Effects of 14 F9 synonymous codon variants on hemophilia B expression: Alteration of splicing along with protein expression
Huayang Zhang and Changming Chen have contributed equally to this study.
Abstract
There is growing evidence that synonymous codon variants (SCVs) can cause disease through the disruption of different processes of protein production. The aim of the study is to investigate whether the 14 SCVs reported in the F9 variant database were the pathogenic causes of hemophilia B. The impacts of SCVs on splicing and protein expression were detected using a combination of in silico prediction, in vitro minigene splicing assay and cell expression detection. The splicing transcripts were identified and quantified by co-amplification fluorescent PCR. The mechanism of splicing was verified by a modified pU1snRNA and pU7snRNA approach. Aberrant splicing patterns were found in eight SCVs. Five of the 8 SCVs produced almost all aberrant splicing isoforms, which were expected to truncate protein, three of them presented a partial defect on both splicing and protein secretion, the overall effects were consistent with the residual Factor IX activity of the affected cases. Neither the pre-messenger RNA (mRNA) splicing process nor the protein function was impaired in the rest six SCVs. In conclusion, our study firstly revealed the pathogenic mechanism of the 14 F9 SCVs and highlighted the importance of performing mRNA splicing analysis and protein expression studies of SCVs in inherited disorders.
1 INTRODUCTION
Hemophilia B (HB) is an X-linked recessive bleeding disorder characterized by quantitative and/or qualitative defects of blood coagulation Factor IX (FIX). Based on FIX activity (FIX:C), HB is classified as severe (<1%), moderate(1%–5%), or mild (5%–40%), and the severity of the disease is mainly determined by the type of variants (White et al., 2001).
More than 1300 unique variants throughout F9 have been reported in the Factor IX Gene (F9) Variant Database (https://f9-db.eahad.org/), the missense/nonsense variants are the most common variants with a frequency of 61%. A total of 17 synonymous codon variants (SCVs) in F9 have been reported. However, the pathogenic mechanism underlying the HB expression has only been verified in a few of them, such as c.87A>G, p.(Thr29Thr) (Odaira et al., 2019).
SCVs, which do not alter encoded amino acids, were previously considered to be biologically silent. Over the past decades, considerable evidence has been accumulated to show that SCVs can lead to human diseases through their effects on the messenger RNA (mRNA) splicing process as well as protein expression, conformation and function (Cheng et al., 2018; Duan et al., 2003; Hunt et al., 2014; Kimchi-Sarfaty et al., 2007; Lazrak et al., 2013). Recent studies focused on F8 and F9 exonic missense variants and SCVs revealed the pathogenic mechanisms of these variants underlying hemophilia phenotypes, which led to developing the tailored therapeutic strategies acting at the rescue of mRNA splicing and protein secretion (Balestra et al., 2015, 2019; Fernandez Alanis et al., 2012; Lombardi et al., 2021).
In our previous HB cohort study, we also found three reported SCVs (c.519A>G, c.711A>G, and c.723G>A) in F9 in three unrelated HB cases (Lu et al., 2019). Therefore, in this context, we comprehensively evaluated the relationship between the 14 SCVs and HB expression, and revealed the pathogenic mechanisms of the 14 SCVs underlying different severity of HB by using a combination of in silico prediction, in vitro minigene splicing assay and cell expression detection. A total of 8 of the 14 SCVs were defined as causative variants for HB and the remaining 6 SCVs were not associated with HB. Intriguingly, 3 of 8 SCVs led to mild and moderate HB symptoms by disrupting the normal splicing along with reducing protein expression.
2 MATERIALS AND METHODS
2.1 Patients with F9 SCVs
The F9 genetic analysis has been performed in a total of 356 unrelated HB patients registered in our center since 2005. Three reported SCVs [c.519A>G, p.(Ala173Ala), c.711A>G, p.(Gln237Gln) and c.723G>A, p.(Gln241Gln)] had been identified in three unrelated patients, respectively. As shown in Figure S1, the segregation analysis of the variants is consistent with the transmission pattern of HB in the three pedigrees. The SCV c.723G>A was associated with severe HB, while both c.519A>G and c.711A>G resulted in moderate HB.
So far, 17 SCVs have been reported in the F9 Variant Database, except for c.87A>G and two uncertain SCVs of c.580A>G and c.1110G>A, a total of 14 SCVs, including the three SCVs mentioned above, were included in this study. As shown in Figure S2, four SCVs were located in the consensus sequences of 5′ splice sites (5′ss), and the other ten SCVs were spread inside the related exons. Variants and alternative transcripts were annotated according to the Human Genome Variation Society (HGVS) guidelines based on the F9 reference sequences (RefSeq NM_000133.4 and NP_000124.1). Sequence variant descriptions were verified by Mutalyzer online tool (https://mutalyzer.nl/; accessed on November 13, 2021).
2.2 Bioinformatics analysis
The Alamut v2.12 (Interactive Biosoftware), which consists of 4 different splice site prediction algorithms (SpliceSiteFinder-like, MaxEntScan, NNsplice, and GeneSplicer), was adopted to predict the effects of 14 SCVs on the splice sites, and Human Splicing Finder version 3.1 (http://www.umd.be/HSF3/) (Desmet et al., 2009) was used to predict the splicing regulatory elements.
2.3 Creation of F9 minigenes
Five F9 wild type fragments (F9-E2,3-WT, F9-E5-WT, F9-E6-WT, F9-E7-WT, and F9-E8-WT) containing the related 14 SCVs exons and both sides of adjacent 100–300 bp intron sequences were amplified from normal genomic DNA and cloned into the splicing plasmid pTB (a gift from Emanuele Buratti) through NdeI restriction sites (F. Pagani et al., 2002). Differently, the inserted fragment of F9-E2,3-WT included the full length of exon 2, intron 2 and exon 3, while the fragment of F9-E8-WT contained the latter part of intron 7 and the full length of exon 8 (Table S1). The 14 SCVs were introduced by site-directed mutagenesis using a QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies) according to the manufacturer's instructions based on the related F9-WT minigenes with their respective primers (Table S2).
Based on the wild type pU1snRNA and pU7snRNA plasmids (kindly provided by Franco Pagani), the sequence of the wild type pU1snRNA between BclI and BglII sites and the histone antisense sequence of wild type pU7snRNA were removed to get the empty vectors of pU1snRNADEL and pU7snRNADEL. The modified pU1snRNA (pU1Ex2 and pU1Ex5) and pU7snRNA (pU7c.153G, pU7c.459G, pU7c.459A, pU7c.484C, pU7c.484A) variant plasmids were created by introducing the targeted oligonucleotides into the pU1snRNADEL and pU7snRNADEL by site-directed mutagenesis with primers (Table S2) as previously described (Meyer & Schümperli, 2012; F. Pagani et al., 2002). As the in silico tool prediction indicated an ESS and a cluster of ESEs upstream of the cryptic 5′ss of F9 exon 2, we also constructed the pU7ESS targeting the ESS and pU7ESE targeting these ESEs.
2.4 Minigene splicing analysis
Human embryonic kidney 293T (HEK293T) cells were seeded in 12-well plates and cultured in 1 ml Dulbecco's modified Eagle's medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS) (Gibco) per well. After the cells were 70%–80% confluent, 500 ng of the minigene alone or with equimolar amounts of the corresponding pU1snRNA or pU7snRNA was transiently transfected in HEK293T cells using 2 μl Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. After 24 h, total RNA was isolated from the cells using TRIzol Reagent (Invitrogen) and phenol-chloroform extraction. First-strand complementary DNA (cDNA) was synthesized from 2 μg of total RNA with the HiScript II First-Strand cDNA Synthesis Kit (Vazyme). Then, transcripts were amplified with a pair of common primers designed on both flanked exons located in the plasmid pTB as previously described (F. Pagani et al., 2003) (Table S2). The PCR products were analyzed by electrophoresis on a 2% Agarose-gel as well as cloned into the pMD19-T Simple Vector (Takara). One hundred clones for each SCV were picked and sequenced to identify all forms of transcripts. In addition, the relative ratio of multiple transcripts of the SCV was also semi-quantified using a co-amplification fluorescent PCR with the same common primers as mentioned above, except for the forward primer labeled with 5′−6-carboxyfluorescein (FAM) (Metabion) as previously described (Liang et al., 2015). FAM-labeled product was separated on an ABI PRISM® 3130xl Genetic Analyzer (Applied Biosystems) and data were analyzed by the GeneMapper 4.0 software (Applied Biosystems). The percentage value of each splicing isoform was calculated by the relative amount of the peak area. Each sample was tested in triplicate and all values were given as the mean of triplicate PCR results including standard deviation.
2.5 FIX in vitro expression analysis
The three SCVs (c.153A>G, c.459G>A, and c.484C>A) exported both normal and aberrant transcripts, and the six SCVs (c.396A>G, c.738T>C, c.819T>C, c.1029C>T, c.1077C>A, and c.1248T>C) presented only normal transcripts detected by minigene assay. In addition, each reported case carried one of the six SCVs simultaneously had a unique missense/nonsense variant [p.(Arg75*), p.(Asn138Asp), p.(Arg75Gln), p.(Pro414Leu), p.(Cys170Tyr), p.(Cys64Tyr)] (Bottema et al., 1991; Chen et al., 1991; Koeberl et al., 1989, 1990; Mahajan et al., 2004; NS et al., 1996), respectively. Therefore, the impacts of the 9 SCVs and the 5 missense variants on the biosynthesis and function of FIX were further calculated by FIX expression in HEK293T cells in vitro. The FIX expression plasmids with the 9 SCVs and the 5 missense variants were generated through site-directed mutagenesis based on the wild-type FIX plasmid with the related primers, respectively (Table S2). HEK293T cells were grown in DMEM (10% FBS). Twenty-four hours before transfections, cultured cells were seeded at 2 × 105 cells in 6-well plates. Cells were transiently transfected with 2.5 µg plasmid DNA per well using 5 µl lipofectamine 2000 transfection reagent (Invitrogen). Transfection media were replaced after 24 h with fresh DMEM (Gibco) with 1× Insulin Transferrin Selenium Supplement (Gibco), and Vitamin K1 (5 μg/ml). Media were harvested after 48 h and centrifuged for 5 min at 3000g. The FIX antigen (FIX:Ag) levels in the supernatant were determined via enzyme-linked immunosorbent assays according to the manufacturer's instructions (Enzyme Research Laboratories). The FIX:C was measured based on a one-stage activated partial thromboplastin time (APTT) assay on the Diagnostica Stago ST4 Coagulation Analyzer (Diagnostica Stago). Due to the supernatant medium containing 1.8 mM of calcium, the medium was replaced with an equal volume of Dade® Owren's Veronal Buffer without calcium (Siemens) using Amicon Ultra-15 30K Centrifugal Filters (Merck Millipore) according to the manufacturer's instructions. Then, a 50 μl sample (further dilution or not according to the expression FIX:C levels) was preincubated with 50 μl FIX-deficient plasma (Instrumentation Laboratory) and 50 μl APTT reagent (Actin FSL; Siemens) for 3 min at 37°C, and the clotting time was measured after addition of 50 μl of 0.025 M CaCl2 (Siemens). A standard curve was derived from serial dilutions (1:10; 1:20; 1:40; 1:80, 1:160, and 1:320) of a pooled normal plasma prepared from 30 normal individuals. The APTT results of samples were calculated using log-log transformation as the percentage of FIX:C compared to standards. All experiments were run in duplicate and repeated three times.
3 RESULTS
3.1 In silico prediction
The scores of authentic and variant splice sites predicted by Alamut v2.12 and the effects on exonic splicing regulatory elements predicted by HSF were listed in Table 1 and Table S3. For the splice site prediction, 41 of 56 predictions (4 algorithms in Alamut v2.12) correctly predicted the effects of the 14 SCVs on splice sites compared to the results of the minigene splicing assay (Table 1). Combined with the 4 algorithms predictions, 3 SCVs disrupted the authentic 5′ss, 3 SCVs created cryptic 5′ss sites, and 6 SCVs had no effect on splicing, which were consistent with minigene mRNA analysis. Among the four algorithms, both SSF and MES were able to accurately predict all the splicing patterns in the 12 SCVs. However, the splice site predictions of c.459G>A and c.484C>A on F9 exon 5 were not so accurate. Alamut v2.12 predicted that the c.459G>A created a new cryptic 5′ss at c.456, and the c.484C>A had no effect on splice sites, while minigene showed exon 5 skipping in both SCVs. Interestingly, NNS and GS algorithms do not even recognize the authentic 5′ss of exon 5, indicating that the Alamut v2.12 tool may have some limitations to predict the impacts of variants related to a weak exon definition. HSF indicated that c.459G>A may create a new ESS, and the c.484C>A might disrupt an ESE, simultaneously create an ESS, which could help to explain the pathogenic mechanisms of exon 5 skipping in mRNA analysis.
| SCVs | Severity | FIX:C (%) | FIX:Ag (%) | Minigene assay | Splice sites predicted by Alamut v2.12 | Concordance | Splicing regulatory elements predicted by HSF | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcripts (%) | Protein changes | SSF | MES | NNS | GS | |||||||
| c.153A>G, p.Lys51Lys | Mi | 7 | Na | NT (11); Del of 104 bp of Ex 2 (89) |
WT; p.G50Dfs*2 |
+ | + | / | ++ | 3/4 | [−] ESE; [+] ESS | [+] 5′ss; [−] ESE; [+] ESS |
| c.396A>G, p.Val132Val* | Na | Na | Na | NT (100) | Wild-type | / | / | / | / | 4/4 | / | / |
| c.459G>A, p.Val153Val | Mi | 10–35 | 14–24 | NT (42); Skipping Ex 5 (58) |
WT; p.D131_A173del |
+ | ++ | + | / | 0/4 | [+] ESS | [+] 5′ss; [+] ESS |
| c.484C>A, p.Arg162Arg | Mo/Mi | 3–16 | 5–7 | NT (12); Skipping Ex 5 (88) |
WT; p.D131_A173del |
/ | / | / | / | 0/4 | [−] ESE; [+] ESS | [−] ESE; [+] ESS |
| c.519A>T, p.(Ala173Ala) | Na | Na | Na | Skipping Ex 5 (100) | p.D131_A173del | -- | -- | / | / | 2/4 | [−] ESE; [+] ESS [+] ESE; [−] ESS |
[−] 5′ss; [−] ESE; [+] ESS |
| c.519A>G, p.(Ala173Ala) | Mo | 3 | Na | Skipping Ex 5 (100) | p.D131_A173del | -- | -- | / | / | 2/4 | [−] ESE; [+] ESS [+]ESE; [-] ESS |
[−] 5′ss; [−] ESE; [+] ESS |
| c.519A>C, p.(Ala173Ala) | Mo | 2/5 | Na | Skipping Ex 5 (100) | p.D131_A173del | -- | -- | / | / | 2/4 | [−] ESE; [+] ESS [+] ESE; [−] ESS |
[−] 5′ss; [−] ESE; [+] ESS |
| c.711A>G, p.(Gln237Gln) | Se/Mo | 3/2/<1 | 2/<3 | Del of 17 bp of Ex 6 (100) | p.Q237Cfs*5 | / | / | / | - | 4/4 | / | [−] 5′ss; [+] 5'ss |
| + | ++ | ++ | ++ | |||||||||
| c.723G>A, p.(Gln241Gln) | Se/Mo | 2/<1 | 2/<1 | Del of 4 bp of Ex 6 (23); Del of 17 bp of Ex 6 (59); Ins of 57 bp of Int 6 (18) |
p.Q241Lfs*3; p.Q237Cfs*5; p.Q241_V242ins(19)# |
-- + |
-- / |
-- / |
-- / |
4/4 | / | [−] 5′ss; [+] 5'ss |
| c.738T>C, p.Gly246Gly* | Se | <1 | Na | NT (100) | WT | / | / | / | / | 4/4 | / | / |
| c.819T>C, p.Val273Val* | Mi | 20–37 | 70 | NT (100) | WT | / | / | / | / | 4/4 | / | / |
| c.1029C>T, p.Asn343Asn* | Na | Na | Na | NT (100) | WT | / | / | / | / | 4/4 | / | / |
| c.1077C>A, p.Val359Val* | Se | <1 | Na | NT (100) | WT | / | / | / | / | 4/4 | / | / |
| c.1248T>C, p.Val416Val* | Se | <1 | Na | NT (100) | WT | / | / | / | / | 4/4 | / | / |
- Note: *, The SCV combined with a missense/nonsense variant; FIX:C, activity of FIX; FIX:Ag, antigen of FIX; Mi, mild; Mo, moderate; Se, severe; Na, not available. (%), the ratio of each transcript was quantified by co-amplification fluorescent PCR. NT, normal transcript; Del, deletion; Ins, insertion; Ex, exon; Int, intron; WT, Wild-type. #, the inserted amino acid sequence is “VLYTDGVSKLELSWQDTGQ”. In silico analysis, the software Alamut v2.12 was used to predict the impacts on splice sites, containing 4 different algorithms, SSF, SpliceSiteFinder-like; MES, MaxEntScan; NNS, NNSplicer; GS, GeneSplicer. Those SCVs creating cryptic splice sites were considered to have “positive” effects on splicing and those SCVs impairing native splice sites were considered to have “negative” effects on splicing; /, no effect; +, a small positive effect (the increased score of the cryptic splice site was less than 50% of the authentic one); ++, a great positive effect (the increased score of the cryptic splice site was higher than 50% of the authentic one); -, a small negative effect (the decreased score of the wild-type splice site was less than 50% of the authentic one); --, a great negative effect (the decreased score of the wild-type splice site was higher than 50% of the score of the authentic one); Concordance, the number of correct predictions predicted by Alamut v2.12 tool for each aberrant splicing isoform. HSF, Human Splicing Finder version 3.1 was used to predict the splicing regulatory elements. 5′ss, 5′ splice site; [−] disruption; [+] creation.
- Abbreviations: ESE, exonic splicing enhancer; ESS, exonic splicing silencer; HSF, human splicing finder; SCVs, synonymous codon variants.
3.2 The effects of 14 SCVS on F9 splicing
As shown in Table 1, the minigene assay showed that eight SCVs had impacts on the pre-mRNA splicing and no visible aberrant transcripts were observed in the remaining six SCVs. Five of the 8 SCVs (c.519A>T, C and G, c.711A>G, and c.723G>A) produced almost all aberrant splicing isoforms, which were expected to generate premature termination codons or in frameshift deletion of essential FIX domains, consistent with the severe phenotypes in reported cases. While three of the 8 SCVs (c.153A>G, c.459G>A, and c.484C>A) showed normal as well as abnormal transcripts co-existing and the ratios of normal transcripts were 11.66%, 42.84%, and 10.58%, respectively (Figure 1a,b).
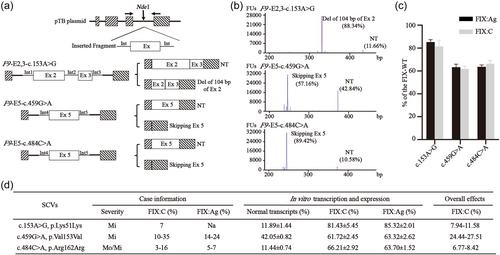
Among the 8 SCVs causing aberrant splicing, 4 SCVs (c.519A>T, C and G, c.723G>A) were located in −2A and −1G of the consensus sequences (CAG/guragu, exon/intron, r means a/g) of 5′ss. The minigene splicing assay showed exon 5 skipped in all the c.519A>T, C and G, while the usage of three cryptic splice sites at c.706, c.719 and c.723+57 were observed in the c.723G>A. In silico tools predicted that the three SCVs (c.519A>T, C, and G) destroyed the authentic 5′ss by reducing splice site score (SSS) from 3.0 to 0 (Table S3). While the c.723G>A destroyed the authentic 5′ss, thus, several cryptic 5′ss sites existing around the authentic 5'ss became stronger than the authentic splice site. These predictions were consistent with the results in the minigene splicing assay. The other four SCVs (c.153A>G, c.459G>A, c.484C>A, and c.711A>G) were spread inside the related exons. Minigene demonstrated that both c.153A>G and c.711A>G caused a partial deletion of the related exon by creating a novel splice site. The same results were predicted by in silico tool, in which the c.153A>G and c.711A>G created a cryptic splice site at c.148 and c.706, respectively, with a higher SSS than the authentic one (10.9 vs. 8.3 and 8.9 vs. 8.2) (Table S3). Both c.459G>A and c.484C>A led to skipping of exon 5 revealed by minigene, while Alamut v2.12 predicted the inconsistent results, in which the c.459G>A activated a new cryptic 5′ss at c.456, and the c.484C>A had no effect on splice site. However, the HSF predicted that both SCVs had impacts on exonic regulatory elements of ESSs and/or ESEs (Table 1).
3.3 FIX expression analysis
The FIX expression in HEK293T cells in vitro showed that the FIX:Ag levels of 3 SCVs (c.153A>G, c.459G>A, and c.484C>A) in the supernatant were reduced to 85.3%, 63.3%, and 63.7% of wild-type FIX, respectively, the similar reduction of FIX:C levels was detected (Figure 1c), indicating that the expressed FIX of the 3 SCVs had normal FIX function. Considering the impaired FIX biosynthesis combined with a partial defect of splicing, the overall FIX:C level of the three SCVs could be calculated by expressed FIX:C corrected for the ratio of normal transcripts, which was approximately consistent with the residual FIX:C levels of the reported cases (Figure 1d).
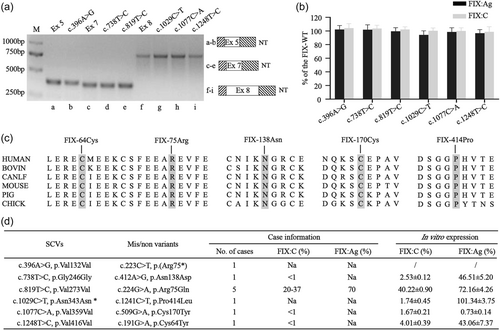
The minigene assay showed that the 6 SCVs (c.396A>G, c.738T>C, c.819T>C, c.1029C>T, c.1077C>A, and c.1248T>C) had no impact on the splicing (Figure 2a), and in vitro FIX expression led us to further demonstrate that both FIX:C and FIX:Ag levels of the 6 SCVs were normal (Figure 2b). The residues of the combined five missense variants [p.(Asn138Asp), p.(Arg75Gln), p.(Pro414Leu), p.(Cys170Tyr) and p.(Cys64Tyr)] were completely conserved across six species (Figure 2c). In vitro expression of the five missense variants showed that 4 variants reduced the FIX expression and the FIX activity in various degrees. The remaining p.(Cys170Tyr) variant severely impaired the FIX expression with FIX:Ag level <1%. Taking together, the one nonsense variant [p.(Arg75*)] and those five missense variants caused detrimental FIX functions and were associated with HB expression in the reported cases. The six SCVs may not contribute to the HB phenotypes (Figure 2d).
3.4 Abnormal splicing related to the interruption of regulation elements
To further investigate whether the aberrant splicing of the three SCVs (c.153A>G, c.459G>A, and c.484C>A) was associated with the interruption of the related regulatory elements, we engineered the pU1snRNA (pU1Ex2 and pU1Ex5) with perfect complementarity to the authentic sequences of 5′ss of F9 exons 2 and 5 to strengthen the definition of the related exons, and we also engineered pU7snRNA contained relative antisense oligonucleotides (pU7c.153G, pU7ESE, pU7ESS, pU7c.459G, pU7c.484C, pU7c.459A, and pU7c.484A) to mask the corresponding potential cryptic splice sites or exonic splicing regulatory elements (Figure 3).
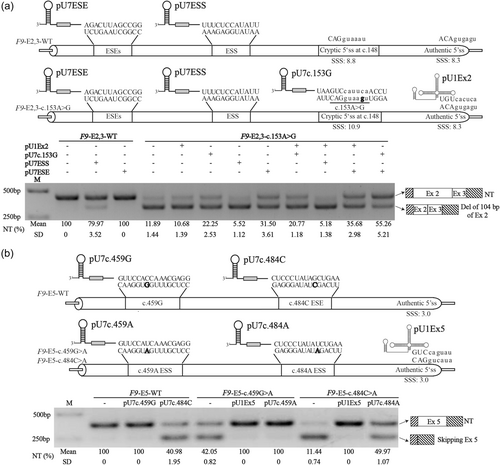
As shown in Figure 3a, when the F9-E2,3-WT minigene was cotransfected with pU7ESS, the ratio of the normal splicing transcripts was decreased from 100% up to approximately 80%, while cotransfected with pU7ESE, no aberrant transcript was detected. These results indicated the ESS predicted by in silico tool disfavored the selection of the cryptic 5′ss, and the predicted ESE may have no regulatory roles to the cryptic and/or authentic 5′ss. When the F9-E2,3-c.153A>G minigene was cotransfected with pU7ESS, the ratio of normal splicing transcripts was decreased from 11.89% to 5.52%, cotransfected with pU7c.153G, pU7ESE or both, the ratio of normal splicing transcripts rose from 11.89% to 22.25%, 31.50%, and 55,26%, respectively. These results revealed the ESE predicted by HSF might strengthen the usage of the cryptic 5′ss. However, cotransfected with pU1Ex2, or pU1Ex2 combined with pU7c.153G, pU7ESS, or pU7ESE, the pU1Ex2 did not improve the definition of exon 2 due to no additional role was shown in any combined transfection, suggesting that pU1Ex2 could not rescue the influence of c.153A>G on splicing due to the authentic 5′ss (SSS: 8.3) already strong enough. Taken together, our results pointed out that there was a competition between the authentic 5′ss and the cryptic 5′ss, which was also regulated by the exonic splicing regulatory elements. The SCV c.153A>G further strengthened the cryptic 5′ss at c.148 (CAGGUAAGU, SSS, 10.9), which shifted the balance to the usage of the cryptic 5′ss.
As shown in Figure 3b, when the F9-E5-c.459G>A and F9-E5-c.484C>A minigenes were cotransfected with pU1Ex5, the pU1Ex5 could increase the normal splicing transcripts' ratio from 42.05% and 11.44% up to 100%, respectively, indicating that both SCVs weakened the definition of exon 5. When the F9-E5-c.459G>A minigene was cotransfected with pU7c.459A, and the ratio of normal splicing transcripts raised up to 100%, while when the F9-E5-c.484C>A minigene was cotransfected with pU7c.484A, the ratio of normal transcripts was improved from 11.44% to 49.47%, indicating that both SCVs created new exonic splicing regulatory elements which decreased the definition of the F9 exon 5, consistent with the in silico prediction of both SCVs creating a novel ESS. When the F9-E5-WT minigene was cotransfected with pU7c.459G, no visible influence on F9-E5-WT splicing was observed, while cotransfected with pU7c.484C, the ratio of normal transcripts of F9-E5-WT was reduced from 100% to 40.98%, suggesting that no regulatory element around c.459G site and an ESE may exist around the c.484C site, consistent with the in silico prediction. Taken together, these results demonstrated the SCV c.459G>A may create a new ESS while the SCV c.484C>A might reverse an original ESE into an ESS, which impaired the definition of the F9 exon 5 and led to the exon skipping.
4 DISCUSSION
Growing evidence reveals that SCVs can cause disease through influencing the pre-mRNA splicing process (Cartegni et al., 2002), mRNA stability (Duan et al., 2003) and/or the protein function (Cheng et al., 2018). The overall effects of SCVs on the severity of causing disease should be determined by the degree of aberrant splicing combined with the functional influence of the protein. Here, we evaluated the effects of 14 SCVs reported in the F9 variant database on splicing along with FIX biosynthesis by minigene splicing assay and in vitro expression, then reclassified that eight SCVs were the causative variants of HB, while the other six SCVs were benign variants in F9 (Table 1). Five of the 8 SCVs produced almost all aberrant transcript forms, which were expected to truncate protein, consistent with the severe HB in reported cases, and three of the 8 SCVs resulted in mild/moderate HB by a partial defect on both splicing and protein expression (Figure 1).
The splicing process requires core splicing signals including the consensus sequences on 5′ss and 3′ss, branch point and polypyrimidine tract sequences. In addition, several auxiliary cis-acting regulatory elements including exonic or intronic splicing silencers (ESS, ISS) and enhancers (ESE, ISE) are also involved (De Conti et al., 2013). The exonic sequences not only encode amino acid sequences but also overlap with splicing consensus sequences and splicing regulatory elements. Therefore, the SCVs may lead to related exon skipping, intron inclusion or alternative splice site usage through disruption of a splice site or a binding site of splicing regulatory factors.
The most straightforward method to identify splicing aberration is based on ectopic transcription using peripheral blood from patients. However, FIX is considered as exclusively synthesized in the liver (Green et al., 2003), which is usually unavailable from patients. Therefore, the functional influences of 14 SCVs in F9 on splicing were analyzed by minigene splicing assay combined with in silico prediction in our study. Surprisingly, all the aberrant splicing patterns of the 8 SCVs were pointed to the poor definition of 5′ss in our minigene assay. Moreover, the previously reported SCV c.87A>G also impaired the splicing process by generating a cryptic splice site at 5′ss (Odaira et al., 2019). Human 5′ss consensus sequences are composed of CAG/guragu, spanning from positions −3 to 6 relative to the exon-intron boundary. These sequences are critical but often insufficient for accurate 5′ss recognition, and may require a series of auxiliary regulatory elements in both intron and exon (Ptok et al., 2019). Four of the 8 SCVs (c.519A>T, C and G and c.723G>A) were located at −2 and −1 of 5′ss consensus sequences, respectively. The c.519A>T, C and G SCVs caused skipping of exon 5, irrespective of the substituting nucleotide, consistent with a position effect (Královicová et al., 2006). However, there were several cryptic splice sites around the position of c.723G, the c.723G>A generated multiple transcripts by the usage of those cryptic splice sites. Actually, the existence of optimistic cryptic splice sites near the variant site is likely to determine if the aberrant splicing pattern is exon skipping or cryptic 5′ss usage (Krawczak et al., 2007).
The remaining four SCVs (c.153A>G, c.459G>A, c.484C>A, and c.711A>G) were away from the 5′ss or 3′ss consensus sequences, belong to the group of variants that had been relatively rare explored (Savisaar & Hurst, 2017). The in vitro minigene assay combined with in silico prediction showed that the c.711A>G introduced a cryptic splice site at c.706 with a higher SSS than that of the authentic 5′ss, resulting in a partial deletion of the related exon. Intriguingly, 3 SCVs of c.153A>G, c.459G>A, c.484C>A exported a normal as well as an aberrant transcript which might be related to the exonic splicing regulatory elements predicted by in silico tools. As shown in Figure 3a, in the wild-type F9 exon 2, there is a comparable cryptic splice site at c.148 with a higher SSS than that of the authentic 5′ss, which might be regulated by a network of an ESS adjacent to the cryptic 5′ss and a cluster of ESEs upstream of the ESS. The overall effects of these regulatory elements disfavor the selection of the cryptic 5′ss at c.148 under the physiology condition (Balestra et al., 2015). The in silico tools predicted the c.153A>G strengthened the cryptic splice site by creating a perfect 5′ss and caused aberrant splicing. We used the modified pU7snRNA to mask the cryptic 5′ss, ESS and ESE, the changes of the splicing pattern supported the presence of the regulatory elements predicted by the HSF tool.
Tajnik et al. (2016) revealed that both the SCVs c.459G>A and c.484C>A increased the inhibitory splicing factors binding, consistent with our results that both SCVs may generate a new ESS. In our study, the modified pU7snRNA masking the wild-type sequences around c.484C decreased the exon 5 inclusion to almost half degree, demonstrating that there might be a cluster of ESEs around the site. Therefore, our results suggested that the c.484C>A may break the fine interplay of active and inhibitory splicing factors by creating a novel ESS simultaneously disrupting the original ESE, which could also explain the more severity of exon 5 skipping in c.484C>A than in c.459G>A. The modified pU1snRNA could rescue the exon 5 inclusion to 100% in both c.459G>A and c.484C>A (Figure 3b). Notably, the in silico tools predicted that F9 exon 5 had a weak authentic 5′ss, our results also emphasized that the splicing regulatory elements played important roles in defining the exons with weak 5′ss (Aissat et al., 2013). Using the constructed pU1snRNA to enhance the definition of exons or the pU7snRNA to offset the negative effects of ESSs both can increase the ratio of normal transcripts, which also provides new treatment strategies for variants that affect splicing regulatory elements.
The 3 SCVs (c.153A>G, c.459G>A, c.484C>A) not only had a partial defect of splicing but also reduced the FIX expression (Figure 1b,c), the overall effects of the three SCVs on splicing along with protein expression were approximately consistent with laboratory phenotypes in the reported cases (Figure 1d). A previous study revealed that the c.459G>A, which is a recurrent variant associated with mild HB reported in Sweden, did not impair the mRNA integrity by ectopic transcription analysis (Knobe et al., 2008). The following study demonstrated that the SCV diminished the secretion of FIX possibly by slowing the FIX translation or altering its confirmation (Simhadri et al., 2017). However, the lower expression level of FIX (40%–80%) in their report was not sufficient to explain the lower residual FIX activity (15%–20%) in the affected patients if the c.459G>A had a negative impact on splicing. To the best of our knowledge, our study is the first time to comprehensively verify alteration of splicing process along with protein biosynthesis abnormality as pathogenic mechanisms of the SCVs in F9. We found that the six SCVs had impacts on neither normal splicing nor FIX expression (Figure 2a,b), suggesting that they may not be associated with HB expression in the reported cases. Actually, all the six mis/nonvariants [p.(Arg75*), p.(Asn138Asp), p.(Arg75Gln), p.(Pro414Leu), p.(Cys170Tyr) and p.(Cys64Thr)] in cases combined with six SCVs are recurrent variants and cause type I or type II HB (https://f9-db.eahad.org/). In addition, the SCV of c.819T>C along with the missense variant of p.(Arg75Gln) was reported in five cases, suggesting that this linkage had the founder effect (Rallapalli et al., 2013). In vitro expression led us to demonstrate that the five missense variants were almost consistent with the clinical symptoms of reported HB cases (Figure 2d). Taken together, all these results suggested that the six SCVs might be benign variants in F9.
There are several limitations in our study, first, the minigene assay is a reliable in vitro splicing analysis using an artificial hybrid gene but may not detect tissue-specific aberrant splicing mimicking in vivo condition of affected patients. Second, some discrepancies between splicing anomalies of patients' RNA and minigene splicing assays had been observed when minigene was constructed with variant related exon and flanking intron sequences (Acedo et al., 2012; Baralle et al., 2006). Recently, larger minigene construction with multiple exons has been confirmed to be more accurate to reflect the real in vivo splicing outcome of variants due to mimicking the natural genomic context (Acedo et al., 2015; Fraile-Bethencourt et al., 2017, 2019). Third, a previous study reported an iPSC-based model of the disease may provide a novel way to investigate the pathogenic mechanism of the splicing site variants at the RNA level (Martorell et al., 2017), especially in F9 with difficulty in ectopic transcription analysis. Fourthly, the clinical and laboratory information of cases carrying the 14 SCVs was collected from our center and F9 variant database, we have some difficulties establishing the relationship between the phenotype and genotype in some cases due to the missing data.
In conclusion, we investigated the pathogenic mechanisms of the 14 F9 SCVs underlying HB expression and defined eight SCVs as HB-related variants and the rest six SCVs might not be the causes of the disease. The in silico tools play important roles in predicting the expected functionality of the analyzed variants. Our study pointed out that the in silico tool may have some limitations to predicting the impacts of variants on splice sites related to a weak exon definition and require comprehensive experimental validations and improvement. Three of the 8 SCVs altered the splicing patterns as well as impaired the FIX biosynthesis, the overall effects of the three SCVs on FIX defect help us to establish the relationship between phenotype and genotype of HB, which highlights that SCVs can result in pleiotropic effects on splicing and protein function, which co-contribute to the clinical symptoms of related disorders. The SCVs can result in detrimental effects on the splicing process by creating or breaking the ESSs and ESEs, which might provide a potential therapeutic strategy through rescuing the aberrant transcript based on a modified pU1snRNA and pU7snRNA approach.
AUTHOR CONTRIBUTIONS
Huayang Zhang and Changming Chen: conceived and designed the experiments. Xi Wu: collected the clinical samples. Can Lou and Qian Liang: performed some of the experiments. Huayang Zhang and Changming Chen: wrote the manuscript. Qiulan Ding, Xuefeng Wang and Wenman Wu: revised the manuscript. Qiulan Ding and Xuefeng Wang: supervised the whole project and provided critical reviews. All authors reviewed and approved the final draft.
ACKNOWLEDGMENTS
We thank Prof. Emanuele Buratti and Prof. Franco Pagani for generously providing the pTB, pU1snRNA, and pU7snRNA plasmids. This study was supported by the General Program of National Natural Science Foundation of China (81770135, 81770136, and 81970126).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article. The variants collected in this study are openly available in the Factor IX Gene (F9) Variant Database at https://f9-db.eahad.org/.




