Expanding the genetic and phenotypic relevance of KCNB1 variants in developmental and epileptic encephalopathies: 27 new patients and overview of the literature
Abstract
Developmental and epileptic encephalopathies (DEE) refer to a heterogeneous group of devastating neurodevelopmental disorders. Variants in KCNB1 have been recently reported in patients with early-onset DEE. KCNB1 encodes the α subunit of the delayed rectifier voltage-dependent potassium channel Kv2.1. We review the 37 previously reported patients carrying 29 distinct KCNB1 variants and significantly expand the mutational spectrum describing 18 novel variants from 27 unreported patients. Most variants occur de novo and mainly consist of missense variants located on the voltage sensor and the pore domain of Kv2.1. We also report the first inherited variant (p.Arg583*). KCNB1-related encephalopathies encompass a wide spectrum of neurodevelopmental disorders with predominant language difficulties and behavioral impairment. Eighty-five percent of patients developed epilepsies with variable syndromes and prognosis. Truncating variants in the C-terminal domain are associated with a less-severe epileptic phenotype. Overall, this report provides an up-to-date review of the mutational and clinical spectrum of KCNB1, strengthening its place as a causal gene in DEEs and emphasizing the need for further functional studies to unravel the underlying mechanisms.
1 BACKGROUND
Developmental encephalopathies constitute a broad and genetically heterogeneous group of neurodevelopmental disorders diagnosed during early childhood and persisting throughout life. The clinical spectrum includes variable degrees of social, cognitive, motor, language, and behavioral impairments. The concept of “developmental and epileptic encephalopathy” (DEE) refers to the frequently associated epileptic activity (seizures and electroencephalogram [EEG] abnormalities) that contributes to developmental impairment and regression (Scheffer et al., 2017). Recent advances in DNA-sequencing methods have highlighted the important role of gene-encoding ion channels in the pathogenesis of DEEs (Wang et al., 2017). Ion channels are crucial in the generation and modulation of excitability in the nervous system (Wei et al., 2017). “Channelopathies” are associated with a wide phenotypic and genotypic spectrum as one gene is often associated with different phenotypes and variants in several genes might result in the same epilepsy phenotype (McTague, Howell, Cross, Kurian, & Scheffer, 2016; Wei et al., 2017). In particular, gene-related potassium channel dysfunction causes a clinical spectrum of DEEs including epilepsy of infancy with migrating focal seizures (KCNT1), early-onset DEEs with suppression-burst (KCNQ2), and nonspecific DEEs (e.g., KCNA2, KCND2, KCND3, KCNH5, KCNJ2, KCNJ10, KCNMA1, KCNQ3, KCNQ5, KCNT2, and KCNV2; Ambrosini et al., 2014; Barcia et al., 2012; Gururaj et al., 2017; Jorge et al., 2011; Lee, Lin, Kornblum, Papazian, & Nelson, 2014; Lehman et al., 2017; Pena & Coimbra, 2015; Sicca et al., 2011; Soldovieri et al., 2014; Tabarki, AlMajhad, AlHashem, Shaheen, & Alkuraya, 2016; Veeramah et al., 2013; Wang et al., 2019; Weckhuysen et al., 2012).
Torkamani et al. (2014) identified de novo variants in the potassium voltage-gated channel subfamily B member 1 (KCNB1) in three sporadic patients affected by early-onset DEE. This study provided the initial evidence of the deleterious effect of variants p.Ser347Arg, p.Thr374Ile, and p.Gly379Arg on KCNB1 function. Following the initial identification of KCNB1 variants (Torkamani et al., 2014), 29 new KCNB1 variants have been reported in 37 patients detected through next-generation high-throughput sequencing in cohorts of individuals with developmental delay and/or DEE (Allen et al., 2016; Fitzgerald et al., 2015; Saitsu et al., 2015; Soden et al., 2014; Srivastava et al., 2014; Thiffault et al., 2015; Torkamani et al., 2014; Calhoun, Vanoye, Kok, George, & Kearney, 2017; de Kovel et al., 2016, 2017; Latypova et al., 2017; Marini et al., 2017; Miao, Peng, Chen, Gai, & Yin, 2018, 2018; Parrini et al., 2017; Samanta, 2018; Zhu et al., 2017). The majority of these patients had epilepsy, intellectual disability, and behavioral problems (MIM# 616056; epileptic encephalopathy, early infantile, 26).
In this mutation update, we aim to present an exhaustive review of patients carrying KCNB1 variants and discuss the evidence for the pathophysiological relevance of these variants. Furthermore, we expand the variant spectrum of KCNB1 with the description of 27 new unrelated patients carrying 18 novel variants.
2 STRUCTURE OF Kv2.1 CHANNEL
KCNB1 (MIM# 600397) is a potassium channel gene located on chromosome 20q13.3 and has a full-length transcript of 11.879 kb (NM_004975) containing two exons. KCNB1 protein is a 96-kDa core protein of 858 amino acids forming the α subunit of the voltage-gated potassium channel subfamily 2 (Kv2.1). Kv2.1 channels are expressed across the central nervous system, especially in large clusters on the soma, proximal dendrites, and axonal initial segment of neurons (King, Manning, & Trimmer, 2014; Trimmer, 1991). Like other voltage-gated potassium channels, they are composed of four α-subunits surrounding the ion conduction pore. Each α-subunit has six transmembrane helices (S1–S6) that include a voltage-sensing domain (S1–S4) and a pore domain (S5–P–S6). The voltage-sensor S4 helix contains a series of positively charged amino acids that senses the change in the membrane potential leading to channel opening and closing. The selectivity filter of the pore is formed by the TVGYG amino acids motif located in the re-entrant pore loop between S5 and S6. In addition, Kv2.1 voltage-gated potassium channels have a N-terminal cytoplasmic region that modulates homotetramerization as well as heterotetramerization with other families of channel-forming subunits, such as the silent α-subunits Kv6 (KCNG), Kv8 (KCNV), and Kv9 (KCNS; Bocksteins et al., 2014; Hugnot et al., 1996; Salinas, Duprat, Heurteaux, Hugnot, & Lazdunski, 1997; Xu, Yu, Jan, Jan, & Li, 1995). Although the functional role of the intracellular C-terminal domain is yet not fully understood, it mediates the restricted and clustered proximal localization of Kv2.1 channel (Lim, Antonucci, Scannevin, & Trimmer, 2000) and interacts with the N-terminal domain to regulate intracellular trafficking, surface expression, voltage-dependent activation gating, and phosphorylation-dependent modulation of the Kv2.1 channel (Ju, Stevens, Leadbitter, & Wray, 2003; Mohapatra, Siino, & Trimmer, 2008). Homotetrameric Kv2.1 channels mediate a delayed-rectifier voltage-dependent outward potassium current which is essential for membrane repolarization during high-frequency firing. Functional properties of heterotetrameric channels are more complex, depending on the channel subunit composition (Ottschytsch, Raes, van Hoorick, & Snyders, 2002; Sano et al., 2002).
3 IDENTIFICATION OF KCNB1 VARIANTS
The database Pubmed was used to search KCNB1 pathogenic variants by combining the terms “KCNB1” and “variants” or “mutations”. Articles were reviewed and crossed with KCNB1 variants listed in professional databases (Human Gene Mutation Database [HGMD], Biobase, and Qiagen). We excluded one patient without Sanger confirmation of de novo inheritance and insufficient clinical data available to validate the variant pathogenicity (Zhu et al., 2017).
We collected 27 new unrelated patients with KCNB1 pathogenic variants through the French reference network for rare epilepsies and international collaborations (Belgium, Italy, Luxembourg, New Zealand, and Australia). KCNB1 variants were detected by targeted next-generation sequencing panels for either epilepsy or intellectual disability (n = 16) or by whole-exome sequencing (n = 11), we classified variants according to the international guidelines of the American College of Medical Genetics (ACMG) Laboratory Practice Committee Working Group (Richards et al., 2015; Table S1). Single-nucleotide variants were confirmed by Sanger sequencing and segregation analysis was completed in each family. In silico predictions supporting evidence of pathogenicity for missense variants reported in our 27 new patients are detailed in Table S2. KCNB1 variants were described according to HGVS variant nomenclature guidelines (http://varnomen.hgvs.org/; Dunnen et al., 2016), using the reference sequence RefSeq NM_ 004975.2. Variants have been submitted to ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). Clinical and EEG data were obtained for all patients, including developmental, neurological, behavioral, and epilepsy history, EEG, and imaging data when available. All parents or legal guardians gave written informed consent for genetic diagnosis procedures and research participation according to the ethics committee of each institution.
4 VARIANT SPECTRUM
We identified 29 pathogenic variants from 37 unrelated patients reported in the literature (Allen et al., 2016; Calhoun et al., 2017; de Kovel et al., 2016, 2017; Fitzgerald et al., 2015; Latypova et al., 2017; Marini et al., 2017; Miao et al., 2017, 2018; Parrini et al., 2017; Saitsu et al., 2015; Samanta, 2018; Soden et al., 2014; Srivastava et al., 2014; Thiffault et al., 2015; Torkamani et al., 2014).
In our cohort of 27 unrelated patients, we found 25 distinct KCNB1 variants including 18 novel variants (Table 1). All patients carried heterozygous missense or truncating variants, arising de novo in 24/27 patients. We report the first inherited variant (p.Arg583*) in Patient 64 and his mother affected by intellectual disability without epilepsy. Study of the maternal grandparents showed that the variant occurred de novo in the proband's mother who carried the variation at the heterozygous state. Segregation could not be completed in both parents for the remaining two variants to confirm their de novo occurrence (Patients 51 and 58), but both were considered pathogenic. The p.Gly395Arg variant in Patient 51 was identified as a de novo variant in Patient 50 in this series. The p.Phe416Leu variant in Patient 58 has been previously reported as likely pathogenic in two patients (Allen et al., 2016; Patient 8; de Kovel et al., 2016; ID-2010D05815).
| Patient ID | Nucleotide change | Amino acid change | Protein effect | Inheritance | Age (years)/Gender | DD | Walk | Verbal skills | Behavioral disorders | Epilepsy/Age at seizure onset (months) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | c.128A>G | p.Glu43Glya | Missense | De novo | 29/M | Yes | Yes | Nonverbal | No | Yes/16 | This study |
| 2 | c.586A>T | p.Ile196Phe | Missense | De novo | 12/F | Yes | NA | Nonverbal | Yes | Yes/18 | Marini et al. (2017; ID-4) |
| 3 | c.595A>T | p.Ile199Phepore domain of the protein | Missense | De novo | NA/M | Yes | NA | NA | Yes | Yes/NA | Calhoun, Vanoye, Kok, George, andKearney (2017) |
| 4 | c.605C>T | p.Ser202Phe | Missense | De novo | 7/M | Yes | Yes | Sentences | Yes | Yes/24 | de Kovel et al. (2017; Patient 26) |
| 5 | c.629C>G | p.Thr210Arg | Missense | De novo | 11/M | Yes | Yes | Words | Yes | Yes/48 | This study |
| 6 | c.629C>T | p.Thr210Met | Missense | De novo | 4/F | NA | NA | NA | NA | No | de Kovel et al. (2017; Patient 24) |
| 7 | c.629C>T | p.Thr210Met | Missense | De novo | 8/F | Yes | NA | Nonverbal | Yes | Yes/12 | Marini et al. (2017; ID-5) |
| 8 | c.629C>A | p.Thr210Lys | Missense | De novo | 7/M | Yes | Yes | Sentences | Yes | Yes/4 | de Kovel et al. (2017; Patient 25) |
| 9 | c.632T>C | p.Leu211Pro | Missense | De novo | 7/F | Yes | NA | NA | No | Yes/11 | de Kovel et al. (2017; Patient 23) |
| 10 | c.682C>T | p.Gln228* | Nonsense | De novo | 3,5/F | Yes | Yes | NA | Yes | Yes/10 | de Kovel et al. (2017; Patient 22) |
| 11 | c.857del | p.Val286Glyfs*58 | Frameshift | De novo | 2/M | Yes | Yes | Sentences | No | Yes/5 | This study |
| 12 | c.916C>T | p.Arg306Cys | Missense | De novo | 8/F | Yes | Yes | Sentences | Yes | Yes/48 | This study |
| 13 | c.916C>T | p.Arg306Cys | Missense | De novo | 22/F | Yes | Yes | Sentences | No | Yes/6 | Marini et al. (2017; ID-3) |
| 14 | c.916C>T | p.Arg306Cys | Missense | De novo | 7/M | Yes | Yes | Words | Yes | Yes/12 | Saitsu et al. (2015; Patient 2) and de Kovel et al. (2017; Patient 20) |
| 15 | c.916C>T | p.Arg306Cys | Missense | De novo | 9/M | Yes | NA | NA | Yes | Yes/12 | de Kovel et al. (2017; Patient 21) |
| 16 | c.934C>T | p.Arg312Cys | Missense | De novo | 23/M | Yes | Yes | Words | Yes | No | This study |
| 17 | c.935G>A | p.Arg312His | Missense | De novo | 9/M | Yes | Yes | Nonverbal | Yes | Yes/10 | de Kovel et al. (2017; Patient 19) |
| 18 | c.935G>A | p.Arg312His | Missense | De novo | 1,2/F | Yes | No | Nonverbal | NA | Yes/14 | Samanta (2018) |
| 19 | c.935G>A | p.Arg312His | Missense | NA | 11/M | Yes | No | Nonverbal | Yes | Yes/14 | de Kovel et al. (2016; ID-KIEL20) and de Kovel et al. (2017; Patient 18) |
| 20 | c.935G>A | p.Arg312His | Missense | De novo | 33/M | Yes | Yes | Nonverbal | Yes | Yes/18 | This study |
| 21 | c.935G>A | p.Arg312His | Missense | De novo | 13/M | Yes | Yes | Nonverbal | Yes | Yes/60 | This study |
| 22 | c.968C>T | p.Thr323Ile | Missense | De novo | 3/M | Yes | Yes | Nonverbal | Yes | Yes/21 | This study |
| 23 | c.984C>G | p.Tyr328* | Nonsense | De novo | 4,5/ F | Yes | Yes | Words | Yes | Yes/7 | This study |
| 24 | c.990G>C | p.Glu330Asp | Missense | De novo | 3,6/F | Yes | NA | NA | NA | Yes/18 | Miao et al. (2018) |
| 25 | c.1001T>C | p.leu334Pro | Missense | De novo | 5/F | Yes | No | Nonverbal | Yes | Yes/24 | This study |
| 26 | c.1041C>G | p.Ser347Arg | Missense | De novo | 10/M | Yes | Yes | Sentences | Yes | Yes/18 | This study |
| 27 | c.1041C>A | p.Ser347Arg | Missense | De novo | 9/F | Yes | Yes | NA | NA | Yes/48 | Torkamani et al. (2014; ID-9) and de Kovel et al. (2017; Patient 17) |
| 28 | c.1045G>T | p.Val349Phe | Missense | De novo | 1,6/F | Yes | No | Nonverbal | Yes | Yes/9 | This study |
| 29 | c.1045G>T | p.Val349Phe | Missense | De novo | 17/M | Yes | NA | Words | Yes | Yes/11 | Marini et al. (2017; ID-6) |
| 30 | c.1088delG | p.Ser363Thrfs*13 | Frameshift | De novo | 2/M | Yes | NA | Nonverbal | No | Yes/14 | de Kovel et al. (2017; Patient 16) |
| 31 | c.1105T>C | p.Trp369Arg | Missense | De novo | 14/M | Yes | No | Nonverbal | Yes | Yes/10 | This study |
| 32 | c.1107G>A | p.Trp369* | Nonsense | De novo | 5/M | Yes | NA | NA | Yes | Yes/18 | de Kovel et al. (2017; Patient 15) |
| 33 | c.1109G>A | p.Trp370* | Nonsense | De novo | 7/M | Yes | NA | Words | No | Yes/9 | Parrini et al. (2017) and Marini et al. (2017; ID-1) |
| 34 | c.1115C>T | p.Thr372Ile | Missense | De novo | 8/M | Yes | Yes | Sentences | No | No | This study |
| 35 | c.1115C>A | p.Thr372Asn | Missense | De novo | 12/F | Yes | Yes | Sentences | Yes | No | This study |
| 36 | c.1121C>T | p.Thr374Ile | Missense | De novo | 5/F | Yes | NA | NA | NA | Yes/6 | Allen et al. (2016; ND27062), Torkamani et al. (2014; Patient 3), and de Kovel et al. (2017; Patient 13) |
| 37 | c.1121C>T | p.Thr374Ile | Missense | De novo | 11/F | Yes | With aids | Nonverbal | Yes | Yes/13 | de Kovel et al. (2017; Patient 14) |
| 38 | c.1130C>A | p.Thr377Asn | Missense | De novo | 2/M | Yes | No | Nonverbal | No | Yes/6 | This study |
| 39 | c.1132G>C | p.Val378Leu | Missense | De novo | 10/F | Yes | Yes | Words | Yes | No | Latypova et al. (2017) |
| 40 | c.1132G>T | p.Val378Phe | Missense | De novo | 8/M | Yes | No | Nonverbal | NA | Yes/14 | This study |
| 41 | c.1133T>C | p.Val378Ala | Missense | De novo | 3/F | Yes | No | Nonverbal | NA | Yes/13 | Soden et al. (2014), Thiffault et al. (2015), and de Kovel et al. (2017; Patient 12) |
| 42 | c.1135G>A | p.Gly379Arg | Missense | De novo | 7/M | Yes | Yes | Nonverbal | Yes | Yes/8 | Torkamani et al. (2014; Individual 2), Srivastava et al. (2014), and de Kovel et al. (2017; Patient 11) |
| 43 | c.1136G>T | p.Gly379Val | Missense | De novo | 2/ND | Yes | NA | NA | NA | Yes/24 | Miao et al. (2017) |
| 44 | c.1139A>G | p.Tyr380Cys | Missense | De novo | 5,5/F | Yes | Yes | Words | No | Yes/6 | This study |
| 45 | c.1141G>A | p.Gly381Arg | Missense | De novo | 7/M | Yes | No | NA | NA | Yes/3 | Allen et al. (2016; Patient 7) and de Kovel et al. (2017; Patient 10) |
| 46 | c.1144G>A | p.Asp382Asn | Missense | De novo | 6/F | Yes | Yes | Nonverbal | No | Yes/8 | This study |
| 47 | c.1153C>A | p.Pro385Thr | Missense | De novo | 17/M | Yes | With aids | Nonverbal | Yes | Yes/13 | de Kovel et al. (2017; Patient 9) |
| 48 | c.1173A>C | p.Lys391Asn | Missense | De novo | NA | NA | NA | NA | NA | NA | Fitzgerald et al. (2015) and de Kovel et al. (2017; Patient 8) |
| 49 | c.1180G>A | p.Gly394Arg | Missense | De novo | 9/F | Yes | Yes | Nonverbal | Yes | Yes/3,5 | This study |
| 50 | c.1183G>A | p.Gly395Arg | Missense | De novo | 9.5/F | Yes | Yes | Nonverbal | Yes | Yes/12 | This study |
| 51 | c.1183G>A | p.Gly395Arg | Missense | NA | 14/M | Yes | No | Nonverbal | Yes | Yes/8 | This study |
| 52 | c.1190G>T | p.Cys397Phe | Missense | De novo | 22/M | Yes | NA | NA | NA | Yes/10 | de Kovel et al. (2017; Patient 7) |
| 53 | c.1201G>A | p.Gly401Arg | Missense | De novo | 4/M | Yes | No | Nonverbal | NA | Yes/17 | Saitsu et al. (2015; Patient 1) and de Kovel et al. (2017; Patient 6) |
| 54 | c.1201G>A | p.Gly401Arg | Missense | De novo | 9/M | Yes | No | Nonverbal | NA | Yes/8 | This study |
| 55 | c.1226T>C | p.Ile409Thr | Missense | De novo | 8/M | Yes | Yes | Sentences | Yes | No | This study |
| 56 | c.1248C>G | p.Phe416Leu | Missense | De novo | 15/F | Yes | NA | NA | Yes | Yes/14 | Allen et al. (2016; Patient 8) and de Kovel et al. (2017; Patient 5) |
| 57 | c.1248C>G | p.Phe416Leu | Missense | De novo | 18/F | Yes | NA | NA | NA | Yes/42 | de Kovel et al. (2016; ID-2010D05815) and de Kovel et al. (2017; Patient 4) |
| 58 | c.1248C>A | p.Phe416Leu | Missense | NA | 14/F | Yes | Yes | Nonverbal | No | Yes/10 days | This study |
| 59 | c.1489G>T | p.Glu497* | Nonsense | De novo | 20/M | Yes | Yes | Sentences | Yes | No | This study |
| 60 | c.1503dup | p.Lys502* | Nonsense | De novo | 10/M | Yes | NA | NA | Yes | No | Fitzgerald et al. (2015) and de Kovel et al. (2017; Patient 3) |
| 61 | c.1599C>A | p.Tyr533* | Nonsense | De novo | 32/M | Yes | Yes | NA | NA | Yes/5 | de Kovel et al. (2016; ID-EP1852) and de Kovel et al. (2017; Patient 2) |
| 62 | c.1747C>T | p.Arg583* | Nonsense | De novo | 8/F | Yes | NA | Nonverbal | No | Yes/5 | Marini et al. (2017; ID-2) |
| 63 | c.1747C>T | p.Arg583* | Nonsense | De novo | 11/F | Yes | Yes | Sentences | Yes | No | de Kovel et al. (2017; Patient 1) |
| 64 | c.1747C>T | p.Arg583* | Nonsense | Maternal | 12/F | Yes | Yes | Nonverbal | Yes | No | This study |
- Note: Numbering is according to the complementary DNA sequence (RefSeq NM_004975.2). Novel mutations are indicated in bold.
- Abbreviation: NA, not available.
- a This patient also carries a de novo variant in GABRA5 (NM_000810:c.902C>T, p.Thr301Met).
Overall, including our data and those of the literature, we reviewed data of 47 distinct pathogenic variants identified in 64 unrelated patients (Table 1 and Figure 1). All variants except one were in exon 2. They included 37 missense variants (37/47; 79%), eight nonsense variants (8/47; 17%), and two frameshift variants (2/47; 4%). Ten variants were recurrent.
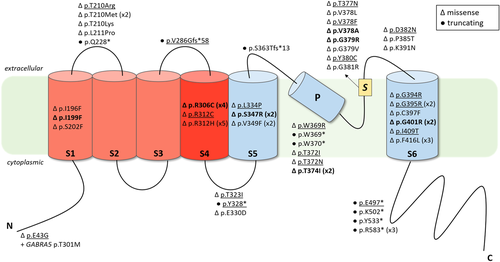
Schematic representation of Kv2.1 protein structure and location of KCNB1 previously described and novel variants. Variants are displayed as changes at protein level (p). Underlined variants correspond to novel variants reported for the first time in this report. For recurrent variants, the number of reported patients is indicated between parentheses next to the variant. Triangles and black circles respectively indicate missense variants and truncating variants (frameshift or nonsense variants). Variants, where functional studies have been previously conducted, are indicated in bold. Functional domains are represented by N: N-terminal domain (residues 1–186), S1: Segment S1 (residues 187–208), S2: Segment S2 (residues 229–250), S3: Segment S3 (residues 260–280), S4: Segment S4 (residues 295–316), S5: Segment S5 (residues 331–351), P: pore helix (residues 365–376), S: Selectivity filter (residues 377–381), S6: Segment S6 (residues 392–420), C: C-terminal domain (residues 421–858)
Most variants (42/47, 89%) were located in the S1 to S6 transmembrane segments of the protein. The K+ selectivity filter located between the S5 and S6 transmembrane segments (amino acid 377–381) had eight distinct missense variants, including three novel variants in our series. Three were at amino acid position 378 (p.Val378Ala; p.Val378Phe; p.Val378Leu), representing a potential variant hotspot. Two recurrent variants affected the voltage sensor domain. The p.Arg306Cys was found in four patients (Patients 12–15) and the p.Arg312His was found in five patients (Patients 17–21). A different amino acid change at this position, p.Arg312Cys, was also found in one patient (Patient 16). Six recurrent variants were localized in the pore domain of the protein (S5–S6). Two were in the S5 transmembrane segment, each one found in two patients (p.Ser347Arg, Patients 26 and 27; p.Val349Phe, Patients 28 and 29). The p.Val349Phe variant found in Patient 29 resulted from somatic mosaicism (Marini et al., 2017). Three recurrent variants were located in the S6 transmembrane segment. The p.Phe416Leu variant was found in three patients (Patients 56–58), whereas the p.Gly395Arg and p.Gly401Arg variants were found in two patients each (Patients 50, 51, and 53, 54).
Patient 1 had a KCNB1 variant in the exon 1, affecting the cytoplasmic N-terminal region (p.Glu43Gly). The highly conserved N-terminal domain is a critical determinant for subunit self-association into tetrameric channels and the p.Glu43Gly variant was thus predicted to be damaging (Xu et al., 1995). This patient also carried a de novo variant in GABRA5 gene (MIM# 137142, p.Thr301Met), which encodes the α5 subunit of the gamma-aminobutyric acid type-A (GABAA) receptor. Recently, Butler et al. (2018) provided functional evidence of the pathogenic effect of a nearby GABRA5 variant (p.Val294Leu) in a patient with severe DEE. Therefore, both variants were considered to be contributing to the patient's phenotype.
All variants located within the intracellular C-terminal domain of the protein were truncating. The non-sense variant p.Arg583* was found in 3 unrelated patients (Patients 62–64; inherited from the mother in Patient 64). Two other nonsense variants were in the pore helix between S5 and S6 (p.Trp369*; p.Trp370*). The last four truncating variants were in the linkers: S1–S2 linker (p.Gln228*), S3–S4 linker (p.Val286Glyfs*58), S4–S5 linker (p.Tyr328*), and S5–S6 linker (p.Ser363Thrfs*13).
5 FUNCTIONAL RELEVANCE AND CHARACTERIZATION OF KCNB1 VARIANTS
To date, seven KCNB1 missense variants have been functionally characterized using different cellular models (Calhoun et al., 2017; Saitsu et al., 2015; Thiffault et al., 2015; Torkamani et al., 2014). Distinct functional effects have been described among studies. The p.Ile199Phe variant, located in the S1 transmembrane segment of the voltage-sensing domain, reduces channel availability due to shift in the voltage dependence of activation compared to the wild-type (WT) channel (Calhoun et al., 2017). A variant in the voltage sensor domain S4, p.Arg306Cys, induces currents similar to those in the WT channel but disrupts sensitivity and cooperativity of the sensor whereas the p.Gly401Arg variant, located in the pore domain S6, has a dominant-negative effect on WT channels and abolishes endogenous currents (Saitsu et al., 2015). Both variants inhibit repetitive neuronal firing by preventing the production of sufficiently deep interspike voltages (Saitsu et al., 2015). Other KCNB1 variants affecting the pore domain result in loss of ion selectivity and gain of inward cation conductance of Kv2.1 channels (Thiffault et al., 2015; Torkamani et al., 2014). Since these mutants also induce reduced current density at more depolarized voltages, they were predicted to result in depolarized resting membrane potential and impaired membrane repolarization leading to increased cellular excitability (Torkamani et al., 2014). Changes in channel expression and localization were also suggested to contribute to the pathophysiology of Kv2.1 pore variants (Thiffault et al., 2015). Therefore, given the biophysical properties of these pathogenic variants on Kv2.1, they should be considered as loss-of-function rather than gain-of-function.
Finally, delayed-rectifier potassium current is diminished in hippocampal neurons cultured from Kcnb1−/− (Kv2.1−/−) mice (Speca et al., 2014). Interestingly, mice lacking Kv2.1 have no spontaneous seizures but display increased seizure susceptibility in response to proconvulsant drugs and exhibit a range of behavioral disorders associated with marked hyperactivity (Speca et al., 2014).
6 EXPANDING THE PHENOTYPIC SPECTRUM OF KCNB1 VARIANTS
The cohort of 64 patients included 34 males and 28 females (sex ratio M/F 1.2; data available for n = 62/64) with pathogenic KCNB1 variants, aged from 1.2 to 33 years (median age at study, 8 years; data available for n = 62/64).
We report here six new patients aged from 8 to 23 years who did not develop seizures (Patients 16, 34, 35, 55, 59, 64).
Overall, 53/63 patients (85%) developed epilepsy with a median age at seizure onset of 12 months (range: 10 days-5 years; mean, 15 months; data available for n = 52/53). All patients had developmental delay before seizure onset. Thirty-seven patients (70%) exhibited several seizure types during follow-up, including generalized tonic–clonic seizures (n = 28), focal seizures (n = 24), epileptic spasms (n = 21), tonic seizures (n = 14), myoclonic seizures (n = 14), atypical absences (n = 13), atonic seizures (n = 9), and clonic seizures (n = 5). Nine patients (18%) developed only epileptic spasms at a mean age of 12 months (range, 5-21 months), consistent with the syndrome of infantile spasms (Pavone, Striano, Falsaperla, Pavone, & Ruggieri, 2014). Behavioral issues occurred in 37/49 patients with available data (76%), including autism spectrum disorder in 26 (53%), aggression in 20 (41%), and hyperactivity in 12 (24%).
Developmental delay was reported in all patients with available data (n = 62/64). A severe expressive language disorder was found in all 42 patients aged 3 years or older in whom some language data was available. Twenty-four patients (57%) were nonverbal whereas the remaining were able to speak some words or short sentences. Data on ambulation was available for 43 out of 61 patients aged 2 years or older. Thirty-one patients (72%) achieved independent walking. Two patients (5%) aged 11 and 17 years walked with assistance and 10 patients (23%) were nonambulatory. The median age of walking reported for 22/31 patients was 24 months (range, 18-54 months). Neurological examination data were available for 52 patients. Hypotonia was the most frequently reported sign (n = 25/52, 48%), followed by spasticity (n = 11, 21%), ataxia (n = 10, 19%), extrapyramidal symptoms including dystonia and choreiform movements (n = 8, 15%) and hyperlaxity (n = 8, 15%).
Electroencephalographic (EEG) data were available for 51 patients with epilepsy. EEG recordings were characterized by slow background activity, with a combination of multifocal (n = 30), focal (n = 12) or generalized spikes and/or spikes and waves (n = 23). Hypsarrhythmia was reported in eight children. Sleep activation of EEG abnormalities was found in 13 patients and photosensitivity in four patients. Thirty-eight patients (72%) had pharmacoresistant epilepsy, whereas 13 patients (26%) responded to antiepileptic drugs. In particular, six out of nine patients who developed only infantile spasms became seizure-free with treatment.
Data on brain magnetic resonance imaging (MRI) was available for 54/64 patients. MRI was normal in 42 of the patients carrying KCNB1 variants (78%). One patient had a normal computed tomography scan. Mild atrophy was reported in seven patients and documented as progressive by serial MRIs in three of them (Patients 47, 53, and 54). Nonspecific periventricular white matter abnormalities were reported in two patients (Patients 37 and 52). One patient had two areas of focal cortical dysplasia associated with focal seizures, multifocal spikes on EEG and responded to carbamazepine (Patient 4). Another patient had small bilateral periventricular heterotopias (Patient 34).
7 GENOTYPE-PHENOTYPE CORRELATION
Patients with KCNB1 variants have a wide phenotypic spectrum including intellectual disability, behavioral disorders, and frequent epilepsy. We examined whether the localization or the type of variant correlated with the phenotype.
We reported the first pathogenic variant in the N-terminal domain in a patient who have a DEE with early developmental delay and a seizure onset at 16 months, evolving into severe cognitive impairment and intractable focal and generalized seizures (Patient 1). Interestingly, a pathogenic variant in GABRA5 was also identified in this patient, located in the pore-forming M2 transmembrane domain of the GABA receptor (p.Thr301Met). A nearby pathogenic GABRA5 variant (p.Val294Leu) was reported in a patient with severe developmental delay who differs from our patient as that patient had earlier seizure onset, autistic behavior and spastic quadriparesis (Butler et al., 2018). As both variants (KCNB1 and GABRA5) were predicted to be damaging, a double hit mechanism may be responsible for our patient's phenotype.
In a previous review of 26 patients with a KCNB1 variant, missense variants in the voltage sensor domain or the pore region of the protein were thought to correlate with a more severe phenotype of epilepsy and global developmental delay (de Kovel et al., 2017). Our results show that nonambulatory patients were more frequent in those with a variant in the S4 to S6 crucial domains (n = 10/31) compared with those with a variant in other regions of the protein (n = 0/10; p = .048, unilateral Fisher's exact test). However, there was no significant difference in terms of epilepsy severity, language acquisition or behavioral issues. In particular, we report here four new variants in the S4 to S6 domains in patients with no epilepsy and a moderate developmental delay (Patients 16, 34, 35, and 55). These findings do not support a clear correlation of mutations in these domains to the most severe phenotype (de Kovel et al., 2017). The epilepsy phenotype was highly variable, as illustrated in patients carrying the same variant. For instance, the recurrent variant p.Arg312His in the voltage sensor domain was associated with infantile spasms (Patients 17 and 18), infantile-onset focal seizures (Patient 19), pharmacoresponsive late-onset focal seizures (Patient 21), and intractable generalized seizures (Patient 20). In addition, a patient carrying a different amino acid change at the same position (p.Arg312Cys, Patient 16) did not develop epilepsy but had a developmental encephalopathy with intellectual disability and behavioral issues. Therefore, no genotype–phenotype correlations can be established based on the localization of the missense variants on the protein domains and more experimental studies are needed to understand the underlying pathophysiology of this phenotype heterogeneity.
Interestingly, all four variants found in the C-terminal part of the protein were truncating. Though these patients had severe developmental delay and behavioral disorders, four out of six did not develop seizures (Patients 59, 60, 63, and 64) and both patients that developed epilepsy had pharmacoresponsive infantile spasms syndrome (Patients 61). As all these truncating variants occur within the last exon, they are not anticipated to result in a nonsense-mediated messenger RNA (mRNA) decay but rather to produce a truncated protein with a potential dominant-negative effect on channel function (Khajavi, Inoue, & Lupski, 2006). C-terminal truncation of Kv2.1 channel has been shown to impact surface expression, voltage-dependent gating function, and phosphorylation-dependent modulation of the channel (Jensen et al., 2017; Mohapatra et al., 2008). Truncated Kv2.1 channel could thus impact trafficking of tetrameric Kv2.1 channel to the cell membrane leading to functional consequences on channel properties, as demonstrated in few other channelopathies (Aizawa et al., 2004; Duarri et al., 2015; Mezghrani et al., 2008; Puckerin et al., 2016). Other truncating variants were also in the last exon of the gene but located upstream the C-terminal domain, either in extracellular loops or in the pore helix, with no obvious phenotypic difference compared to missense variants. We speculate that they may also escape nonsense-mediated mRNA decay and result in either a nonfunctional or even deleterious truncated protein missing vital channel domains. Indeed, most functionally studied missense variants displayed a range of dominant-negative effects (Calhoun et al., 2017; Saitsu et al., 2015; Thiffault et al., 2015; Torkamani et al., 2014) that might thus represent the main underlying pathogenic mechanism of KCNB1 variants, as for some other potassium channelopathies-related DEE (Jorge et al., 2011; Masnada et al., 2017; Orhan et al., 2014; Smets et al., 2015). The phenotype severity might be related to the extent of mutation-induced functional Kv2.1 channel impairment, in addition to other (genetic or environmental) modulating factors that could interact with the mutation during development shaping the phenotype. More electrophysiological studies and animal models are now needed to confirm the pathogenic mechanism of truncating KCNB1 variants.
8 CLINICAL AND DIAGNOSTIC RELEVANCE
Even though targeted therapy is not yet available for KCNB1 encephalopathies, genetic diagnosis is important in clinical practice to stop unnecessary diagnostic tests, to correctly inform parents about prognosis and allow accurate genetic counseling.
All the KCNB1 variants identified occurred de novo except in one patient who inherited her p.Arg583* variant from her affected mother. Her mother had an intellectual disability with delayed language skills, no reading and writing abilities but could live independently. She did not develop epilepsy. Her 12-year-old daughter did not have seizures but had a severe neurodevelopmental disorder with no language acquisition, autism spectrum disorder and behavioral disorders. We thus report the first inherited KCNB1 variant, associated with intrafamilial variable expressivity. This observation that individuals harboring a KCNB1 variant with a “less-severe” phenotype can transmit a severe disease is important for accurate interpretation of inherited variants, prenatal diagnosis, and genetic counseling.
In addition, Patient 1 carrying KCNB1 and GABRA5 variants suggests the co-occurrence of two deleterious variants in the same patient. This 30-year-old patient had a severe phenotype of DEE with social withdrawal, no language acquisition, and daily seizures. Additional data from exome and genome studies will enable us to better understand such double hit genotypes and will help in genetic counseling. In some patients, seizure frequency attenuates and disappears over time with prolonged periods of remission and the possibility of withdrawn antiepileptic drugs (Marini et al., 2017). In this study, we also reported six new patients without epilepsy, further strengthening the importance of KCNB1 in neurodevelopmental disorders, beyond DEEs.
9 CONCLUSIONS AND PERSPECTIVES
We review the clinical and molecular spectrum of patients with KCNB1 variants through the description of 18 unreported pathogenic variants in 27 new unrelated patients and an exhaustive review of the literature increasing the number of patients to 64 and of pathogenic variants to 47. KCNB1 encephalopathies encompass a wide spectrum of neurodevelopmental disorders, including early-onset global developmental delay with predominant language difficulties and behavioral impairment. Epilepsy is frequent, including the DEEs, but syndrome type and prognosis are variable. Identification of new patients is thus important to fully delineate the phenotypic spectrum of KCNB1 dysfunction. Most variants occur de novo and mainly consist of missense variants with some hotspots located in the voltage sensor and the pore domain of the protein. Few truncating variants are reported with one hotspot in the C-terminal domain associated without epilepsy or with a mild epilepsy. However, available data do not inform further genotype–phenotype correlations, especially in terms of neurodevelopmental outcome. The variety of reported functional effects might contribute to the heterogeneous phenotype. Potential modulation by coexisting pathogenic variants in other genes is likely to be another modifier of the phenotype. Beyond the variant effects on ion currents, new animal models reproducing KCNB1 variants are needed to explore more accurately the associated pathophysiological mechanisms of disrupted neurodevelopmental pathways and to develop targeted therapies.
ACKNOWLEDGMENTS
The authors thank the association “KCNB1 France” as well as patients and their families for their participation in this study. This study was supported by State funding from the Agence Nationale de la Recherche under “Investissements d'avenir” program (ANR-10-IAHU-01) and the Fondation Bettencourt Schueller (C. B. and R. N.), the ERC Consolidator Grant (E. K.), the Curekids New Zealand and the Health Research Council of New Zealand (L. S. and I. E. S.), the National Health and Medical Research Council of Australia (I. E. S.), the European Commission Seventh Framework Programme under the project DESIRE (Grant agreement No. 602531, R. G.).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.