considering the given SD. This somehow contradicts the findings of others (Sprenger, Leistert, Gimbel, & Weiler, 2016; Vargas et al., 2017) that tightly bound soil water quickly exchanges with mobile water in soils. Barbeta et al. (2020) demonstrated in a drought experiment with Fagus sylvatica an opposite isotope trend for δ2H and δ18O in soil water when the permanent wilting point had been reached and water potentials fell below -1 MPa. The soils used in their study were coarse sands and a sandy clay loam. They argue that soil evaporative enrichment creates a stronger enrichment in 18O than in 2H and surface isotope effects are much stronger for 2H than for 18O (Chen et al., 2016; Lin et al., 2018). Given this, it is possible that soil water δ18O enriches while soil water δ2H becomes depleted, at least when the soil water balance is dominated by root water uptake. However, the observed differences in the isotopic compositions of stem, root and soil water in the study by Barbeta et al. (2020) were not affected by soil type.
Gaj and McDonnell (2019) hypothesized that the soil water and soil vapour fractionation at high soil tension are driven by the surface properties and the ionic strength of the remaining soil solution. This implies that for example, the interlayer space of clay minerals and mineral surfaces impact the amount and strength at which water is held in the soil (Gaj, Kaufhold, Koeniger, et al. (2017); Oerter et al., 2014). Further, water retention and O and H interactions with the soil matrix are higher for clay soils than for sandy soils (Thielemann et al., 2019). Adams et al. (2019) showed that the retention increased with increasing clay and silt contents. Our silty sand consisted of 92.7% sand and only 4.8% silt but our clayey loam had a clay fraction of 41.9%, which is rich in Vermiculite (43.4%) (see Orlowski, Winkler, McDonnell, & Breuer, 2018). This is a 2:1 clay with a medium shrink-swell capacity but high cation exchange capacity. This has been shown to affect mineral-water interactions and thus cause isotope fractionation effects (Gaj, Kaufhold, Koeniger, et al. (2017); Meißner et al., 2014; Oerter et al., 2014). This might explain why in our experiment 2, the clayey loam water extracts showed a different extraction behaviour to the silty sand when exposed to the highest pressure level (Figures 5 and 6) and the clayey loam soil water most likely interacted with the ceramic plate water. We speculate that the 7 days extraction time might not have been sufficient for recovering the introduced LW from the clayey loam. Our results clearly showed an extraction time-dependent effect on soil water held at 15 bar. This might have implications on how we sample and interpret plant available soil water. If at a certain point in time plants would apply a constant tension to take up soil water, the timing of sampling for studying plant water uptake patterns would be highly relevant; since given our findings at 15 bar pressure, the soil water isotopic composition would change over time. We admit that this is highly speculative but underlines the need for more research on time-variant changes in soil water pools relevant for plant water supply by for example, simultaneously applying high-resolution in-situ isotope measurements at the soil and plant level.

3.4 Isotopic variation over time in dual isotope space
While testing whether soil water collected sequentially over a period of 7 days at 15 bar, we observed different effects on 2H and 18O when compared to the cryogenically extracted samples and the introduced labels (to the soils and ceramic plates). For δ18O, the DIW showed no statistically significant differences to the clayey loam extracts and the cryogenically extracted silty sand. However, for δ2H, DIW was significantly different to all tested subgroups (Figure 6). This might become clearer when comparing the regression lines. The regression line of the cryogenically extracted silty sand is shifted parallel to the right of the regression line from the pressure plate extracts. Interestingly, the LW was statistically similar to the cryogenically extracted water from the ceramic plates for both isotopes. This indicates that the water used for rewetting the ceramic plates might have interacted with the LW used for spiking the soil water samples. Additionally for δ2H there were no significant differences to the cryogenically extracted silty sand samples (p = .41). The clayey loam extracts were statistically similar to the cryogenically extracted silty sand samples for δ18O, which was not true for δ2H (p = .001). For δ18O, the cryogenically extracted clayey loam samples showed no significant differences to the silty sand samples from the pressure extractor but were significantly different to the clayey loam samples (p = .006). The cryogenically extracted silty sand samples on the other hand did not differ significantly from the silty sand samples from the pressure extractor. This did not hold true for δ2H. But the cryogenically extracted silty sand samples were statistically similar to the cryogenically extracted ceramic plates and the cryogenically extracted clayey loam samples for δ2H. Given the statistical differences between the LW (used for spiking the soil samples) and the pressure plate extracts, we had to reject our null hypothesis. Soil water collected sequentially over 7 days at a constant pressure of 15 bar did not show the same isotopic composition as the water used for spiking the samples. The water draining from the clayey loam surprisingly showed statistical similarities to the δ18O of the DIW (p = .67) used for rewetting the ceramic plates of the extractor. This was not the case for δ2H. However, over time the LW must have exchanged the DIW of the ceramic plates, since we did not find statistical differences between the cryogenically extracted ceramic plate water and the LW for both isotopes. This leads to the conclusion that there was an isotopic exchange between the ceramic plate water and the water to be extracted from the saturated soil samples. Surprisingly, the exchange did not occur in experiment 1, which leads us to the conclusion that it is pressure level dependent. If it would have occurred, the extracted water isotopic composition would have plotted closer to the spiked isotopic label as we used the same water for spiking the soil samples and rewetting the ceramic plates.
Through the cryogenic extraction of the silty sand, the isotopic composition of the water changed in a way that the δ2H values became more negative and the δ18O values became more positive in comparison to the water extracted from the pressure plates. Interestingly, the cryogenically extracted water from the clayey loam was more depleted in both heavy isotopes (2H and 18O) than the clayey loam water extracted via the pressure plate but also the cryogenically extracted water from the silty sand was more depleted than the silty sand water from the pressure plate extraction. For a spiking experiment with the same soil types, Orlowski et al. (2013) observed that the cryogenically extracted silty sand water was more enriched in heavy isotopes and showed a smaller deviation from the spike water than the clayey loam, which plotted furthest away from the spike water. Here we saw a similar behaviour with the cryogenically extracted clayey loam samples being more depleted but only the hydrogen isotopic composition of the silty sand extracts did not differ significantly from the introduced LW. Thus, we observed a deviation from the water used for spiking for both soil types.
When plotting the data in dual isotope space, we found statistically significant linear regressions for the samples of the different subgroups. The cryogenically extracted silty sand samples showed a much higher correlation among each other (R2 = 0.97) than the cryogenically extracted clayey loam samples (R2 = 0.62). The same was true for the clayey loam (R2 = 0.65) and silty sand samples (R2 = 0.96) from the pressure extractor (Figure 6). The slopes of the different regression lines were very similar: The silty sand's line had a slope of 4.01, the clayey loam's of 4.50 and the cryo silty sand's of 4.14; only the cryo clayey loam's slope of 5.81 was slightly higher than the others. The intercepts of the regression lines from the cryogenically extracted samples and the pressure plate extraction for both soil types showed a difference of approximately 5. In the study by Gaj and McDonnell (2019), which included the same soil types, the slope of the regression lines decreased with decreasing grain size. Their sandy soils plotted on a regression line with a slope of 3 and the clayey soils' regression line had a slope of 2. Such strong grain size dependency was not reflected in the slopes of the regression lines in our study. Much rather was there a cryogenic extraction induced effect on the clayey loam as previously observed by Orlowski et al. (2013).
In summary, the soil water sampled sequentially over 7 days at a constant pressure of 15 bar deviated from the spiking water. The observed deviations changed over the time of the experiment and were larger for the clayey loam than for the silty sand and also different for the two isotopes (2H and 18O).
4 CONCLUSIONS
Recent literature has called for a discrete isotopic sampling along the pF curve (e.g., Gaj et al. (2019); Gaj and McDonnell (2019); McDonnell (2014)). We have investigated the effect of water retention characteristics on the water isotopic composition of soil pore water sampled along the pF curve for two different soil types. The null hypothesis guiding our work was that water held at different tensions has the same isotopic composition. We tested this in two different experiments where soils where spiked with a known isotopic label. We collected soil water along the pF curve (Exp. 1) and sequentially over a period of 7 days under a 15 bar pressure (Exp. 2). Our work has shown that the sampled soil water differed isotopically from the introduced isotopic label over time and sequentially along the pF curve.
Our results provide valuable insight into how soil water retention characteristics affect the soil water isotopic composition sampled along the pF curve. This has implications for interpreting the water isotopic composition of mobile and bulk water in soils (with respect to the tension water is held in the soil) and further modelling of the fast and slow flow domain in the vadose zone (Sprenger et al., 2018).
Clearly, more research needs to be done. Future studies should consider testing retention characteristics on a variety of different soil types, so that retention curve approach parameters as in Gaj et al. (2019) can be applied to predict soil water fractionation effects under natural and non-stationary conditions. As such pF curve experiments are extremely time-consuming, we call for a community approach to tackle this problem.
Studies using soil water extraction techniques (e.g., suction cups vs. cryogenic vacuum extraction) that apply different pressure levels for extraction should consider the various effects pressure can have on the soil water isotopic composition. This is particularly important when different soil water pools are compared.
Our work and previous studies (e.g., Adams et al., 2019; Gaj et al., 2019) implies caution in interpreting isotope results of extracted soil water and a need to better characterize processes that govern soil water fractionation with respect to soil water retention characteristics. We hope that with our study we contribute to stimulate much needed new research in these areas.
ACKNOWLEDGEMENTS
We thank student assistant Julian Drescher for his support during the lab experiment and Marcel Gaj for fruitful discussions along the way. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Natalie Orlowski and Lutz Breuer: Designed the experiment. Natalie Orlowski: Performed the experiment and data analysis. Both authors prepared the manuscript.
Appendix A:
| Soil pore size [μm] | Description | Pressure [hPa] | pF-value |
|---|---|---|---|
| >120 | Very wide macrospores | 25 | 1,4 |
| 120–50 | Wide macrospores | 60 | 1,8 |
| 50–30 | Medium macrospores | 100 | 2,0 |
| 30–10 | Thigh macrospores | 300 | 2,5 |
| 10–3 | Wide medium pores | 1,000 | 3,0 |
| 3–0,2 | Thigh medium pores | 15,000 | 4,2 |
| <0,2 | Fine pores | >15,000 | >4,2 |
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




