Mutations in PLS1, encoding fimbrin, cause autosomal dominant nonsyndromic hearing loss
[Note Added in Proof: During the evaluation process of this article, a paper was published by Schrauwen et al. (2019) that reported similar findings of hearing impairment related to a variant in PLS1. This result further validates the findings reported here.]
Abstract
Nonsyndromic hearing loss (NSHL), a common sensory disorder, is characterized by high clinical and genetic heterogeneity (i.e., approximately 115 genes and 170 loci so far identified). Nevertheless, almost half of patients submitted for genetic testing fail to receive a conclusive molecular diagnosis. We used next-generation sequencing to identify causal variants in PLS1 (c.805G>A, p.[E269K]; c.713G>T, p.[L238R], and c.383T>C, p.[F128S]) in three unrelated families of European ancestry with autosomal dominant NSHL. PLS1 encodes Plastin 1 (also called fimbrin), one of the most abundant actin-bundling proteins of the stereocilia. In silico protein modeling suggests that all variants destabilize the structure of the actin-binding domain 1, likely reducing the protein's ability to bind F actin. The role of PLS1 gene in hearing function is further supported by the recent demonstration that Pls1−/− mice show a hearing loss phenotype similar to that of our patients. In summary, we report PLS1 as a novel gene for autosomal dominant NSHL, suggesting that this gene is required for normal hearing in humans and mice.
1 INTRODUCTION
Hearing loss (HL) is the most common sensory disorder (Azadegan-Dehkordi, Ahmadi, Koohiyan, & Hashemzadeh-Chaleshtori, 2019) affecting more than 5% of the world's population (World Health Organization; http://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss), with approximately half of cases attributed to a genetic cause (Nishio et al., 2015). It can be classified according to several factors, such as the age of onset (congenital or acquired), the type (conductive, sensorineural, or mixed), the pattern of inheritance (autosomal recessive, autosomal dominant, X-linked, or mitochondrial cases), and the presence or not of other clinical features, with syndromic HL (SHL) and nonsyndromic HL (NSHL) accounting for 30% and 70% of cases, respectively (Alford et al., 2018; Dror & Avraham, 2009; Parker & Bitner-Glindzicz, 2015; Rehm & Morton, 1999; Smith, Shearer, Hildebrand, & Camp, 2017).
To date, approximately 115 genes and 170 loci have been linked to NSHL (http://hereditaryhearingloss.org/). Because of this genetic heterogeneity, providing a molecular diagnosis for patients with HL is challenging. Furthermore, current genetic tests fail to provide a diagnosis for ~40% of cases, suggesting that many novel HL genes remain to be discovered (Castiglione, Busi, & Martini, 2013). In this light, next-generation sequencing (NGS) of undiagnosed HL patients and families, together with validation using animal models offer a powerful approach to identify and characterize new causative genes (Azaiez et al., 2015; Girotto et al., 2013; Morgan et al., 2019).
The identification of new genes is essential for further understanding the molecular mechanisms of the hearing system, as well as providing genetic counseling, recurrence risk estimation, and novel therapeutic options.
In this study, we report PLS1 as a novel gene for autosomal dominant HL and describe causative mutations in three independent families of European ancestry, precisely one from Italy, one from the United States, and one from France.
2 MATERIAL AND METHODS
2.1 Ethical statement
For Family 1, approval was obtained before study onset from the Institutional Review Board of IRCCS Burlo Garofolo, Trieste, Italy. All patients provided written informed consent forms for both genetic counseling and molecular genetic testing. Written informed consent was obtained from the next of kin on behalf of the minors/children involved in this study.
For Family 2, written consent was obtained before enrolling subjects into a research protocol approved by the Institutional Review Board at Nationwide Children's Hospital (IRB11–00215 Study: Using Genome Sequencing to Identify Causes of Rare Birth Defects and Rare Disorders).
For Family 3, informed consent to genetic testing was obtained from all members after the explanation of the nature and its possible implications for the patients and family. This study was approved by the Institutional Review Board (IRB) from CHU Montpellier under the number 2018-IRB-MTP_05-05.
All research was conducted according to the ethical standard as defined by the Helsinki Declaration.
2.2 Subjects
2.2.1 Family 1
An Italian family affected by NSHL with a likely dominant pattern of inheritance was enrolled in this study (Figure 1a). All the affected family members underwent a careful clinical examination. Pure-tone audiometry evaluation showed bilateral sensorineural symmetric medium-high frequency hearing loss. Conductive hearing impairment was excluded thanks to bone conduction thresholds (similar to the air conduction thresholds), tympanometry (all affected patients present with type A tympanogram), and acoustic reflex (ipsilateral and contralateral in both ears ranging from 90 to 95 dB at 500 Hz, 1 kHz, 2 kHz, and 4 kHz). Clinical examination and clinical history were negative for the presence of vestibular dysfunction as well as for any kind of syndromic hearing loss. The presence of Cytomegalovirus infections and autoimmune phenotypes was also ruled out.
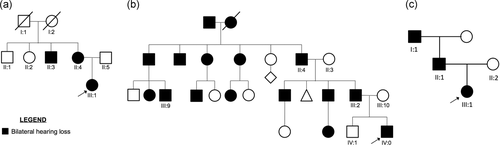
Pedigrees of Family 1, 2, and 3. (a) Pedigree of Family 1. (b) Pedigree of Family 2. (c) Pedigree of Family 3. Filled symbols represent affected individuals. Probands are indicated with an arrow. Individuals with Roman numeric labels were analyzed in this study
Figure S1 displays the latest audiological examination of the affected patients. Pure-tone audiometry of patient III:1 (performed at the age of 12) displays bilateral symmetric mild to severe medium-high frequency hearing loss, first detected at the age of eight. The proband's mother (II:4) at the age of 48 shows a similar down-sloping audiometric pattern (moderate hearing loss at the 4,000 Hz and severe at the high frequencies) that was originally discovered around the age of 30. Finally, the proband's uncle (II:3) reports medium-high frequency hearing loss, identified in his adulthood (~ 30 years old). The proband's father (II:5) and two maternal uncles (II:1, II:2) show normal hearing. No information is available for the proband's maternal grandparents.
All patients were negative for mutations in GJB2, GJB6, and MTRNR1 and in 96 HL genes (Vozzi et al., 2014).
Genomic DNA was extracted from peripheral blood using QIAsymphony instrument (Qiagen), quantified using Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies) and checked on a 0.8% agarose gel, stained with ethidium bromide.
2.2.2 Family 2
Family 2 is a large American kindred of Irish and German ancestry with bilateral hearing loss segregating in dominant fashion (Figure 1b). The proband is a male with congenital bilateral sensorineural hearing loss of profound severity. He also had failure to thrive and a pericardial effusion. Figure S1 displays the first audiological examination performed in the proband at 1 month of age. Further clinical evaluations at 1 year and 2 years of age did not show any progression of the hearing thresholds, indicating stable NSHL affecting all frequencies. The patient underwent panel testing for hearing loss genes (University of Iowa) and clinical exome sequencing (Baylor), both of which failed to provide a diagnosis. He had a strong family history, including a father with congenital HL and multiple affected relatives on the paternal side (Figure 1b). His mother and brother were unaffected. Genomic DNA was extracted from peripheral blood and isolated using the Purgene method (Qiagen) in the proband, while the other family members submitted saliva samples (Oragene). DNA was quantified using Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies).
2.2.3 Family 3
A French family was referred from ENT and Genetic departments with NSHL segregating in autosomal dominant fashion (Figure 1c). Patient III:1, a full-term baby, presented with a congenital moderate NSHL, revealed by abnormal neonatal otoacoustic emission during neonatal hearing screening. Hearing loss was confirmed by increased auditory brainstem responses (clicks stimuli record; ABR thresholds at 60 dB in both ears, at 2 and 4 months old). A 10-year follow-up confirmed the hearing loss stability. Pure-tone audiometry at 10 years old showed a moderate down-sloping sensorineural hearing loss (air and bone conduction thresholds were similar; Figure S1). Age of walking was 18 months old. Vestibular assessment at 3 years old was normal (vestibular ocular reflex in rotary chair and video head impulse test). Cochlear–vestibular MRI was normal. The proband's father (patient II:1) reported a moderate down-sloping SNHL diagnosed in his twenties during systematic military service assessment (unknown age of onset). The proband's grandfather also reported a moderate hearing loss, diagnosed in his forties (unknown age of onset). The grandfather's audiogram showed a more steeply down-sloping configuration (Figure S1). The mother's proband was unaffected.
Genomic DNA was extracted from peripheral blood and isolated using standard techniques. DNA was quantified using Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies) and Qubit dsDNA HS Assay (Invitrogen, Carlsbad, CA). The patients I:1, II:1, and III:1 underwent NSHL panel testing as described in Baux et al. (2017), which failed to provide any candidate variants in the screened genes.
2.3 Genetic analysis
2.3.1 Family 1
The proband (III:1) and her relatives (II:2, II:3, II:4, and II:5) were selected for whole exome sequencing (WES).
WES was carried out using the Ion ProtonTM platform (Thermo Fisher Scientific). Briefly, 1 μg of genomic DNA was used to construct DNA libraries using the Ion AmpliSeqTM Exome Kit. Libraries were sequenced with the Ion ProtonTM System (Thermo Fisher Scientific), according to the manufacturer's protocols.
2.3.2 Family 2
The proband (IV:0) and his unaffected brother (IV:1) were enrolled for whole genome sequencing (WGS) under a research protocol along with the affected father (III:2), unaffected mother (III:10), and affected paternal first cousin once removed (III:9). Sequencing libraries were constructed using the Illumina TruSeq DNA PCR-Free Library Preparation Kit. Paired-end 150 base pair reads were generated on an Illumina HiSeq. 4000 to a minimum depth of 30 × coverage.
2.3.3 Family 3
The proband (III:1) and her affected relatives (I:1 and II:1) were selected for WES. Sequencing libraries were constructed using the SureSelect Human All Exon V6 and SureSelectXT Target Enrichment kits (Agilent), according to the manufacturer's protocols. Prepared libraries were sequenced with 2 × 125 bp paired-end reads on a HiSeq. 2500 platform (Illumina).
2.4 Data analysis
2.4.1 Family 1
WES data were analyzed with Ion Torrent SuiteTM v4.0 software, set up with standardized parameters. Single Nucleotides Variations (SNVs) and Small Insertions and Deletions (INDELs) were collected into a standardized Variant Call Format (VCF) version 4.1 (Danecek et al., 2011). SNVs and INDELS were then annotated with ANNOVAR (Wang, Li, & Hakonarson, 2010) using human genome build 19 (hg19) as the reference.
SNVs leading to synonymous amino acids substitutions not predicted as damaging and not affecting highly conserved residues were excluded, as well as SNVs/INDELs with quality score (QUAL) < 20 and called in off-target regions.
A comparison between the identified genetic variants and data reported in NCBI dbSNP build150 (http://www.ncbi.nlm.nih.gov/SNP/) as well as in gnomAD (http://gnomad.broadinstitute.org/), and NHLBI Exome Sequencing Project (ESP) Exome Variant Server (Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP), Seattle, WA) led to the exclusion of those variants previously reported as polymorphism. In particular, a Minor Allele Frequency (MAF) cutoff of 0.0001 was used.
The pathogenicity of known genetic variants was evaluated using ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), Deafness Variation Database (http://deafnessvariationdatabase.org/) as well as The Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php).
Several in silico tools, such as PolyPhen-2 (Adzhubei, Jordan, & Sunyaev, 1999), SIFT (Ng & Henikoff, 2003), MutationTaster (Schwarz, Rödelsperger, Schuelke, & Seelow, 2010), LRT (Chun & Fay, 2009), and CADD score (Kircher et al., 2014) were used to evaluate the pathogenicity of novel variants. Moreover, the evolutionary conservation of residues across species was evaluated by PhyloP (Pollard, Hubisz, Rosenbloom, & Siepel, 2010) and GERP (Cooper et al., 2005) scores.
Human Splicing Finder (HSF) version 2.4.1 (http://www.umd.be/HSF/; Desmet et al., 2009) and Splice Site Prediction by Neural Network (NNSPLICE) version 9 (www.fruitfly.org) were used to predict the effect of the splice-site mutations.
We manually investigated the raw sequence reads for all the candidate pathogenic variants using the Integrative Genomics Viewer (IGV; Thorvaldsdottir, Robinson, & Mesirov, 2013) with the purpose of excluding likely false positive calls due to read misalignment.
Finally, on a patient-by-patient basis, identified variants were discussed in the context of phenotypic data at interdisciplinary meetings and the most likely disease-causing SNVs/INDELs were analyzed by direct Sanger sequencing on a 3500 Dx Genetic Analyzer (Applied Biosystems), using ABI PRISM 3.1 Big Dye terminator chemistry (Applied Biosystems).
Sanger sequencing was employed also to perform the segregation analysis within the family.
2.4.2 Family 2
FASTQ files were generated and secondary data analysis was performed using Churchill, a pipeline that implements a best-practices workflow for variant discovery and genotyping (Kelly et al., 2015). SNPeff, ANNOVAR, and custom in-house scripts were used to annotate the variant call set with mutation and gene information, protein functional predictions, and population allele frequencies. Variants filtering have been applied following the criteria reported above; variants consistent with autosomal dominant inheritance have been considered. Sanger sequencing of the PLS1 variant confirmed it as heterozygous in the proband (IV:0), father (III:2), paternal grandfather (II:4), and paternal first cousin once removed (III:9); it was wild-type in the unaffected sibling (IV:1) and mother (III:10).
2.4.3 Family 3
FASTQ files were generated using Illumina bcl2fastq utility, and secondary and tertiary analyses were performed with an in-house pipeline called nenufaar (https://github.com/beboche/nenufaar) based on GATK best practices for germline DNA variant discovery (https://software.broadinstitute.org/gatk/best-practices/workflow?id=11145). ANNOVAR (Wang et al., 2010) is the main annotation tool used in nenufaar annotation module.
Variants filtering have been applied following the criteria reported above. Only Human Splicing Finder (HSF) version 2.4.1 (http://www.umd.be/HSF/; Desmet et al., 2009) was used to predict the effect of the splice-site mutations. Variants consistent with autosomal dominant inheritance have been considered.
Sanger sequencing was finally performed to validate the segregation of identified PLS1 variant within the family.
2.5 Gene prioritization framework
To further prioritize genes discovered by NGS analyses, we considered the following features: (a) Signature of natural selection (prioritizing the genes with the highest number of SNPs with false discovery rate (FDR)< 0.1), (b) observed/expected missense and observed/expected loss of function (LOF) ratios in gnomAD database (prioritizing the genes with lowest score), and finally (c) the RVIS score (Residual Variation Intolerance Score; Petrovski, Wang, Heinzen, Allen, & Goldstein, 2013). The rationale behind our approach is that if a gene harbors any signature of natural selection this could hint to some important biological functions, and then some deleterious variation in this gene could show phenotypic effect.
Since all families have an European ancestry, the following methodology has been applied: (a) We investigated the pattern of natural selection using PCAdapt (Luu, Bazin, & Blum, 2017) in an European data set (using 1000 Genomes Phase 3 data; Gibbs et al., 2015); this methodology has been developed to detect genetic variants and genes involved in biological adaptation adjusting for population structure. This method allows to ascertain if the difference in frequency between two population is due to substructure or natural selection (such as North or South European populations).
(b) We collected the following metrics of gene constrains: Observed/expected missense loss of function ratios from gnomAD database and finally the RVIS score. This second step was done for each gene that has at least five SNPs putatively under selection in the European population.
The best candidate gene has been chosen according to the following criteria: Among the genes with the highest number of variants under selection we choose those with the lowest observed/expected missense, loss of function ratio, and RVIS score to finally obtain the candidate with the highest level of constrain.
In addition, analyses of protein–protein interaction (PPI) for the best candidate were done with STRING v 10.5 (Szklarczyk et al., 2017) focusing on those interactions with medium confidence (value> =0.4) and using as interaction sources: co-occurrence, experiments, databases, coexpression, neighborhood, and gene fusion. Then information about network enrichment and functional enrichment has been collected.
2.6 Building of fimbrin homology model and in silico mutagenesis
The homology model of human fimbrin was built with the MODWEB-MODBASE server version r198 (Pieper et al., 2011) using the sequence of human PLS1 as the input (UNIPROT entry: Q14651). Briefly, five out of 142 structural models were retained based on the MPQS, TSVMOD, LONGEST_DOPE, and DOPE criteria. Among the most reliable generated models, the one corresponding to the 120–373 amino acid segment of the target PLS1 was chosen for further structural analysis, which corresponds to the first actin-binding domain (ABD1). The model was built using as a template the X-ray structure of the 121–375 amino acid stretch of the N-terminal actin-crosslinking domain from human fimbrin (PDB: 1AOA, chain A; Goldsmith et al., 1997) which shares high (83.0%) sequence identity with the selected segment of the query sequence.
In silico amino acid substitutions (E264 to K; L238 to R, or F128 to S) were performed using PyMol (The PyMOL Molecular Graphics System, Version 1.8 Schrödinger, LLC) and the resulting structural models of each fimbrin mutant were energy-minimized in two steps, similarly to what was done previously (Marino & Dell’Orco, 2016; Marino, Sulmann, Koch, & Dell’Orco, 2015), the only difference being that the minimization was performed with unconstrained motion for backbone and side-chain atoms both for the first steepest descent algorithm step and for the following steps performed according to the conjugate gradients algorithm.
3 RESULTS
In this study, we report three unrelated families, identified using GeneMatcher website (Sobreira, Schiettecatte, Valle, & Hamosh, 2015), affected by autosomal dominant NSHL and carrying rare missense segregating variants in PLS1 gene. All the variants here described have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/).
3.1 Family 1
After targeted re-sequencing of 96 known deafness genes did not reveal any disease-causing variant, we performed WES on all three affected individuals and two healthy subjects (II:2, II:3, II:4, II:5, and III:1). The overall mean-depth base coverage for WES was 99X, while, on average, 92% of the targeted region was covered at least 20-fold. A total of 83.412 genetic variants were called among the five subjects included in the WES study. After data filtering, six candidate missense variants, segregating with the disease in a dominant fashion, were considered. These have been first prioritized according to the role of the gene based on literature research. Three missense variants, located within genes associated with specific phenotypes not present in any of our patients, were excluded (i.e., MC1R associated with analgesia from kappa-opioid receptor agonist, female specific [MIM# 613098], skin/hair/eye pigmentation 2, blond/red hair/fair skin, UV-induced skin damage [MIM# 266300], albinism, oculocutaneous, type II, modifier [MIM# 203200], melanoma, cutaneous malignant, 5 [MIM# 613099]; ALOXE3 associated with ichthyosis, congenital, autosomal recessive 3 [MIM# 606545]; and PGAM2 associated with glycogen storage disease X [MIM# 261670]). Among the remaining SNVs, one involves a gene of unknown function, C14orf132, which has been recently proposed as a candidate for pre and early postnatal developmental delay (Tiirats et al., 2016), another one involved AVL9 gene that is a cancer driver candidate gene (Li et al., 2014) and the last one affects PLS1 gene that has recently been described as causing HL in mouse (Taylor et al., 2015). The c.805G>A (NM_002670.2) PLS1 allele, predicted as damaging by all the in silico tools, causes the amino acidic substitution p.(E269K), which affects a highly conserved residue (Figure 2b).
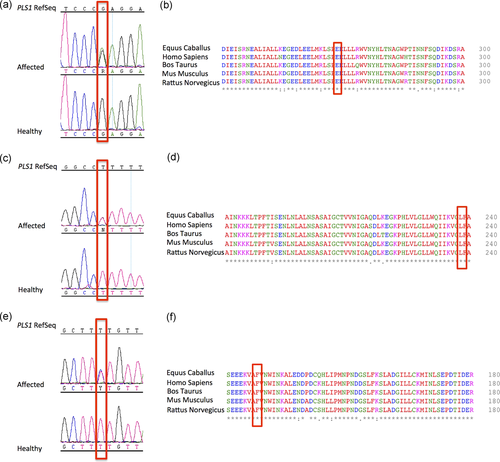
DNA sequence chromatograms and protein sequence alignment. (a) The figure displays DNA sequence chromatograms showing the nucleotide variant identified in Family 1. (b) Protein alignment showing conservation of residue E269 across species. (c) DNA sequence chromatograms of the nucleotide variant identified in Family 2. (d) Protein alignment showing the conservation of L238 residue across species. (e) DNA sequence chromatograms of the nucleotide variant identified in Family 3. (f) Protein alignment showing the conservation of F1288 residue across species
This variant was not described in any public database and was not present in our internal database of 1,000 WGS data. Sanger sequencing demonstrated the correct segregation of this allele within the two-generation family (PLS1_Family_1_For: 5′-CATTGACATGTACTTGCTATGG-3′; PLS1_Family_1_Rev: 5′-CTGCACATCCTTATTTCATGTC-3′; Figure 2a). Taking into account these data, the PLS1 variant was considered as likely causative.
3.2 Family 2
The family was enrolled in a rare disease research study and WGS was performed on the proband, unaffected sibling, both parents, and a distant affected relative, achieving 20–33x haploid coverage per individual.
Of the ~7.36 million SNVs and small indels uncovered by WGS, some 6,705 were rare variants (MAF< 0.05) in or near coding regions. Of these, only three were nonsynonymous changes, segregated with disease consistent under an autosomal dominant inheritance model (heterozygous in proband, father, and paternal cousin, but absent from sister and mother), and were sufficiently rare (MAF< 0.0001 in the gnomAD database) to underlie a dominant disorder. The first was a missense variant in PPP2R2A (NM_00217.3:c.896A>G, p.Y299C). PPP2R2A encodes an isoform of regulatory subunit B of phosphatase 2, which is one of four major serine/threonine phosphatases and implicated in the control of cell growth and division. It is ubiquitously expressed, and had no obvious connection to auditory pathways. The second variant was a missense change in TEX15 (NM_001350162.1:c.4258 T>C, p.[C420R]). It has been observed in 11 gnomAD individuals (MAF= 0.00004396) and is predicted to be damaging by only 2/10 in silico algorithms. TEX15 encodes a protein required for DNA double-strand break repair, chromosome synapsis, and meiotic recombination in spermatocytes. Loss-of-function mutations in TEX15 cause spermatogenic failure 25 (MIM #617960), an autosomal recessive disorder characterized by small testes and infertility. Given the gene's established function and the variant properties, we consider it is likely to be benign. The third candidate variant was a missense change in PLS1 (NM_002670.2:c.713 T>G, p.[L238R]) that has never been observed in gnomAD populations, making it extremely rare.
The variant is predicted to be damaging by all (10/10) in silico algorithms (Kopanos et al., 2019) and affects a highly conserved residue (Figure 2d); thus, we considered PLS1 to be the strongest candidate variant.
While this gene was likely detected in the proband's clinical WES, it would not have been reported since PLS1 was not associated with disease in the OMIM database. Sanger sequencing demonstrated the correct segregation of this allele within the family (PLS1_Family_2_For: 5′-TTTGGCAGTCCATTGTTTCTT-3′; PLS1_Family_2_Rev: 5′-TCGGGAGAAAGCTTCATCAG-3′; Figure 2c).
3.3 Family 3
The analysis of the affected patients with a targeted panel including 63 known NSHL genes did not reveal any disease-causing variant (Baux et al., 2017). We then performed WES on all three affected individuals (I:1, II:1, and III:1). The overall mean-depth base coverage for WES was 134X, while on average 94% of the bases was covered at least 15-fold (89% > 20x).
A total of 110,000 genetic variants were called per patient. Variants not in common between affected individuals were filtered out, remaining 4,717 variants with a MAF < 0.005. Among them, 250 variants were nonsynonymous and 16 variants localized in six different genes remained after filtering. Five of these six genes were reported with functions not related to hearing loss: ABCB6 (MIM# 605452) associated with dyschromatosis universalis hereditaria 3 (MIM# 615402) and microphthalmia isolated with coloboma 7 (MIM# 614497), ZNF717 (MIM# 618405) implicated in osteogenic differentiation, ABTB1 (MIM# 608308) involved in PTEN signaling pathway, KRT18 (MIM# 148070) related to cirrhosis, and CTBP2 (MIM# 602619; in which 11 of 16 variants remained) associated with prostate cancer. As a result, only the PLS1 (MIM# 602734) gene remained as putative responsible for the phenotype as a Pls1 knockout mouse model had been associated with a hearing loss phenotype.
The c.383 T>C, p.(F128S) PLS1 variant (NM_002670.2), is predicted as damaging by all the in silico tools and impacts a highly conserved residue (Figure 2f).
Sanger sequencing validated the correct segregation of this allele within the family (PLS1_Family_3_For: 5′-GGAAGTGCTTTGGGAAGTGC-3′; PLS1_Family_3_Rev: 5′-GACAATGGACAGCTGAATTGG-3′; Figure 2e).
3.4 PLS1 prioritization framework
In addition to the available literature data, and to further help the prioritization process, a population-based approach aimed at the detection of signal of selection among WES candidates was carried out. Analyses of Natural Selection among the genes discovered in this study revealed that only three of them harbors signature of selection (i.e., AVL9, PLS1, and CTBP2), being PLS1 the gene with highest number of markers under selection (see Table S1). Furthermore, PLS1 is the one with the lowest observed/expected missense ratio and lowest RVIS score (the lower the values the higher the constrain). In this light, according to our prioritization framework, we choose PLS1 as the best candidate gene in our study.
Furthermore, PPI analyses using PLS1 as core showed that this gene forms a network functionally enriched for actin binding and actin filament binding in humans, thus confirming results obtained in the animal model. In particular, analyses using STRING revealed a network of interaction for PLS1 with ten different proteins with a significant PPI enrichment (p-value 2.07e−10), indicating that proteins linked to PLS1 are somewhat biologically connected, as a group. These proteins belong to the following functional groups defined by Gene Ontology (GO) analyses: Regulation of cellular component size (GO:0032535, FDR = 0.00018), cytoskeletal protein binding (GO:0008092, FDR= 0.000292), structural constituent of cytoskeleton, (GO:0005200, FDR= 0.000314), actin binding (GO:0003779, FDR= 0.00218), and actin filament binding (GO:0003779, FDR= 0.00611).
3.5 Structural details of the first ABD domain of fimbrin and predicted effects of the missense mutations
PLS1 encodes the 629 amino acid protein, fimbrin also known as plastin-1, an actin bindling protein abundant in stereocilia of cochlear hair cells whose complete three-dimensional structure is currently unknown. Overall, the three new variants here identified affect all three isoforms of PLS1, and in particular, the p.(E269K) variant affects the second calponin-homology (CH2) domain of the protein, which is thought to be involved in actin binding (Sjöblom et al., 2008), whereas the p.(L238R) and p.(F128S) variants map within the first CH domain (CH1; Figure 3).

Schematic of fimbrin protein. Fimbrin contains a N-terminal regulatory region that includes two EF-hand domains, followed by two tandem actin-binding domains (ABD1 and ABD2), each consisting of two calponin-homology (CH) domains. The p.(F128S), p.(L239R), and p.(E269K) variants map in the first actin-binding domain (ABD1)
Fimbrin presents the unique feature of possessing in the same polypetide chain two tandem repeats of domains capable of binding F-actin (ABD) in contrast to other homolog proteins such as spectrin and alpha-actinin, which contain only one such domain and thus require dimerization to crosslink F-actin (Bañuelos, Saraste, & Djinović Carugo, 1998). To assess the potential structural consequence of the novel NSHL-associated mutations, which are located in the first ABD, we built a homology model covering the 120–373 region of fimbrin, including the first two CH domains (CH1 and CH2, Figure 4) and the interconnecting low-complexity region, altogether forming the ABD1 domain. In line with what has been reported by high-resolution structures (Keep, Norwood, Moores, Winder, & Kendrick-Jones, 1999), each individual CH domain is an assembly of four alpha helices, three of which bundle parallel to one another, and the fourth one, respectively αA in CH1 (CH1–α1A) and in CH2 (CH2–α2A), is faced perpendicular to the bundle (Figure 4a). The overall topology is that of a tightly packed ABD made of two complementary, tandemly-arranged CH domains, which interact with one another by means of both polar and apolar interactions. In particular, a salt bridge between E269 and K235 ensures a strong electrostatic interaction between CH2–α2A and CH1–α1F, which adds to the stabilizing effect of a network of hydrophobic interactions between the side chains of L238, I234, W131, and F128 in CH1 and A366 and F350 in CH2 (Figure 4b).

Structural model of the first actin binding domain (ABD) of human fimbrin (expressed by PLS1) based on homology modeling. (a) The first ABD is formed by the optimal interaction between the first CH domain (CH1, orange) and the second CH domain (CH2, blue) arranged tandemly and separated by the low-complexity linker (pink). Residues that have been found to be mutated in NSHL, namely E269, L238, and F128, are represented in sticks and labeled. Nomenclature for the secondary structure of each CH domain is according to Klein et al. (2004). (b) Zoomed-in view of the interaction between CH2–α2A and CH1–α1F mediated by a salt bridge between residues E269 and K235. The hydrophobic cluster involving L238 and F128 further stabilizes the interaction of CH2–α2F with CH1–α1F and CH1–α1A, respectively. (c) The Glu–>Lys substitution in position 269 breaks up the stabilizing salt bridge shown in (b) and induces an electrostatic repulsion between CH2–α2A and CH1–α1F. (d) Insertion of a positive charge and amine groups (R238) as in the discovered mutation partly disrupts the optimal hydrophobic network involving the native L238 residue. (e) Substitution of Phe for Ser in position 128 weakens the hydrophobic interaction between CH1–α1A and CH2–α2F and perturbs the stabilizing interdomain hydrophobic network. NSHL, nonsyndromic hearing loss
The structural model allows a prediction of the structural effects of the three point mutations observed in this study. The substitution of the negatively charged E269 with a positively charged lysine (E269K) is predicted to break up the important salt bridge contributing to the stabilization of the CH1–CH2 domains interaction, as the physiologically charged amine groups will generate interdomain electrostatic repulsion (Figure 4c). On the other hand, replacing the hydrophobic leucine at position 238 with a polar and positively charged arginine (L238R) may severely perturb the optimal packing of hydrophobic residues observed in the WT protein (Figure 4d), eventually resulting in a perturbed conformation of the CH1–CH2 interface, with severe consequences for the whole ABD1 stability. A similar effect is predicted for the F128S substitution, where a substitution of the bulky aromatic side chain of a phenylalanine with the small and polar hydroxyl group of serine is likely destabilizing the network of hydrophobic interactions at the CH1–CH2 interface (Figure 4d,b), especially by weakening the interaction between CH1–α1A and CH2–α2F, mediated by F350 in CH2 and F128 in CH1in the WT protein.
In conclusion, although perturbing substantially different physical interactions, the E269K, L238R, and F128S mutations associated with NSHL might all similarly perturb the overall stability of the ABD1 by reducing the optimal interaction between the CH1–CH2 domains, with consequences on the capability of binding F actin.
4 DISCUSSION
In this study we identified missense variants in PLS1 in three unrelated families of European ancestry affected by autosomal dominant NSHL. This protein belongs to the human plastin family (i.e., PLS1, PLS2, and PLS3), a group of evolutionary conserved proteins that noncovalently crosslink actin filaments (F actin) into tight bundles (Shinomiya, 2012). Fimbrin is one of the most abundant actin-bundling proteins of the stereocilia (Taylor et al., 2015) in mice, and is required for stereocilia to grow to their normal width as well as maintain their integrity and function (Roy & Perrin, 2018). Furthermore, Pls1 (MGI: 104809) knockout mice have a moderate and progressive form of hearing loss associated with defects in stereocilia morphology; Taylor et al. (2015) speculated that mutations in the human PLS1 gene could be associated with mild and progressive forms of hearing loss. Despite the mouse model displays a recessive pattern of inheritance, our patients are affected by autosomal dominant NSHL. This is not surprising, since it is well known that, in some cases, mouse models can differ from the human phenotype, both in terms of clinical features, as well as in terms of inheritance mode. For instance, in case of hearing loss, some mouse models of genes that in humans show both an autosomal dominant and recessive pattern of inheritance, in mouse are only inherited in a recessive fashion and thus the heterozygous animals do not show any phenotype or only a slight defect (i.e., Ceacam16 [Cheatham et al., 2014], or Myo6 [Wong, Xu, Brahmachary, & Xu, 2016]).
Furthermore, our PPI analysis confirmed the assertions from animal models, suggesting that PLS1 forms a network functionally enriched for actin filament binding also in humans. Finally, population data indicate that PLS1 show some evidence of constrains against missense variation, being one with the highest number of variants (among the tested genes) harboring signal of natural selection and thus providing further evidence that this gene is biologically relevant.
The PLS1 variants identified in the present study are located in ABD1, a domain which binds one actin monomer in the filament and comprising two adjacent CH domains. Previous studies (Stradal, Kranewitter, Winder, & Gimona, 1998) highlighted that in proteins containing calponin-homology domains, both CH domains are necessary for achieving a completely functional protein which binds actin. Indeed, although proteins containing a single CH domain are in principle able to bind actin, it has been shown that the full function can be achieved only following protein dimerization (Keep et al., 1999), where the amino terminal CH domain is intrinsically able to bind actin with low affinity, and the carboxy terminal CH domain does bind actin extremely weakly or not at all. Only the complementary assembly of CH domains into an ABD domain allows fully functional F actin binding. Therefore, in the case of fimbrin, the CH1 domain may bind actin while the CH2 domain thus contributes to the overall stability of the complete ABD through interdomain helix–helix interactions. The localization of the three NSHL-associated variants at the CH1–CH2 interface (Figure 4) suggests a perturbation of the structural stability of the whole ABD1 via disruption of an essential electrostatic interaction (p.[E269K]) or hydrophobic core network (p.[F128S]), and p.[L238R]). This may result in general domain destabilization, which is expected to modify the F-actin binding capability of PLS1. It is possible to speculate that these changes may eventually cause an abnormal stereocilia formation, leading to the hearing defect identified in all patients.
Our study further reinforces the association of PLS1 variants and autosomal dominant NSHL in humans. The age of onset and audiometric features vary somewhat among mutation carriers in our families. The proband of Family 1 displays an early-onset NSHL that mainly affects the high frequencies. Similarly, the proband's mother shows comparable hearing thresholds, even though the onset of hearing deficit was around the age of 30. Also in Family 3, both the proband and the father show a similar down-sloping audioprofile despite the age of onset variability (congenital in the proband and around 20 years old in the father).
On the other hand, the proband of Family 2 presents with congenital HL (like the proband of Family 3) even though with a different phenotype (severe to profound hearing loss affecting all frequencies). Taken together, these observations suggest that a high variable expressivity (which is one of the main features of autosomal dominant diseases) may be characteristic of PLS1-associated hearing loss. Additional studies with greater sample numbers will be required to fully characterize the phenotypic spectrum of this disease.
Overall, all these observations (i.e., the identification of missense variants in three unrelated autosomal dominant NSHL families, our in silico protein modeling, and the previous work on PLS1 in mouse model) clearly support that PLS1 is required for normal hearing and is a novel gene for autosomal dominant HL.
ACKNOWLEDGMENT
We are very grateful to David Baux for his help in bioinformatics and we would like to thank the MGX plateform (Dr. Laurent Journot, Emeric Dubois, and Hugues Parrinello) for NGS facilities.




