Dominant-negative SOX9 mutations in campomelic dysplasia
Abstract
Campomelic dysplasia (CD) is an autosomal dominant, perinatal lethal skeletal dysplasia characterized by a small chest and short long bones with bowing of the lower extremities. CD is the result of heterozygosity for mutations in the gene encoding the chondrogenesis master regulator, SOX9. Loss-of-function mutations have been identified in most CD cases so it has been assumed that the disease results from haploinsufficiency for SOX9. Here, we identified distal truncating SOX9 mutations in four unrelated CD cases. The mutations all leave the dimerization and DNA-binding domains intact and cultured chondrocytes from three of the four cases synthesized truncated SOX9. Relative to CD resulting from haploinsufficiency, there was decreased transactivation activity toward a major transcriptional target, COL2A1, consistent with the mutations exerting a dominant-negative effect. For one of the cases, the phenotypic consequence was a very severe form of CD, with a pronounced effect on vertebral and limb development. The data identify a novel molecular mechanism of disease in CD in which the truncated protein leads to a distinct and more significant effect on SOX9 function.
1 INTRODUCTION
Campomelic dysplasia (CD) is a rare, usually perinatal lethal, autosomal dominant skeletal dysplasia, initially distinguished from other skeletal disorders by bowed lower extremities (Maroteaux et al., 1971). The phenotype is mainly characterized by an under mineralized skeleton with a small chest, shortened long bones, club feet, hypoplastic scapulae, 11 pairs of ribs, cleft palate, and micrognathia. Two-thirds of the affected males present with complete sex reversal or a lesser degree of genital defects. Most affected individuals die of respiratory insufficiency shortly after birth or in the neonatal period; the survivors usually die within the first few years of life but longer-term survivors have been reported (Mansour, Hall, Pembrey, & Young, 1995).
CD is caused by dominant mutations in the SRY-box containing gene 9 (SOX9), a key regulator of endochondral skeletal development (Foster et al., 1994; Kwok et al., 1995; Wagner et al., 1994). Most cases of CD are sporadic and caused by a de novo mutation, although families with recurrence due to parental germline mosaicism have been described (Gentilin et al., 2010). The SOX9 protein consists of 509 amino acids and contains several domains; a dimerization domain (DIM), a DNA-binding domain (high-mobility group, HMG), a small domain composed solely of proline, glutamine, and alanine (PQA), the function of which is unknown, and a C-terminal transactivation domain (TA; reviewed in Lefebvre & Dvir-Ginzberg, 2016). CD primarily results from missense, nonsense, frameshift, or consensus splice site, loss-of-function mutations in one of the three SOX9 exons. More rarely CD can result from chromosomal rearrangements or mutations in SOX9 regulatory regions (Foster et al., 1994; Kwok et al., 1995; Velagaleti et al., 2005; Wagner et al., 1994; Wirth et al., 1996) that reduce SOX9 expression. Despite the different types of mutations, an obvious correlation between the genotype and the severity of the condition has not been reported (Meyer et al., 1997). Because mutations in a single allele of SOX9 cause such severe skeletal abnormalities, it is evident that the dosage of SOX9 is critical for its normal function and, particularly based on the loss-of-function mutations, CD is believed to be caused by haploinsufficiency of SOX9 (Foster et al., 1994; Wagner et al., 1994). Moreover, heterozygous Sox9 knockout mice phenocopy most of the skeletal abnormalities of CD (Bi et al., 2001).
SOX9 is essential for chondrogenesis and chondrocyte differentiation; the gene is initially expressed in mesenchymal condensations of the early skeletal precursors and subsequently becomes exclusively expressed in chondrocytes. In the growth plate, SOX9 is at its highest levels in proliferating and prehypertrophic chondrocytes; its expression decreases as chondrocytes undergo hypertrophy (reviewed in Kozhemyakina, Lassar, & Zelzer, 2015). SOX9 functions as a transcriptional activator, directly targeting genes encoding key cartilage-specific extracellular matrix components, such as collagen types II, IX, and XI, other members of its own family like SOX5 and SOX6, and key signaling pathway mediators in cartilage, including FGFR3. SOX9 regulates chondrocyte-specific transcription by binding enhancer elements of its target genes, for example, it controls COL2A1 expression by directly binding an enhancer present in intron 1 (Bell et al., 1997; Lefebvre, Huang, Harley, Goodfellow, & de Crombrugghe, 1997; Liu, Li, Tanaka, Tsumaki, & Yamada, 2009). SOX9 also works as a transcriptional repressor, probably via indirect mechanisms such as activation of downstream transcriptional repressors (Ohba, He, Hojo, & McMahon, 2015).
Here, we describe four unrelated individuals with CD in which the phenotype resulted from heterozygosity for nonsense mutations in the terminal exon of SOX9, disrupting the transactivation domain but leaving the DNA-binding domain intact. We demonstrate the presence of a truncated protein in chondrocytes derived from three of these cases and show that the transactivation of COL2A1 expression was decreased relative to a CD case resulting from SOX9 haploinsufficiency, consistent with a dominant-negative effect. In two of the cases, the affected individuals had severe forms of CD that were not initially recognized as due to reduced SOX9 function.
2 MATERIALS AND METHODS
2.1 Cell culture
Primary chondrocytes were isolated from distal femurs of the affected individuals (International Skeletal Dysplasia Registry reference numbers R83–090, R89-086, R92–124, R09–315, and R14–170) or age-matched normal controls by incubation of fragmented cartilage with 0.03% bacterial collagenase II. HEK293T cells were used for luciferase assays. All cells were grown in Dulbecco-Vogt Modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). For protein analyses, cells were collected in IP Lysis Buffer (Thermo Fisher Scientific, 87787) supplemented with proteinase inhibitors. For the differentiation experiments, primary chondrocytes were treated with 50 ng/ml BMP-2 every day for 7 days. Cells were collected in TRIzol reagent (Life Technologies) for RNA extraction.
2.2 Exome analysis
DNA was isolated and library preparation and exome sequencing were performed as previously described (Lee et al., 2012). The samples were barcoded, captured using the NimbleGen SeqCap EZ Exome Library v2.0 probe library targeting 36.5 Mb of genome, and sequenced on the Illumina GAIIx platform with 50 bp bidirectional reads. Novoalign was used to align the sequencing data to the human reference genome (NCBI build 37) and the Genome Analysis Toolkit (GATK) was used for postprocessing and variant calling according to GATK Best Practices recommendations. For each sample, at least 90% of targeted bases were covered by at least 10 independent reads. Variants were filtered against dbSNP137, NIEHS EGP exome samples (v.0.0.8), exomes from the NHLBI Exome Sequencing Project (ESP6500), 1000 genomes (release 3.20120430), and in-house exome samples. Mutations were further compared with known disease-causing mutations in HGMD (2012.2). Variants were annotated using VAX34. All variants have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) with submission ID: SUB6071188.
2.3 Western blot
For western blot analyses, protein lysates were separated by electrophoresis on 10% or gradient (4–20%) sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE), transferred to polyvinylidene difluoride membranes, blocked in 5% milk and probed with primary antibodies (anti-SOX9 antibody, 1:1,000 Cell Signaling 82630; anti-GAPDH 1:2,000 Cell Signaling 2118S). Peroxidase-conjugated secondary antibody (Cell Signaling 7071) was used and immunocomplexes were identified using the enhanced chemiluminescence detection reagent (Cell Signaling 7003). Experiments were replicated three times.
2.4 Histological analyses
For histology, human tissues were fixed in 4% paraformaldehyde, decalcified using Immunocal decalcification solution and then paraffin embedded. Paraffin blocks were sectioned at 10 μm and sections were stained with Picrosirius Red. For Picrosirius Red staining, deparaffinized and rehydrated sections were stained in a 0.1% Direct Red 80 (Sigma, 43665)/saturated picric acid (Sigma, P6744) solution followed by counterstaining using hematoxylin QS.
2.5 Plasmid construction
HA-SOX9WT and HA-SOX9Q412X were custom designed by GenScript (https://www.genscript.com). pGF1–4eCOL2A1-Luc vector was a gift from Ryan Porter (Addgene plasmid #97210).
2.6 Luciferase reporter assay
COL2A1 promoter response to SOX9 was analyzed in HEK293T cells by transducing the vector pGF1–4eCOL2A1-Luc and Renilla Control (Lenti CIGNAL, Qiagen). Two days after transduction cells were transduced with expression vectors containing SOX9WT or SOX9Q412X using Lipofectamine 3000 (Thermo Fisher Scientific). Forty-eight hours later, cells were lysed and luciferase activity was determined using a Dual-Luciferase Reporter Assay (Promega).
2.7 RNA extraction and qPCR
RNA was extracted from chondrocytes using the TRIzol reagent (Life Technologies). Complementary DNA (cDNA) was prepared from 1 µg of RNA using the RevertAid First-strand cDNA synthesis kit (Thermo Fisher Scientific) and amplified using Maxima SYBR Green/ROX quantitative polymerase chain reaction (qPCR) Master Mix (Thermo Fisher Scientific). Gene expression was calculated using the 2–∆∆Ct of analysis against the stable housekeeping gene beta-2-microglobulin (B2M). Two biological replicates were performed with two technical replicates each. qPCR primers were (a) COL2A1 Fw: CTCCTGGAGCATCTGGAGAC and Rev: ACCACGATCACCCTTGACTC; (b) SOX9 Fw: TACGACTACACCGACCACCA and Rev: TCAAGGTCGAGTGAGCTGTG; and (c) B2M Fw: TGACTTTGTCACAGCCCAAG and Rev: AGCAAGCAAGCAGAATTTGG.
2.8 Peptide analysis
Proteins were extracted from frozen cartilage, cleaved with cyanogen bromide (CB), and the resulting peptides separated by SDS-PAGE using previously published methods (Bogaert et al., 1992; Mortier et al., 2000).
3 RESULTS
3.1 Clinical findings
Four cases in the International Skeletal Dysplasia Registry had diagnoses of unclassified types of CD due to the severity and unusual features of their phenotypes. All four cases were diagnosed during the prenatal period and the families elected to terminate the pregnancies between 14 and 20 weeks gestation.
Case 1.International Skeletal Dysplasia Registry (ISDR) reference number R83–090. Ultrasound detected abnormal bending of the long bones in a 20-week gestation, 46, XX female fetus. Following termination of the pregnancy at 21 weeks gestation, radiographs (Figure 1b,g) revealed a small skull, micrognathia, and slightly hypoplastic iliac wings. The lower extremities exhibited the most significant findings with disproportionally short and bent femora and tibiae. Bone density was normal and no fractures were observed. Based on these findings a diagnosis of an unclassified form of campomelic dysplasia was made.
Case 2.R89-086. This 20-week gestation fetus (sex and karyotype unknown) exhibited severe bending of the long bones, especially the tibiae and, on one side, the radius and ulna. There was normal ossification and no evident fractures (Figure 1f). Studies were limited and there was little information about the femora or skull, but other findings included hypoplastic fibulae, sagittal clefts in the thoracic spine, hypoplasia of the thumbs and halluces, and precocious ossification of the carpal centers. A diagnosis of an unclassified form of CD was made.
Case 3.R09–315. This 14-week gestation, 46, XX female fetus was identified by ultrasound with a cystic hygroma along with micromelic bowed limbs. Post-termination radiographs were limited but revealed significant shortening and bending of all bones of the upper and lower extremities (Figure 1e). Both feet appeared clubbed. Based on this limited information, a diagnosis of an unclassified bent bone dysplasia was made.
Case 4.R14–170. The fetus was identified by ultrasound at 12-weeks gestation with a large cystic hygroma, severe micromelia, a poorly ossified skull and spine, a hypoplastic midface, and micrognathia. The following termination at 14-weeks gestation, radiographs revealed bending of all of the long bones, micromelia, generalized osteopenia, and limited ossification of the vertebral bodies (Figure 1c,h). A diagnosis of an unclassified lethal skeletal dysplasia was made.
Although differing in overall severity, these four cases shared the common features of short long bones with severe bending, especially of the distal extremities. In this respect, they differed from classic cases of CD due to haploinsufficiency for SOX9, in which milder bending of the femurs was a prominent feature. Consequently, sequence analysis was used to identify the molecular basis of disease in these cases.
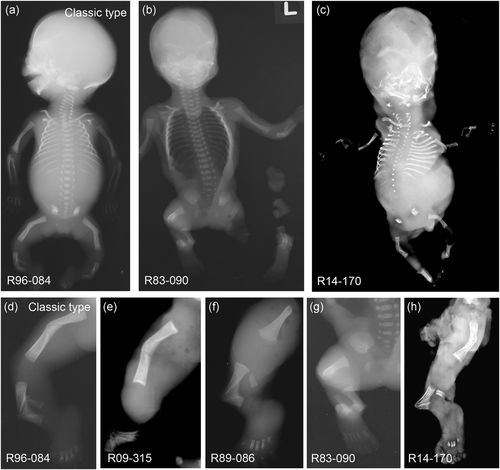
Radiological findings of four cases of CD. The four CD cases (b), (c), (e–h) were compared with a case with the classic type of CD (a), (d). Full body X-rays were available for two of the cases: R83–090 (b) and R14–170 (c). Lower limb radiographs show bending of the tibiae and sometimes the femora (d–h). Cases R83–090, R89-086, and the classic type case, R96-084, were studied at 20 weeks of gestational age while cases R09–315 and R14–170 were at 14 weeks of gestational age. CD, campomelic dysplasia
3.2 Molecular findings
For affected individuals R09–315 and R14–170, in which a radiographic diagnosis of CD was not initially made, exome sequencing was carried out. Both individuals were heterozygous for mutations in SOX9: R09–315, c.957C>G predicting the protein sequence change p.Y319X; and R14–170, c.1234C>T predicting the protein sequence change p.Q412X (Figures 1 and 2a). For R89-086 and R83–090, the radiographic diagnosis suggested candidate sequence analysis of SOX9, which identified heterozygosity for mutations in both cases: R89-086, c.1177C>T, predicting p.Q391X and R83–090, c.1180C>T, predicting p.R394X. The variants found in three of the cases, R09–315, R89-086, and R14–170, were novel, not being present in any databases: Ensembl (http://ensembl.org), ExAC (http://exac.broadinstitute.org), 1000 genomes (http://www.1000genomes.org), or UW (http://evs.gs.washington.edu). The variant for case R83–090 (rs1057518216) has been reported as pathogenic two times before by clinical testing laboratories.
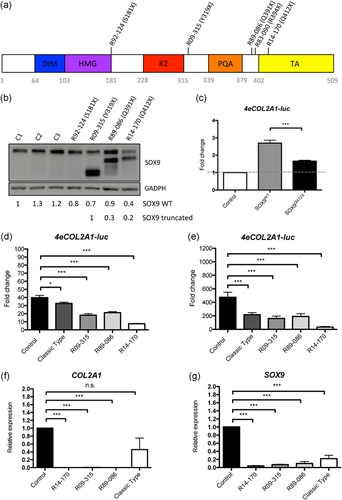
The SOX9 mutations resulted in the production of truncated proteins. (a) Diagram of SOX9 showing the different protein domains and the positions of the mutations in five cases: R92–124 (p.S181X), classic type; R09–315 (p.Y319X), R89-086 (p.Q391X), R83–090 (p.R394X), and R14–170 (p.Q412X). (b) Western blot analysis of SOX9 protein in control and patient chondrocytes. Three different control chondrocyte lines were used and compared with cells from a CD classic type, R92–124 (p.S181X) and three of the four patients with truncating mutations, R09–315 (p.Y319X), R89-086 (p.Q391X), and R14–170 (p.Q412X). The numbers below reflect densitometric quantification of the upper and lower bands. (c) HEK293T cells were transduced with a COL2A1-luciferase reporter plasmid (4eCOL2A1-luc) and then transfected with SOX9WT or SOX9Q412X; luciferase levels were measured and compared with basal levels in control cells transfected with an empty vector. (d), (e) Primary chondrocytes from control and cases were transduced with 4eCOL2A1-luc and luciferase levels were determined without induction (d) and after adding BMP-2 (e). Luciferase was measured in three independent experiments; the graph shows the average ± SD. (f), (g) Endogenous levels of SOX9 (f) and COL2A1 (g) in primary chondrocytes from control and cases were determined by qPCR. The graph shows the average ± SE of two independent experiments. In all experiments statistical analyses were performed using Student's t test; asterisks indicate significant differences between samples, *p < .05, ***p < .001. CD, campomelic dysplasia; qPCR, quantitative polymerase chain reaction; SE, standard error; SD, standard deviation
To determine whether the mutations resulted in SOX9 haploinsufficiency or led to synthesis of truncated SOX9, primary chondrocytes derived from three of the four cases (cells from case R83–090 were not available) were cultured and proteins were extracted and analyzed by western blot. As shown in Figure 2b, the chondrocytes each synthesized a protein corresponding to WT SOX9 and a second protein consistent with synthesis of a truncated protein, with each corresponding to the expected size based on the location of the mutation. This pattern was distinct from a case of CD (R94–124) due to heterozygosity for a more 5′ point mutation, implying a p.S181X change, in which the cells synthesized a reduced amount of exclusively WT SOX9 (Figure 2b). For two of the cases, R09–315 and R14–170, there was an apparently higher abundance of the truncated compared with the WT protein (Figure 2c). The presence of a truncated protein suggested the hypothesis that these mutations might act through a dominant-negative effect rather than haploinsufficiency.
To determine the functionality of the truncated proteins, a SOX9-responsive COL2A1-luciferase reporter plasmid (4eCOL2A1-Luc) was transduced into HEK293T cells and then either a WT SOX9 construct (SOX9WT) or a SOX9 construct with the Q412X mutation (SOX9Q412X) was transfected into the cells previously transduced with the reporter. SOX9Q412X activation of COL2A1 was only 38% of that of SOX9WT (Figure 2c), demonstrating that the activity of the truncated protein was severely compromised. Second, to determine whether SOX9 truncating mutations have a dominant negative effect on WT SOX9, cultured primary chondrocytes derived from the three cases from which such cells were available were transduced with the SOX9 reporter. The reduction of SOX9 activation of COL2A1 was more severe in the cells synthesizing a truncated protein compared with the haploinsufficiency case; COL2A1 activity was 82% of that of WT in R94–124 compared with 45%, 53%, and 19% in R09–315, R89-086, and R14–170, respectively (Figure 2d). When BMP-2 was added to the cells to induce chondrocyte differentiation, R09–315 and R89-086 cells did not show a significant reduction in COL2A1 activity compared with the haploinsufficient cells (34% and 40% of the WT activity compared with 45%), whereas the severe case, R14–170 showed a much more pronounced reduction, with only 7% of COL2A1 transactivation activity retained (Figure 2e).
To determine if the endogenous levels of COL2A1 remained low in cells after differentiation, we cultured primary chondrocytes from CD and control cells and treated them with BMP-2 for 7 days. RNA was extracted from the cells and the levels of COL2A1 were determined by qPCR. COL2A1 expression was almost undetectable in the CD cases with truncated proteins, whereas they were not significantly reduced in the classic type of CD compared with control cells (Figure 2f). This result shows that chondrocyte differentiation was more severely impaired in CD cases with truncated SOX9 proteins compared with CD classic type. We also measured the expression levels of SOX9 and found a great decrease in all CD cases; no statistically significant differences in SOX9 expression were observed between dominant-negative and haploinsufficiency types of CD (Figure 2g).
In addition to more significant abnormalities of the long bones, the most severe case, R14–170, exhibited decreased ossification of the vertebral bodies reminiscent of achondrogenesis type II, which results from dominant negative missense mutations in COL2A1 and synthesis of very little type II collagen (Vissing et al., 1989). This suggested that the R14–170 SOX9 mutation might result in severely decreased type II collagen in cartilage. To test this hypothesis, we used cyanogen bromide (CB) cleavage of cartilage from both fetal and adult controls, a case of classic CD (R18–100A), case R14–170, and a case of achondrogenesis type II (R18–095A). The abundance of type II collagen was estimated from the ratio of the density of α1(II) CB10 (derived from type II collagen) to α2(I) CB3,5 (derived from the α2 chain of type I collagen). As expected, the achondrogenesis type II case showed near absence of type II collagen, with less than 2% type II collagen (Figure 3a,b) relative to type I collagen. Case R14–170 had about 30% type II collagen, much reduced relative to the abundance of type II collagen in the fetal control and the classic CD case (59% and 58%, respectively).
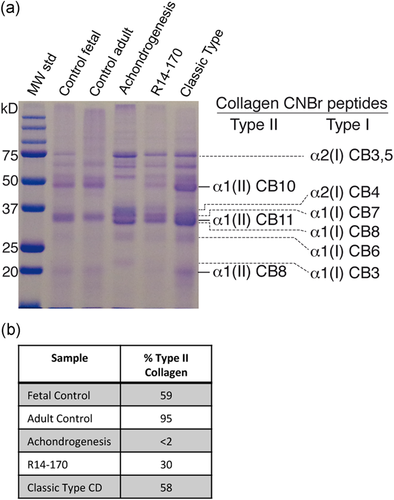
Type II collagen quantification in control and CD cartilage. (a) SDS-PAGE of cyanogen bromide (CB) peptides extracted from cartilage of cases and controls. Shown to the right are the positions of the CB peptides derived from type II and type I collagen, respectively. (b) Quantification of type II collagen from SDS-PAGE in (a). CD, campomelic dysplasia; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis
3.3 Histological findings
To determine the tissue consequences of these new dominant-negative mutations, we studied growth plate morphology using femora and/or tibiae from the cases. The four cases in which truncating mutations were found, as well as the case due to haploinsufficiency, all showed a disruption of the growth axis of the long bones, as observed by the angle of the growth plate compared with the main axis of the bone (Figure 4b–f). In some cases, the cortical bone seemed to grow beneath the growth plate, inside the trabecular bone, causing the curvature of the bone (arrow). In two cases this was accompanied by a midshaft fracture-like occurrence at the location of the maximum angle of bending (Figure 4e,f asterisk).
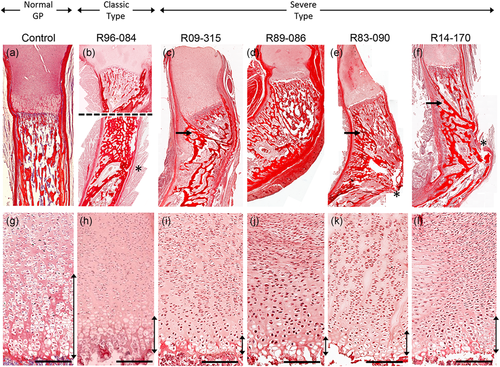
Histological findings. Picrosirius red staining of long bones in control (a), (g), a case of classic type CD (b), (h), and CD due to truncating SOX9 mutations (c–f), (k), (l). (a–f) Macroscopic details of bending at the growth plate and midshaft levels. Image (b) is a composite of two images due to size restrictions. (g–l) Growth plates show reduction of the hypertrophic region (vertical bars). Control, R96-084, R89-086, and R83–090 are tibiae at 20 weeks of gestational age. R09–315 and R14–170 are femurs of 14–15 weeks of gestational age. Bars represent 50 μm. CD, campomelic dysplasia
At the growth plate level, all cases of CD showed a similarly reduced hypertrophic region compared with control bones. This observation is consistent with the known disruption in the differentiation of chondrocytes at the growth plate due to reduced SOX9 (Akiyama, Chaboissier, Martin, Schedl, & de Crombrugghe, 2002).
4 DISCUSSION
In this study, we identified four unrelated CD cases heterozygous for SOX9 null mutations that led to synthesis of truncated SOX9 proteins from the mutant alleles. The mutations all occurred within the terminal exon of SOX9, and presence of truncated proteins derived from these alleles indicated that the mutant transcripts escaped nonsense-mediated decay (NMD). The possibility that some previously described SOX9 mutations might result in the production of truncated proteins derived from transcripts that evade NMD has been proposed (Meyer et al., 1997; Stoeva et al., 2011), but not experimentally demonstrated. All of the mutations in the four cases described here fell within the C-terminal domain of the protein, leaving the dimerization and DNA-binding domains intact. For two of the cases, a dominant-negative effect on transactivation of COL2A1 expression supports the conclusion that the truncated proteins can dimerize with WT SOX9 and exacerbate the effect of mutant SOX9 on transcriptional activity. This conclusion likely reflects that the transactivation domains of the truncated proteins are missing, limiting the ability of SOX9 to activate its targets. One previously reported mutation (p.Y440X) that presumably produces a truncated protein but leaves a longer fraction of the TA domain has been described in a nonlethal form of CD. The two patients reported with this mutation survived for several years (Meyer et al., 1997), suggesting a lesser effect on transactivation than the mutations in the cases described here. However, as the pregnancies of the cases we studied were all terminated, we do not know the clinical outcome had these fetuses developed to term.
The radiographic phenotype resulting from the p.Q412X mutation was particularly severe and the dramatic effect on transactivation noteworthy. Indeed the phenotype was so severe that we did not recognize that it was due to a SOX9 mutation. In retrospect, the short long bones and lack of vertebral ossification is reminiscent of achondrogenesis type II, which results from near total loss of type II collagen (Vissing et al., 1989). As the SOX9 TA domain is known to bind to elements of the chromatin machinery, including Med25, a component of the Mediator complex, that is necessary for SOX9 activation of Col2a1 (Nakamura et al., 2011), disruption of the TA domain by these mutations could affect COL2A1 transcription in part by disrupting this interaction. The reduced synthesis of type II collagen in this case provided a functional readout of the effect of the mutation, providing an explanation for the more severe effect of the mutation on skeletal development.
The alteration of chondrocyte differentiation at the growth plate corresponds with reduced SOX9 activity and leads to deviation of the polarity of the growth plate relative to the main axis of the growing bone. What is still a mystery is how the chondrocyte differentiation defects so dramatically alter the architecture of the bone to cause bending. Different hypotheses have speculated that there may be two mechanisms that lead to either “curved” bending or “angulated,” fracture-like bending (Bi et al., 2001; Sharir, Stern, Rot, Shahar, & Zelzer, 2011). Curved bending could be explained by mechanical forces from tendons and muscles gradually changing the direction of the axis of growth (Bi et al., 2001; Sharir et al., 2011); in addition to mechanical forces, angulated bending might be explained by fractures during development due to fragility of the bone, perhaps related to the location of the insertion of the midshaft blood vessels (Sharir et al., 2011; Sivaraj & Adams, 2016). CD cases show a phenotypic range between these two bending types suggesting that angulated bending is a severe form of the curved bending. Whether the mechanical forces and other possible causes like the fragility around the midshaft vessel insertion site are factors resulting in bending is still to be determined.
In conclusion, we report several new mutations in SOX9 that each result in synthesis of a truncated protein and a dominant-negative effect on the ability of SOX9 to transactivate its targets in CD. In one case, a severe dominant-negative effect led to a unique CD phenotype, resulting in decreased type II collagen synthesis and resembling achondrogenesis type II. Overall the data indicate that the severity of the clinical and radiographic phenotype depends on the extent to which SOX9 mutations affect target transactivation.
ACKNOWLEDGMENTS
We thank the families for their participation in this study. We also thank the following clinicians who cared for the families, John Mann, Robert M. Cardelli, Lee P. Shulman, Carol M. Meyers, Thomas Pinckert, Jill S. Fonda, Tobie Beckerman, Kim Guida, Matt Tschirigi, and Reinaldo Acosta, for referral of the cases.
FUNDING
D. H. C and D. K. are supported by NIH RO1 AR066124, R01 AR062651, and RO1 DE019567. I.D. is supported by a Geisman Fellowship award from the OIF. M.B. and the University of Washington Center for Mendelian Genomics are supported by NIH HG006493.




