Incorporation of semi-quantitative analysis of splicing alterations for the clinical interpretation of variants in BRCA1 and BRCA2 genes
Abstract
BRCA1 and BRCA2 (BRCA1/2) genetic variants that disrupt messenger RNA splicing are commonly associated with increased risks of developing breast/ovarian cancer. The majority of splicing studies published to date rely on qualitative methodologies (i.e., Sanger sequencing), but it is necessary to incorporate semi-quantitative or quantitative approaches to accurately interpret the clinical significance of spliceogenic variants. Here, we characterize the splicing impact of 31 BRCA1/2 variants using semi-quantitative capillary electrophoresis of fluorescent amplicons (CE), Sanger sequencing and allele-specific assays. A total of 14 variants were found to disrupt splicing. Allelic-specific assays could be performed for BRCA1 c.302−1G>A and BRCA2 c.516+2T>A, c.1909+1G>A, c.8332–13T>G, c.8332−2A>G, c.8954−2A>T variants, showing a monoallelic contribution to full-length transcript expression that was concordant with semi-quantitative data. The splicing fraction of alternative and aberrant transcripts was also measured by CE, facilitating variant interpretation. Following Evidence-based Network for the Interpretation of Germline Mutant Alleles criteria, we successfully classified eight variants as pathogenic (Class 5), five variants as likely pathogenic (Class 4), and 14 variants as benign (Class 1). We also provide splicing data for four variants classified as uncertain (Class 3), which produced a “leaky” splicing effect or introduced a missense change in the protein sequence, that will require further assessment to determine their clinical significance.
1 INTRODUCTION
Hereditary breast and ovarian cancer (HBOC) accounts for 5–10% of the total breast/ovarian cancer cases, and pathogenic variants in the high-penetrance genes BRCA1 (MIM# 113705) and BRCA2 (MIM# 600185; BRCA1/2) explain about 15–20% of HBOC cases (Couch, Nathanson, & Offit, 2014; Shiovitz & Korde, 2015), although this proportion varies depending on the ascertainment criteria for BRCA1/2 testing and between ethnic groups. BRCA1/2 genetic testing is generally recommended to individuals with a personal or family history of breast/ovarian cancer, with two or more family members affected, the presence of male breast cancer and/or the presence of triple-negative breast cancer cases, among other indicative criteria (Valencia et al., 2017).
The estimated average risk of breast cancer in carriers of BRCA1/2 pathogenic variants ranges from 57% to 65% for BRCA1, and from 35% to 57% for BRCA2; and the risk of ovarian cancer ranges from 20% to 50% for BRCA1, and from 5% to 23% for BRCA2 (Kuchenbaecker et al., 2017). The identification of pathogenic variants in BRCA1/2 genes has been demonstrated to be clinically relevant. Carriers and their families can benefit from risk-reducing strategies such as increased surveillance and surgical intervention (Domchek & Weber, 2006; Valencia et al., 2017), and from therapeutic choices specifically designed to target BRCA1/2 deficient tumors, such as PARP inhibitors (Lord & Ashworth, 2017; Somlo et al., 2017; Weren et al., 2017).
A successful genetic diagnosis relies on the correct interpretation of genetic results. However, BRCA1/2 genetic testing in clinical laboratories commonly identifies variants of unknown significance (VUS) from which sequencing information alone is not sufficient for variant clinical classification, failing to provide a clear genetic diagnosis (Eccles et al., 2015; Wallace, 2016). One group of VUS is represented by variants that have the potential to alter the messenger RNA (mRNA) splicing process. A recent study reported that variants affecting splicing, namely spliceogenic, are the third most common type occurring in BRCA1 and BRCA2 genes, accounting for 10.1% and 7.6% of the total, respectively (Rebbeck et al., 2018). Although multiple spliceogenic variants producing abnormal transcripts have been identified in HBOC families, the clinical relevance of the splicing outcomes can be sometimes difficult to determine (de Caputo et al., 2018; Colombo et al., 2018; la Hoya et al., 2016; Montalban et al., 2018). Furthermore, numerous BRCA1/2 alternative splicing events have been described in several tissues and cell lines, adding complexity to the interpretation of splicing outcomes generated from variant alleles (Colombo et al., 2014; Fackenthal et al., 2016).
Splicing analysis of sequence variants is relatively easy to assess using biological material from variant carriers, and it is in fact a common approach used in the clinical setting (Anna & Monika, 2018). Numerous works have characterized BRCA1/2 spliceogenic variants in patient RNA or minigene assays, demonstrating the benefits of performing RNA analysis to better understand the clinical consequences of splicing variation (Brandão, Roozendaal, Tserpelis, García, & Blok, 2011; Colombo et al., 2013; Thomassen et al., 2012; Whiley et al., 2011; Baert et al., 2018; Fraile-Bethencourt et al., 2017; Garibay et al., 2014). However, discrepant splicing results have been detected for several BRCA1/2 variants, as well as discordant interpretations across multiple reviewers, leading to differences in variant classification (Walker et al., 2013). Such differences were attributed to a variety of methodological issues: the lack of splicing information occurring in noncarrier controls and the lack of quantitative splicing data, that is, the levels of reference full-length transcript generated by the variant allele, as well as the levels of alternative and aberrant transcripts, detected. In this regard, previous works have demonstrated that the incorporation of capillary electrophoresis into variant analysis provides high-resolution and high-sensitivity detecting reverse transcription-polymerase chain reaction (RT-PCR) products, and provides semi-quantitative data that facilitates the clinical interpretation of BRCA1/2 germline variants according to existing guidelines (Garibay et al., 2014; Whiley et al., 2014).
In the present study, we analyze the splicing outcomes of 31 BRCA1/2 variants in patient RNA using capillary electrophoresis of fluorescent amplicons, providing a comprehensive evaluation at qualitative and semi-quantitative level, and we also discuss the utility of incorporating semi-quantitative splicing information for the clinical interpretation of spliceogenic variants according to classification guidelines established by the Evidence-based Network for the Interpretation of Germline Mutant Alleles (ENIGMA).
2 METHODS
2.1 Editorial policies and ethical considerations
This study was approved by the Clinical Research Ethics Committee from the Hospital Universitari Vall d'Hebron, Barcelona, Spain. All individuals received genetic counseling and provided written informed consent for BRCA1/2 genetic analysis and research studies.
2.2 Clinical features from probands and families
Families affected with hereditary breast/ovarian cancer were recruited between years 2008 and 2016 following clinical eligibility criteria for BRCA1/2 genetic testing (Llort et al., 2015). BRCA1/2 coding exons and exon-intron boundaries were screened by Sanger sequencing or massively parallel sequencing with the BRCA MASTR Dx Multiplicom panel in a Miseq platform (Illumina), and large genomic rearrangements were determined by Multiplex Ligation-dependent Probe Amplification (MRC-Holland). Clinical information from the probands and families analyzed in the present study are summarized in Table S1.
2.3 Variant selection and in silico analysis
BRCA1/2 variants located within the 20 exonic and 20 intronic nucleotides adjacent to exon-intron boundaries were selected. From these, two BRCA1 variants (c.81−14C>T and c.591C>T) and nine BRCA2 variants (c.68−7T>A, c.516+4C>T, c.7617+1G>A, c.7976+5G>T, c.7976+12G>A, c.9116C>T, c.9117G>A, c.9257−16T>C, and c.9502−17T>C) were excluded from RNA analysis due to the existence of previous works in the literature, or due to patient sample unavailability (see Table S2 for further details). After exhaustive gene-database and literature search, we selected for RNA analysis 31 BRCA1/2 variants with a minor allele frequency in non-Finish European (NFE) population <0.1%, that were either novel and/or had not been previously characterized in patient RNA using semi-quantitative capillary electrophoresis and allele-specific assays.
Variants were assessed in silico using prediction scores from Human Splicing Finder (HSF), Splice Site Finder-like (SSF) and MaxEntScan (MES), ascertained through Alamut Visual software v2.10 (Interactive Biosoftware). Score changes between variant (VAR) and wild-type (WT) sequences were calculated as ([VAR−WT]/WT × 100). HSF, SSF, and MES scores were used to assess variants located near or within the consensus 5′ donor sites, whereas SSF was used for variants located near or within the 3′ acceptor sites, as recommended in previous work from our laboratory (Moles Fernández et al., 2018). Alamut's algorithm to determine the creation/activation of splice sites (“LocalSpliceEffect”) was also applied for each variant. This algorithm interprets MES, SSF, and NNSPLICE scores. Briefly, if in a given genomic position at variant vicinity at least two scores are significant in the VAR sequence but not in the WT sequence, Alamut will predict a new splice site creation. Also, if at least two scores are significant in both VAR and WT sequence, with VAR scores being 3% greater on average than those from the WT sequence, Alamut will predict the activation of a cryptic splice site (https://www.interactive-biosoftware.com/doc/alamut-visual/2.6/splicing.html).
Probabilities of pathogenicity were also obtained using Breast Cancer Genes Prior Probabilities tool (http://priors.hci.utah.edu/PRIORS/index.php; Vallée et al., 2016), to determine the probability of consensus splice site disruption, the formation of de novo splice sites and/or damage to protein sequence, when applicable.
2.4 RT-PCR and Sanger sequencing
Total RNA from variant carriers and 8–16 noncarrier controls was isolated from 5 to 10 ml of peripheral blood using Trizol reagent (Invitrogen). RNA was cleaned-up using RNeasy Mini Kit (QIAGEN) following the manufacturer's protocol with an additional step of DNase digestion using RNase-Free DNase Set (QIAGEN). A total of 300–500 ng of RNA were retrotranscribed using PrimeScript RT reagent kit (Takara), combining random and oligo-dT primers. PCR primers were designed to amplify a whole exon upstream and downstream from the exon containing the variant of interest, when possible. PCR assays were performed in 25 ul reaction volume containing 100 ng of cDNA as template, using EcoTaq DNA Polymerase (Ecogen). Samples were denatured at 95º for 10 min, followed by 35 cycles consisting of 95º for 30 s, 56–58º for 30 s, and 72º for 1–7 min; and a final extension step at 72º for 7 min. A detailed description of PCR primers and conditions is provided in Table S3A.
RT-PCR products were qualitatively assessed by capillary electrophoresis in a QIAxcel instrument using the QIAxcel DNA High-resolution kit (QIAGEN). Electrophoresis methods 0M500 and 0M1200 were used for short amplicons (<2 kb) and long amplicons (>2 kb), respectively, with injection times of 8 s. Products were also qualitatively assessed by capillary electrophoresis of fluorescent amplicons in a Genetic Analyzer (ABI3130xl). Electrophoresis conditions are described in the following section. RT-PCR products were purified using ExoSAP-IT® PCR Product Cleanup (Affimetrix, Thermo Fisher Scientific) and bidirectionally sequenced using BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing products were run in an ABI3130xl Genetic Analyzer (Applied Biosystems) and analyzed using SeqScape v2.6 and Sequencing Analysis v6.0 softwares (Applied Biosystems). Reference transcripts BRCA1 NM_007294.3 and BRCA2 NM_000059.3 from GRCh37/h19 genome build were used for sequence alignment and transcript annotation.
2.5 Semi-quantitative analysis by capillary electrophoresis of fluorescent amplicons
BRCA1/2 variants producing abnormal splicing profiles were assessed by semi-quantitative capillary electrophoresis of fluorescent amplicons to measure the relative abundance of each transcript detected. RT-PCR assays for semi-quantitative purposes were performed in triplicate using the primers described in Table S3A, labeled with a FAM molecule at the 5′ end. RT-PCR products were diluted (1:10–1:30) and 0.5 ul were run in an ABI3130xl Genetic Analyzer instrument. GeneScan ROX 500 and GeneScan ROX 1,000 (Applied Biosystems) were used as internal size-standards for amplicons <500 bp and >500 bp, respectively. The following electrophoresis conditions were used: temperature 60ºC, 12 s injection at 1.2 kV and 2,000 s run at 12 kV. GeneMapper software v5 (Applied Biosystems) was used for data visualization and peak size-calling.
The relative abundance of the reference full-length transcript (FL) was calculated in variant carriers and noncarrier controls using peak areas corresponding to the FL product. FL areas of all samples were normalized using the FL mean calculated from controls, and the mean of the normalized values from the control group was used as a reference to compare FL relative levels between samples. The splicing fraction (SF) of each transcript was also estimated by dividing the peak area of the individual transcript with the Σ of all peak areas (all transcripts) detected.
Peaks above 8,000 relative fluorescent units (RFUs) and below 100 RFUs were not considered. Data graphs were built using the GraphPad Prism software.
2.6 Allele-specific assays and allelic imbalance assessment using informative exonic variants
Allele-specific RT-PCR assays were performed for variants with altered splicing profiles to determine the contribution of variant alleles to reference transcript expression. For each variant, primers were designed to anneal to exonic sequences skipped in altered mRNA products, to specifically amplify reference transcripts without abnormal splicing. The amplified region included an informative exonic variant found to be heterozygous in the corresponding gDNA from the carrier. RT-PCR assays were performed with 100 ng of cDNA as template, under the following cycling conditions: denaturing step at 95º for 10 min, followed by 40 cycles consisting of 95º for 30 s, 54–56º for 30 s, and 72º for 1 min 30 s–7 min; and a final extension step at 72º for 7 min (see primers and PCR conditions in Table S3B).
BRCA1/2 allelic imbalance was determined for variants producing normal splicing profiles to rule out the presence of aberrant transcripts degraded by the nonsense-mediated decay system. A region containing a heterozygous exonic variant was amplified by RT-PCR (see primers and PCR conditions in Table S3C).
RT-PCR products were sequenced by Sanger and visual inspection of informative variants was performed using SeqScape v2.6 (Applied Biosystems).
3 RESULTS
A total of 31 BRCA1/2 variants were included for RNA analysis (Figure 1). To our knowledge, 13 variants had been previously analyzed inpatient RNA or minigene constructs using qualitative approaches (i.e., agarose gel + Sanger sequencing), and 18 variants had not been previously characterized at the RNA level, from which five are novel and not reported in the literature or gene databases. Evidence reported for the 31 BRCA1/2 variants is described in Table S4. The 31 variants have been submitted in the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/).
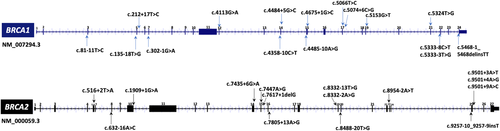
Location of BRCA1/2 variants selected for mRNA characterization. Variants within the 20 exonic/intronic nucleotides adjacent to canonical splice sites were selected. Gene diagrams were obtained from UCSC genome browser (https://genome.ucsc.edu/). mRNA, messenger RNA
3.1 In silico splicing analysis of BRCA1/2 variants
Recommendations for in silico variant assessment were followed as described in Moles Fernández et al. (2018). Variant score changes and predictions of splice site creation/activation are described in Table 1. In brief, all variants located in the donor region were correctly predicted by HSF, MES, and SSF, with the exception of BRCA2 c.9501+4A>G (false positive) which was predicted to disrupt the donor splice site but no alteration was observed experimentally. The majority of variants in the acceptor region were correctly predicted by SSF, with the exception of BRCA1 c.81–11T>C (false positive), c.135–18T>G (false negative) and c.5153G>T (false positive). A total of 10 variants were predicted to create/activate splice sites, but only a small effect was observed experimentally for BRCA2 c.7447A>G variant (see the following section for further details). This variant is predicted to create a new acceptor site 12nt downstream from the canonical site (Figure S1).
| GENE | Variant (HGVS)a | Location | Nearest splice site | In silico analysis (Alamut)b | Transcripts detected | HGVS nomenclature of abnormal transcripts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HSF score change (%) (−2%) | MES score change (%) (−15%) | SSF score change (%) (−5%) | Local splice effect (SSF, NNSPLICE, MES) | Region amplified | Controls | Carriers | mRNA | Protein | Functional annotation | ||||
| BRCA1 | c.81–11T>C | Intron 2 | Acceptor | −2.39 | −11.21 | −5.85 | – | Exons 1–7 | FL, Δ1q, Δ5q, Δ5 | FL, Δ1q, Δ5q, Δ5 | – | – | – |
| c.135–18T>G | Intron 3 | Acceptor | 0.00 | −15.09 | 0.00 | – | Exons 1–7 | FL, Δ1q, Δ5q, Δ5 | FL, Δ1q, Δ5q, enhanced Δ5 | r.135_212del (Δ5) | p.Phe46_Arg71del | In-frame | |
| c.212+17T>C | Intron 5 | Donor | 0.00 | 0.00 | 0.00 | – | Exons 1–7 | FL, Δ1q, Δ5q, Δ5 | FL, Δ1q, Δ5q, Δ5 | – | – | – | |
| c.302–1G>Ad | Intron 6 | Acceptor | −100.00 | −100.00 | −100.00 | – | Exons 3–11 | FL, Δ8p, Δ5q, Δ5, Δ9,10 | FL, Δ8p, Δ5q, Δ5, Δ9,10; Δ7p (del10nt) | r.302_311del (Δ7p) | p.Tyr101Serfs*15 | Frameshift | |
| c.4113G>A (p.G1371G) | Exon 12 | Acceptor | 0.00 | 0.00 | 0.00 | Cryptic donor weakly activated | Exons 11–13 | FL, Δ13p | FL, Δ13p | – | – | – | |
| c.4358–10C>T | Intron 13 | Acceptor | 0.89 | −3.53 | 4.39 | Cryptic acceptor strongly activated | Exons 12–16 | FL, Δ13p, Δ14p, ▼13A | FL, Δ13p, Δ14p, ▼13A | – | – | – | |
| c.4484+5G>C | Intron 14 | Donor | −12.42 | −15.61 | −13.44 | – | Exons 12–16 | FL, Δ13p, Δ14p, Δ15 | FL, Δ13p, Δ14p, Δ14, Δ14,15c | r.4358_4484del (Δ14) r.4358_4675del (Δ14,15) |
p.Ala1453Glyfs*10 p.Ala1453_Leu1558del |
Frameshft In-frame |
|
| c.4485–10A>G | Intron 14 | Acceptor | 0.13 | 4.76 | 0.00 | – | Exons 12–16 | FL, Δ13p, Δ14p | FL, Δ13p, Δ14p | – | – | – | |
| c.4675+1G>Cd | Intron 15 | Donor | −100.00 | −100.00 | −100.00 | – | Exons 12–16 | FL, Δ13p, Δ14p, Δ15 | FL, Δ13p, Δ14p, Δ15q (del11nt), enhanced Δ15, Δ14,15c | r.4665_4675del (Δ15q) r.4485_4675del (Δ15) r.4358_4675del (Δ14,15) |
p.Gln1556Glyfs*14 p.Ser1496Glyfs*14 p.Ala1453_Leu1558del |
Frameshift Frameshift In-frame |
|
| c.5066T>C (p.Met1689Thr) | Exon 17 | Donor | 0.00 | 0.00 | 0.00 | – | Exons 14–20 | FL | FL | – | – | – | |
| c.5074+6C>G | Intron 17 | Donor | 0.31 | 16.70 | 1.04 | – | Exons 14–18 | FL | FL | – | – | – | |
| c.5153G>T (p.Trp1718Leu) | Exon 19 | Acceptor | −5.13 | −27.48 | −7.62 | – | Exons 16–24 | FL | FL | – | – | – | |
| c.5324T>G (p.Met1775Arg) | Exon 21 | Donor | 0.00 | 0.00 | 0.00 | New acceptor site | Exons 16–24 | FL | FL | – | – | – | |
| c.5333–8C>T | Intron 21 | Acceptor | −0.76 | 8.42 | 2.4 | – | Exons 16–24 | FL | FL | – | – | – | |
| c.5333–3T>G | Intron 21 | Acceptor | −3.25 | −90.26 | −6.36 | – | Exons 16–24 | FL | FL, Δ22 | r.5333_5406del (Δ22) | p.Asp1778Glyfs*27 | Frameshift | |
| c.5468–1_5468delinsTT | Intron 23 | Acceptor | −100.00 | −100.00 | −100.00 | – | Exons 20–24 | FL | FL, Δ24p (del11nt) | r.5468_5478del (Δ24p) | p.Ala1823Aspfs*3 | Frameshift | |
| BRCA2 | c.516+2T>A | Intron 6 | Donor | −100.00 | −100.00 | −100.00 | – | Exons 3–10 | FL, Δ6q,7 | FL, Δ6, Δ5,6, Δ6q,7 | r.476_516del (Δ6) r.426_516del (Δ5,6) |
p.Val159Glyfsc*10 p.Ser142Argfs*13 |
Frameshift Frameshift |
| c.632–16A>C | Intron 7 | Acceptor | 0.00 | 12.08 | 0.00 | – | Exons 3–10 | FL, Δ6q,7 | FL, Δ6q,7 | – | – | – | |
| c.1909+1G>A | Intron 10 | Donor | −100.00 | −100.00 | −100.00 | – | Exons 1–11 | FL, Δ9,10 | FL, enhanced Δ9,10 | r.682_1909del (Δ9,10) | p.Asn228Valfs*7 | Frameshift | |
| c.7435+6G>A | Intron 14 | Donor | −0.54 | −16.61 | −0.45 | – | Exons 12–16 | FL | FL | – | – | – | |
| c.7447A>G (p.Ser2483Gly) | Exon 15 | Acceptor | 0.00 | 0.00 | 0.00 | New acceptor site | Exons 14–17 | FL | FL, Δ15p (del12nt) | r.7436_7447del (Δ15p) | p.Asp2479_Ser2483delinsGly | In-frame | |
| c.7617+1delG | Intron 15 | Donor | −100.00 | −100.00 | −100.00 | New acceptor site | Exons 14–17 | FL | FL, del1nt, enhanced Δ15 | r.7617del (Δ15q) r.7436_7617del (Δ15) |
p.Gln2539Hisfsc*12 p.Asp2479Alafs*8 |
Frameshift Frameshift |
|
| c.7805+13A>G | Intron 16 | Donor | 0.00 | 0.00 | 0.00 | New acceptor site | Exons 14–18 | FL | FL | – | – | – | |
| c.8332–13T>G | Intron 18 | Acceptor | 0.00 | −87.20 | −100.00 | New acceptor site | Exons 16–20 | FL, Δ19, Δ18, Δ17,18 | FL, Δ18, Δ17,18, enhanced Δ19 | r.8332_8487del (Δ19) | p.Ile2778_Gln2829del | In-frame | |
| c.8332–2A>G | Intron 18 | Acceptor | −100.00 | −100.00 | −100.00 | – | Exons 16–20 | FL, Δ19, Δ18, Δ17,18 | FL, Δ18, Δ17,18, Δ19p (del14nt), enhanced Δ19 | r.8332_8345del (Δ19p) r.8332_8487del (Δ19) |
p.Ile2778Valfs*15 p.Ile2778_Gln2829del |
Frameshift In-frame |
|
| c.8488–20T>G | Intron 19 | Acceptor | 0.00 | −60.33 | 0.00 | – | Exons 18–21 | FL | FL | – | – | – | |
| c.8954–2A>T | Intron 22 | Acceptor | −100.00 | −100.00 | −100.00 | – | Exons 19–24 | FL,▼20A, Δ23p | FL,▼20A, enhanced Δ23p, Δ22,23p | r.8954_9004del (Δ23p) r.8755_9004del (Δ22,23p) |
p.Val2985_Thr3001del p.Gly2919Lysfs*26 |
In-frame Frameshift |
|
| c.9257–10_9257–9insT | Intron 24 | Acceptor | 0.00 | 1.95 | 0.00 | – | Exons 22–25 | FL | FL | – | – | – | |
| c.9501+3A>T | Intron 25 | Donor | −8.36 | −57.58 | −10.74 | Cryptic acceptor strongly activated | Exons 24–27 | FL | FL, Δ25 | r.9257_9501del (Δ25) | p.Gly3086Glufs*3 | Frameshift | |
| c.9501+4A>G | Intron 25 | Donor | −8.66 | −24.02 | −10.96 | Cryptic acceptor strongly activated | Exons 24–27 | FL | FL | – | – | – | |
| c.9501+9A>C | Intron 25 | Donor | 0.00 | 0.00 | 0.00 | Cryptic acceptor strongly activated | Exons 24–27 | FL | FL | – | – | – | |
- Abbreviations: CE, capillary electrophoresis of fluorescent amplicons; FL, full-length transcript; HSF, human splicing finder; MES, MaxEntScan; SSF, Splice-Site Finder-like.
- a Sequence variants were annotated using RefSeq NM_007294.3 for BRCA1 and RefSeq NM_000059.3 for BRCA2; HGVS guidelines were followed for variant nomenclature (Den Dunnen et al., 2016).
- b Variant score changes were obtained as described in the methods section. Thresholds to consider a damaging effect are detailed between brackets and were obtained from Moles Fernández et al. (2018).
- c RNA products imputed based on the length of the product observed in CE experiments.
- d Variants producing other minor transcripts with co-occurring splicing events (see Figure 2b,c).
- * The asterisk indicates a stop codon.
3.2 Qualitative and semi-quantitative analysis of BRCA1/2 transcripts
For the qualitative approach, RT-PCR products were visualized in a QIAxcel instrument and confirmed by Sanger sequencing. For the semi-quantitative analysis, data from capillary electrophoresis of FAM-labelled RT-PCR experiments (CE) was used, which also provided a qualitative visualization of all products. Splicing results obtained for the 31 variants are summarized in Table 1. In brief, normal splicing patterns were observed for 17 variants (BRCA1 c.81–11T>C, c.212+7T>C, c.4113G>A, c.4358–10C>T, c.4485–10A>G, c.5066T>C, c.5074+6C>G, c.5333–8C>T, c.5153G>T, c.5324T>G; and BRCA2 c.632–16A>C, c.7435+6G>A, c.7805+13A>G, c.8488–20T>G, c.9257–10_9257–9insT, c.9501+4>G, c.9501+9A>C), that were confirmed by Sanger sequencing and CE (see Figures S2 and S3).
In contrast, altered splicing patterns were observed for 14 variants and CE data were used to determine the relative abundance of each transcript detected. BRCA1 c.135–18T>G variant slightly upregulated isoform Δ5 (SF mean in carrier = 7% vs. controls = 2.6%; Figure 2a). BRCA1 variants c.302–1G>A, c.4675+1G>C and c.5468–1_5468delinsTT produced predominant transcripts not reported in control tissues (Colombo et al., 2014; Davy et al., 2017; Lattimore et al., 2018), that consisted in splice site shift events: a 10 nucleotides (nt) deletion from 5′ end of exon 7 (Δ7p; (SF = 19.9%), 11nt deletion from 3′ end of exon 15 (Δ15q; (SF = 24%), and 11nt deletion from 5′end of exon 24 (Δ24p; (SF = 30.4%), respectively (Figure 2b–d). BRCA1 c.4484+5G>C variant generated a major transcript Δ14 (SF = 41.2%) and a minor transcript Δ14,15, imputed by size-calling (SF = 5%; Figure 2e); c.5333–3 T>G variant promoted a major transcript Δ22 (SF = 52.2%; Figure 2f). Although Δ14 and Δ22 transcripts have been previously reported as minor alternative transcripts occurring in control tissues (Colombo et al., 2014), none were detected in peripheral blood from our control group.
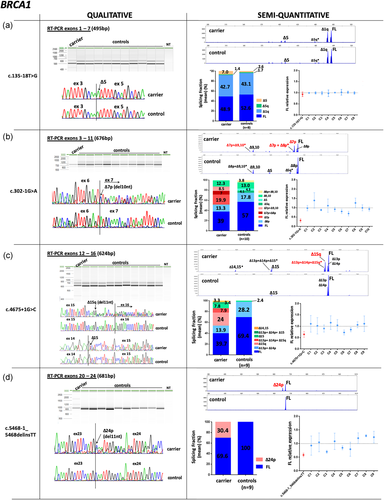
Qualitative and semi-quantitative analysis of BRCA1 variants with altered splicing profiles (a–f). Qualitative data from QIAxcel electrophoresis and Sanger sequencing are shown on the left. Semi-quantiative data obtained from capillary electrophoresis of fluorescent amplicons (CE) are shown on the right. The abundance of each transcript is expressed as a splicing fraction (SF; mean values are shown). Full-length (FL) transcript data were used to compare FL levels between carriers and the reference control group (mean = 1, grid line). Mean ± SEM is shown. FL data for variant BRCA1 c.4484+5G>C were obtained from QIAxcel electrophoresis.
*Transcripts imputed based on the length of the product observed and not confirmed by Sanger
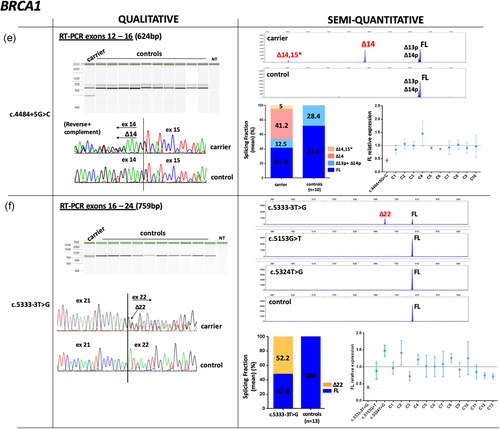
Qualitative and semi-quantitative analysis of BRCA1 variants with altered splicing profiles (a–f). Qualitative data from QIAxcel electrophoresis and Sanger sequencing are shown on the left. Semi-quantiative data obtained from capillary electrophoresis of fluorescent amplicons (CE) are shown on the right. The abundance of each transcript is expressed as a splicing fraction (SF; mean values are shown). Full-length (FL) transcript data were used to compare FL levels between carriers and the reference control group (mean = 1, grid line). Mean ± SEM is shown. FL data for variant BRCA1 c.4484+5G>C were obtained from QIAxcel electrophoresis.
*Transcripts imputed based on the length of the product observed and not confirmed by Sanger
For BRCA2, four variants generated predominant exon skipping events. Variant c.516+2T>A produced Δ6 and Δ5–6 (SF = 31.6% and 20.1%, respectively; Figure 3a); c.1909+1>A enhanced Δ9,10 (SF = 63.2%) and produced additional minor products that could not be either imputed or confirmed by Sanger (Figure 3b); variants c.8332–13T>G and c.8332–2A>G generated Δ19 (SF = 57.7%, 21.7%, respectively; Figure 3c). An additional transcript was detected in c.8332–2A>G carrier consisting of a splice acceptor shift event that caused the deletion of 12nt at 5′end of exon (Δ19p; (SF = 18%; Figure 3c). Analysis of c.8954–2>T variant also identified a major splice acceptor shift event, consisting of the 51nt deletion at 5′ end of exon 23 (Δ23p; SF = 33.8%), and a mixed splicing biotype Δ22,23p (SF = 10.8%; Figure 3d). This variant was identified in a man affected with two breast cancers at ages 72 and 80, and segregated in his affected daughter, diagnosed with breast cancer at age 44 (Table S1). All the mentioned BRCA2 transcripts (Δ6, Δ5,6, Δ9,10, Δ19, Δ19p, Δ23p, and Δ22,23p) were confirmed by Sanger sequencing. Previous works have detected these products in control tissues at lower expression levels, with the exception of Δ19p, which was not previously reported (Davy et al., 2017; Fackenthal et al., 2016; Lattimore et al., 2018). Similarly, transcripts Δ9,10, Δ19, and Δ23p were detected in our control group at lower abundancies, whereas Δ6, Δ5,6, Δ19p, and Δ22,23p were not detected.
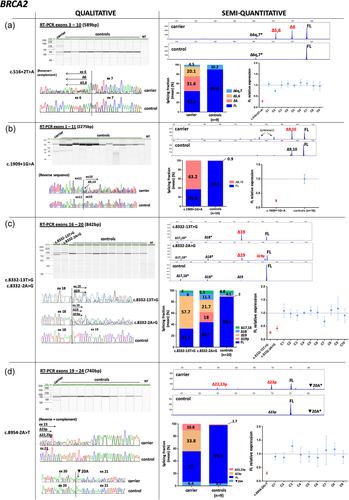
Qualitative and semi-quantitative analysis of BRCA2 variants with altered splicing profiles (a–g). Splicing data are depicted as described in Figure 2. Semi-quantitative results were obtained from CE experiments, with the exception of variants c.1909+1G>A, c.9501+3A>T, c.9501+4A>G, and c.9501+9A>C, for which QIAxcel data were used. RT-PCR experiments of variant c.1909+1G>A and controls (n = 16) were performed independently (Figure 3b). Experimental replicates for variant c.7447A>G could not be performed due to sample unavailability (Figure 3f). Splicing products from c.7617 + 1delG carrier could not be quantified because peak areas from the two major transcripts overlapped (Figure 3g). CE, capillary electrophoresis; RT-PCR, reverse transcription-polymerase chain reaction.
*Transcripts imputed based on the length of the product observed and not confirmed by Sanger
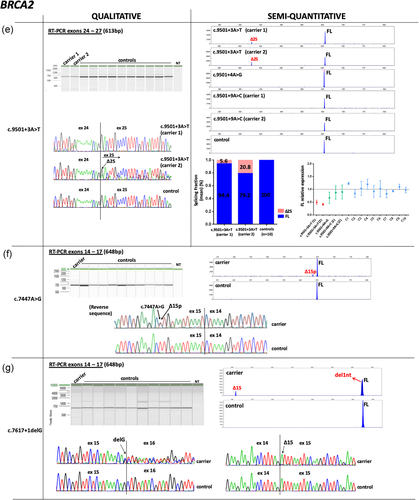
Qualitative and semi-quantitative analysis of BRCA2 variants with altered splicing profiles (a–g). Splicing data are depicted as described in Figure 2. Semi-quantitative results were obtained from CE experiments, with the exception of variants c.1909+1G>A, c.9501+3A>T, c.9501+4A>G, and c.9501+9A>C, for which QIAxcel data were used. RT-PCR experiments of variant c.1909+1G>A and controls (n = 16) were performed independently (Figure 3b). Experimental replicates for variant c.7447A>G could not be performed due to sample unavailability (Figure 3f). Splicing products from c.7617 + 1delG carrier could not be quantified because peak areas from the two major transcripts overlapped (Figure 3g). CE, capillary electrophoresis; RT-PCR, reverse transcription-polymerase chain reaction.
*Transcripts imputed based on the length of the product observed and not confirmed by Sanger
BRCA2 c.9501+3A>T was detected in two independent families and analyzed in parallel with BRCA2 c.9501+4A>G and BRCA2 c.9501+9A>C carriers, under the same FAM-labelled RT-PCR and capillary electrophoresis conditions. Exon 25 skipping was detected only in c.9501+3A>T carriers (carrier 1 SF = 5.6%; carrier 2 SF = 20.8%; Figure 3e).
BRCA2 c.7447A>G variant generated a minor in-frame event Δ15p lacking 12nt from the 5′end of exon 15, absent in controls (Figure 3f). Although we could not estimate the fraction of this transcript due to sample unavailability, data obtained from one RT-PCR experiment indicates a small proportion corresponding to <10%.
Finally, BRCA2 c.7617+1delG variant located at the intron 15 donor site and adjacent to an exonic G, promoted the shift of the canonical donor site to one nucleotide upstream, producing a major frameshift event and a minor transcript Δ15 (Figure 3g). Accordingly, in silico analysis predicted the creation of a donor site one nucleotide upstream, although at reduced strength (Figure S4).
3.3 Semi-quantitative analysis of the reference full-length transcript
We next compared the relative abundance of the reference FL transcript between variant carriers and noncarrier controls, obtained from semi-quantitative capillary electrophoresis experiments. Full-length levels from the control group (mean = 1) were used as the reference value to compare FL levels between samples. BRCA1 c.302–1G>A, c.4484+5G>C, and c.5333–3T>G variants yielded FL levels <0.5, with mean values ranging from 0.32 to 0.44, suggesting that variant alleles do not contribute to FL transcript expression (Figure 2b,e,f, respectively). BRCA1 c.5333–3T>G variant was analyzed in parallel with the exonic variants c.5153G>T and c.5324T>G under the same FAM-labelled RT-PCR experiments, which yielded similar levels compared to controls (Figure 2f). In contrast, FL levels from BRCA1 c.4675+1G>C and BRCA1 c.5468–1_5468delinsTT carriers were >0.5 (mean = 0.64 and 0.62, respectively) suggesting that variant alleles may still produce some amount of normal transcript (Figure 2c,d). However, this could not be confirmed by allele-specific assays due to the absence of informative exonic variants in these carriers.
Full-length levels were also measured for BRCA2 variants generating altered splicing profiles. Levels were notably reduced in c.516+2T>A, c.1909+1G>A, c.8332–13T>G, c.8332–2A>G and c.8954–2A>T carriers, with values ranging from 0.25 to 0.38 (Figure 3a–d). In contrast, FL levels from BRCA2 c.9501+3A>T carriers were also reduced (carrier 1 mean = 0.50; carrier 2 mean = 0.41), but standard error overlapped with two nonsplicing BRCA2 variants c.9501+4A>G and c.9501+9A>C, which were analyzed in parallel under the same RT-PCR experiments and electrophoresis conditions (Figure 3e).
3.4 Allelic contribution to full-length transcript expression and allelic imbalance assessment
Variant allele ability to generate normal full-length transcript could be determined for 7 out of the 14 BRCA1/2 variant carriers with abnormal splicing profiles. The full-length transcript was specifically amplified with primers located in skipped exons and variant allele contribution was determined by visual inspection at heterozygous exonic loci. A monoallelic contribution was observed for BRCA1 c.302–1G>A variant and for BRCA2 c.516+2T>A, c.1909+1G>A, c.8332–13T>G, c.8332–2A>G, c.8954–2A>T variants, consistent with semi-quantitative data. In contrast, biallelic contribution was observed for BRCA2 c.9501+3A>T carrier, although not at equal signal intensity (Figure 4).
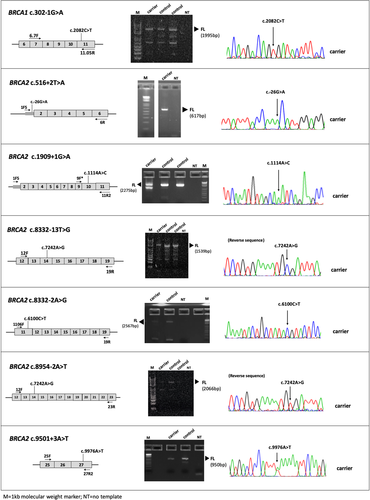
BRCA1/2 allele-specific RT-PCR experiments. PCR primers were designed to target skipped exons and amplify a region containing an exonic variant known to be heterozygous in the patient's genomic DNA (data not shown). Sanger sequencing revealed a monoallelic contribution to normal transcript expression for all variants except for BRCA2 c.9501+3A>T variant, in which a biallelic contribution was observed. PCR, polymerase chain reaction
To rule out the presence of aberrant transcripts that could be degraded by the nonsense-mediated decay system and escape CE detection, allelic imbalance was determined in variant carriers displaying normal splicing profiles. Visual inspection of Sanger peaks at heterozygous exonic sites showed equal allelic expression for BRCA1 c.4113G>A, c.5066T>C, c.5153G>T and c.5324T>G variants (Figure S2), and BRCA2 c.632–16A>C, c.7435+6G>A and c.9501+9A>C variants (Figure S3). The remaining variants could not be assessed due to the lack of informative loci.
3.5 Clinical classification of the 31 BRCA1/2 variants following ENIGMA guidelines
3.5.1 Class 5 variants
BRCA1 c.302–1G>A and BRCA2 c.516+2T>A, c.1909+1G>A, c.8332–13T>G, c.8332–2A>G, c.8954–2A>T variants are located in the highly conserved donor/acceptor splice sites. Results obtained from semi-quantitative CE experiments and allele-specific assays showed a monoallelic contribution to full-length transcript expression in variant carriers (Figure 4), as well as the presence of predominant nonfunctional transcripts absent in control samples (Table 2). Taken together, these variants qualify for a pathogenic classification according to ENIGMA criteria.
| GENE | Variant (HGVS)a | Control population frequencies (gnomAD) | Probability of pathogenicityb (Breast Cancer Genes Priors tool) | Semi-quantitative analysis by CE | Clinical classification## | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Splice site damage | Creation of a de novo splice site | Protein sequence damage | Major abnormal mRNAs detected by CE | Relative FL expression levels (mean ± SEM) | Allele-specific analysisc | Allelic imbalance assessment | Previous to this studyd | After this study | |||||
| Transcript | Splicing fraction (carriers/controls) | Functional annotation | |||||||||||
| BRCA1 | c.81–11T>C | ALL:0.00041%; NFE:0.00090% | Moderate (0.34) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | – | Class 1 (Benign) |
| c.135–18T>G | ALL:0.00042%; NFE:0.00090% | Moderate (0.34) | NA | NA | Δ5 | 7%/2.6% | In-frame | 0.93 ± 0.02 | ND (absence of informative loci) | ND (absence of informative loci) | Uncertain | Class 3 (Uncertain) | |
| c.212+17T>C | EA: G=0.00%; AA: G=0.02% | NA | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Likely benign | Class 1 (Benign) | |
| c.302–1G>A | – | High (0.97) | NA | NA | Δ7p | 25,8%/Absent | Frameshift | 0.32 ± 0.097 | Monoallelic | ND | Pathogenic | Class 5 (Pathogenic) | |
| c.4113G>A (p.G1371G) | ALL:0.048%; AFR:0.49%; AMR:0.032%; NFE:0.0024%; OTH:0.016% | NA | Weak/Null (0.02) | Weak/Null (0.02) | – | – | – | ND | NA | No imbalance | Likely benign (***) | Class 1 (Benign) | |
| c.4358–10C>T | ALL:0.032%; AFR:0.0083%; AMR:0.12%; NFE:0.034%; OTH:0.031% | Moderate (0.34) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Likely benign | Class 1 (Benign) | |
| c.4484+5G>C | – | Low (0.04) | NA | NA | Δ14 | 41,2%/Absent | Frameshift | 0.45 ± 0.07 | ND (absence of informative loci) | ND (absence of informative loci) | Pathogenic | Class 4 (Likely pathogenic) | |
| c.4485–10A>G | ALL:0.0012%; AMR:0.0060%; NFE:0.00090% | Improved (0.04) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Likely benign | Class 1 (Benign) | |
| c.4675+1G>C | – | High (0.97) | NA | NA | Δ15q | 24%/Absent | Frameshift | 0.65 ± 0.13 | ND (absence of informative loci) | ND (absence of informative loci) | Pathogenic | Class 4 (Likely pathogenic) | |
| c.5066T>C (p.Met1689Thr) | – | NA | Weak/Null (0.02) | Low (0.29) | – | – | – | ND | NA | No imbalance | Uncertain | Class 3 (Uncertain) | |
| c.5074+6C>G | ALL:0.0058%; AFR:0.0042%; AMR:0.012%; NFE:0.0087% | Improved (0.04) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Conflicting | Class 1 (Benign) | |
| c.5153G>T (p.Trp1718Leu) | – | Moderate (0.34) | Weak/Null (0.02) | Moderate (0.66) | – | – | – | 0.88 ± 0.25 | na | No imbalance | – | Class 4 (Likely pathogenic) | |
| c.5324T>G (p.Met1775Arg) | ALL:0.0014%; AFR:0.017% | NA | Weak/Null (0.02) | Moderate (0.66) | – | – | – | 1.5 ± 0.17 | NA | No imbalance | Pathogenic (***) | Class 5 (Pathogenic) | |
| c.5333–8C>T | ALL:0.0018%; AMR:0.0058%; NFE:0.0024% | Improved (0.04) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Conflicting | Class 1 (Benign) | |
| c.5333–3T>G | – | High (0.97) | NA | NA | Δ22 | 52,2%/Absent | Frameshift | 0.4 ± 0.03 | ND (absence of informative loci) | ND (absence of informative loci) | Pathogenic | Class 4 (Likely pathogenic) | |
| c.5468–1_5468delinsTT | – | NA | NA | NA | Δ24p | 30,4%/Absent | Frameshift | 0.60 ± 0.07 | ND (absence of informative loci) | ND (absence of informative loci) | – | Class 4 (Likely pathogenic) | |
| BRCA2 | c.516+2T>A | – | High (0.97) | NA | NA | Δ6 Δ5,6 |
31,6%/Absent 20,1%/Absent |
Frameshift frameshift |
0.28 ± 0.05 | Monoallelic | ND | Pathogenic | Class 5 (Pathogenic) |
| c.632–16A>C | ALL:0.00085%;AMR:0.0061% | Improved (0.04) | NA | NA | – | – | – | ND | NA | No imbalance | Conflicting | Class 1 (Benign) | |
| c.1909+1G>A | – | Capped (0.5) | NA | NA | Δ9,10 | 63,2%/0.9% | Frameshift | 0.24 ± 0.05 | Monoallelic | ND | Pathogenic/Likely pathogenic | Class 5 (Pathogenic) | |
| c.7435+6G>A | ALL:0.028%; AFR:0.23%; AMR:0.029%; SAS:0.0065%; NFE:0.0048%; OTH:0.047% | Moderate (0.34) | NA | NA | – | – | – | ND | NA | No imbalance | Conflicting | Class 1 (Benign) | |
| c.7447A>G (p.Ser2483Gly) | ALL:0.0016%; AMR:0.0089%; NFE:0.00090% | NA | Weak/Null (0.02) | Weak/Null (0.03) | Δ15p | ND | In-frame | ND | ND | No imbalance | Uncertain | Class 3 (Uncertain) | |
| c.7617+1delG | – | NA | NA | NA | del1nt | – | Frameshift | ND | NA | ND (absence of informative loci) | – | Class 4 (Likely pathogenic) | |
| c.7805+13A>G | ALL:0.0065%; AFR:0.054%; SAS:0.0032%; NFE:0.0032% | NA | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Conflicting | Class 1 (Benign) | |
| c.8332–13T>G | – | High (0.97) | NA | NA | Δ19 | 57,7%/2% | In-frame | 0.26 ± 0.04 | Monoallelic | ND | – | Class 5 (Pathogenic) | |
| c.8332–2A>G | – | High (0.97) | NA | NA | Δ19p Δ19 |
18%/Absent 21,7%/2% |
Frameshift in-frame |
0.42 ± 0.1 | Monoallelic | ND | Pathogenic/Likely pathogenic | Class 5 (Pathogenic) | |
| c.8488–20T>G | – | High (0.97) | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | – | Class 1 (Benign) | |
| c.8954–2A>T | – | High (0.97) | NA | NA | Δ23p Δ22,23p |
33,8%/1,7% 10,8%/Absent |
In-frame frameshift |
0.29 ± 0.06 | Monoallelic | ND | – | Class 5 (Pathogenic) | |
| c.9257–10_9257–9insT | ALL:0.0047%; AMR:0.015%; SAS:0.0034%; NFE:0.0047% | NA | NA | NA | – | – | – | ND | NA | ND (absence of informative loci) | Likely neutral | Class 1 (Benign) | |
| c.9501+3A>Te | ALL:0.011%; AFR:0.0042%; AMR:0.017%;NFE:0.018% | Moderate (0.34) | NA | NA | Δ25 | 13,2%/Absent | Frameshift | Carrier 1: 0.50 ± 0.08 carrier 2: 0.41 ± 0.04 |
Biallelic | ND | Conflicting | Class 3 (Uncertain) | |
| c.9501+4A>G | ALL:0.0012%; NFE:0.0027% | Moderate (0.34) | NA | NA | – | – | – | 0.70 ± 0.22 | NA | ND (absence of informative loci) | Conflicting | Class 1 (Benign) | |
| c.9501+9A>C | ALL:0.0061%; AMR:0.0087%; NFE:0.010%; OTH:0.015% | NA | NA | NA | – | – | – | Carrier 1: 0.88 ± 0.26 carrier 2: 0.89 ± 0.27 |
na | No imbalance | Conflicting | Class 1 (Benign) | |
- Abbreviations: CE, capillary electrophoresis; FL, full-length transcript; NA, not applicable; ND, not determined.
- ##Clinical classifications ascertained according to ENIGMA criteria (www.enigmaconsortium.org).
- a Sequence variants were annotated using RefSeq NM_007294.3 for BRCA1 and RefSeq NM_000059.3 for BRCA2; HGVS guidelines were followed for variant nomenclature (Den Dunnen et al., 2016).
- b Breast Cancer Genes Prior Probabilities and scores obtained through http://priors.hci.utah.edu/PRIORS/index.php.
- c Results obtained from specific amplification of reference transcripts. Monoallelic = variant allele does not contribute to normal transcript expression; Biallelic = variant allele contributes to normal transcript expression.
- d ClinVar classification ascertained by January 2019. Only variants marked as (***) have been reviewed by an expert panel, providing high-confidence clinical classifications.
- e Splicing fraction mean obtained from two unrelated carriers.
Interestingly, BRCA2 c.516+2T>A variant was previously reported to promote a major exon 6 skipping identified by agarose gel analysis and Sanger sequencing (Rodríguez-Balada et al., 2016), but CE analysis using the same patient RNA detected an additional deleterious event consisting of exons 5–6 skipping (Figure 3a). In this sense, our results were consistent with the splicing defects observed for another variant affecting intron 6 donor site (BRCA2 c.516+1G>T; Whiley et al., 2011), remarking the importance of standardizing RNA protocols and implementing high-sensitive methodologies to provide accurate clinical interpretations.
Finally, although RNA analysis of BRCA1 c.5324T>G (p.Met1775Arg) variant showed no splicing alteration and allelic imbalance could be ruled out (see Figures 2 and S2), previous multifactorial likelihood analysis classified this variant as pathogenic (posterior probability = 0.9978; Tavtigian, Byrnes, Goldgar, & Thomas, 2008) and functional studies reported a deleterious impact on homologous recombination repair activity (Towler et al., 2013; Findlay et al., 2018)
3.5.2 Class 4 variants
In vitro analysis of BRCA1 variants c.4484+5G>C and c.5333–3T>G identified two major frameshift transcripts Δ14 and Δ22, respectively, that would contribute to variant pathogenicity by producing nonfunctional BRCA1 proteins totally or partially lacking the BRCT domains (Lee et al., 2010). Furthermore, the semi-quantitative analysis indicated a two-fold reduction of full-length transcript levels, suggesting a monoallelic contribution, although this could not be confirmed by allele-specific assays due to the lack of informative exonic variants in carriers. Taken together, our results support classification as likely pathogenic (Class 4; Table 2).
The effect observed for BRCA2 c.7617+1delG should also be considered likely pathogenic according to ENIGMA criteria: (a) the variant is located in position IVS+1; (b) it produces a major frameshift transcript lacking 1nt that would contribute to variant pathogenicity; (c) the variant is untested for in vitro assays of patient RNA that asses allele-specific transcript expression. A similar splicing defect was previously reported for BRCA1 c.5193+1delG variant, described as severely impacting splicing and suggesting a likely pathogenic classification (Wappenschmidt et al., 2012).
BRCA1 c.4675+1G>C and c.5468–1_5468delinsTT variants also meet the criteria to be classified as likely pathogenic. However, semi-quantitative estimation of the full-length transcript in carriers was above >0.5, and standard error of the mean (SEM) overlapped with controls SEM (see Figure 2c,d) suggesting that variant alleles might be able to produce some amounts of FL transcript. However, notable differences on FL transcript levels were also observed among controls, suggesting that these differences could also apply for variant carriers if data were available from additional samples. In this regard, a methodological analysis assessing the accuracy of CE data would be required to assume 0.5 as a threshold for determining monoallelic or biallelic contribution to FL expression. Therefore, weighing the evidence collected for these two variants (Table 2) we consider them as likely pathogenic.
RNA analysis of the BRCA1 missense variant c.5153G>T (p.Trp1718Leu) showed normal splicing in variant carrier, as well as biallelic expression at variant site (see Figures 2 and S2). However, a deleterious impact on protein function has been recently observed by saturation genome editing analysis (Findlay et al., 2018), and a previous study also reported a deleterious effect on the transcriptional activity of BRCA1 (Quiles et al., 2013). This variant was identified in a woman affected with ovarian cancer at age 63 and segregated with her sister, also affected with ovarian cancer at age 60 (Table S1). Furthermore, this variant is not present in control populations from gnomAD. Although a multifactorial analysis should be carried out to ascertain a final clinical classification, we consider that all evidence collected for this variant supports a likely pathogenic classification.
3.5.3 Class 3 variants
A “leaky” splicing effect was observed for BRCA1 c.135–18T>G, BRCA2 c.7447A>G and BRCA2 c.9501+3A>T variants. Semi-quantitative analysis of BRCA1 c.135-18T>G detected similar levels of full-length transcript compared to controls, but higher levels of the in-frame Δ5 were detected in variant carrier (Figure 2a). Accordingly, real-time PCR data obtained in a previous study detected similar levels of full-length in carrier and controls, but Δ5 was upregulated (Wappenschmidt et al., 2012). This in-frame transcript is predicted to encode for a protein that would lack part of the RING domain, which is crucial for BRCA1 protein stability and homologous recombination repair activity (Ransburgh, Chiba, Ishioka, Toland, & Parvin, 2010). Given the lack of conclusive evidence and the possibility that c.135–18T>G could act as a risk-modifier allele, this variant should be classified as uncertain (Class 3) according to ENIGMA criteria.
Semi-quantitative analysis of BRCA2 c.7447A>G (p.Ser2483Gly) detected a minor in-frame transcript lacking 12 nucleotides, compatible with the creation of a new acceptor site in exon 15 (Figure 3f). As expected, allelic imbalance was not detected at the variant site because the in-frame isoform would not be degraded by the nonsense-mediated decay mechanism. This transcript is predicted to encode for a protein lacking four aminoacids of the DBD domain, and although it is present at a low abundance, we cannot exclude a modifier effect. Moreover, the effect of this missense variant has not been functionally assessed, thus we consider this variant as uncertain (Class 3) until more data are collected.
Inconclusive evidence was also obtained for BRCA2 c.9501+3A>T variant. Different amounts of exon 25 skipping were detected in the two carriers (5.6% and 20.8%; Figure 3e), and allele-specific analysis performed in one carrier showed biallelic contribution to FL transcript expression (Figure 4). Previous studies using a minigene approach or RNAseq technology have also reported a partial splicing effect, with exon 25 skipping levels ranging from 10% to 20% (Acedo, Hernández-Moro, Curiel-García, Díez-Gómez, & Velasco, 2015; Farber-Katz et al., 2018). Although the authors suggested a questionable deleterious impact for c.9501+3A>T variant, they considered it as a potential low-penetrance or disease-modifier allele. However, authors from the second study considered this variant as non-pathogenic (Farber-Katz et al., 2018). Furthermore, a previous multifactorial likelihood analysis yielded a probability of pathogenicity of 0.64, classifying the variant as uncertain (Whiley et al., 2011). Given the reported inconsistencies and the lack of conclusive evidence from our study, we classify this variant as uncertain (Class 3).
RNA analysis of the BRCA1 missense variant c.5066T>C (p.Met1689Thr) showed normal splicing in the variant carrier, as well as biallelic expression at the variant site. This variant is located in the BRCT domain of BRCA1 but no functional impact was observed by saturation genome editing analysis (Findlay et al., 2018). However, this variant was identified in a patient (male) affected with breast cancer and segregated with her sister affected with breast cancer at age 42. Furthermore, previous multifactorial likelihood analysis classified this variant as uncertain (Thompson et al., 2016). Thus, given the reported inconsistencies, further clinical data needs to be collected to clarify the risk associated with c.5066T>C allele.
3.5.4 Class 1 variants
No mRNA alteration was observed for BRCA1 c.81–11T>C, c.212+17T>C, c.4113G>A, c.4358–10C>T, c.4485–10A>G, c.5074+6C>G, c.5333–8C>T variants, and BRCA2 c.632–16A>C, c.7435+6G>A, c.7805+13A>G, c.8488–20T>G, c.9257–10_9257–9insT, c.9501+4A>G, c.9501+9A>C variants, which showed the same splicing patterns and peak signal intensities observed in noncarrier controls (Figures S2 and S3). Accordingly, in silico analysis indicated a low probability of splicing alteration (Table 1). Furthermore, BRCA1 c.4358–10C>T was detected in co-occurrence with the pathogenic variant BRCA1 c.4195_4196delAC (p.Thr1399Hisfs*; Judkins et al., 2005), and at MAF = 0.12% in AMR control population, supporting a benign classification. BRCA2 c.7435+6G>A variant was detected at MAF = 0.23% in AFR population, also supporting a benign classification. In all, these variants meet the criteria to be classified as benign according to ENIGMA guidelines (Table 2).
Splicing analysis of the synonymous variant BRCA1 c.4113G>A showed the same patterns compared to noncarrier controls, and Sanger inspection at variant site ruled out BRCA1 allelic imbalance. Furthermore, this variant was detected at a higher frequency in the African population (MAF = 0.49%). Thus, this variant should also be considered of no clinical significance.
4 DISCUSSION
Genetic variants that affect mRNA splicing are the molecular basis for many inherited diseases (Faustino & Cooper, 2003). Sequence variants have the potential to disrupt cis-elements involved in proper exon recognition of pre-mRNA molecules and promote the generation of altered mRNA products leading to disease.
The main objective of the present study was to assess the splicing impact of 31 BRCA1/2 variants using capillary electrophoresis and allele-specific analysis and provide an accurate clinical classification for each variant following ENIGMA guidelines (Table 2). Previous studies have demonstrated the utility of using capillary electrophoresis of fluorescent amplicons (CE) for the characterization of BRCA1/2 splicing variants, as it provides high sensitivity and resolution (1–2 bp; Acedo et al., 2015; Garibay et al., 2014; Whiley et al., 2014). Accordingly, CE analysis in our samples provided higher resolution compared to QIAxcel electrophoresis, allowing the detection of all abnormal RNA products regardless of amplicon size (see BRCA1 c.302–1G>A, c.4675+1G>C, c.5468–1_5468delinsTT variants in Figure 2b–d, respectively; and BRCA2 c.8332–2A>G and c.7617+1delG in Figure 3c,f respectively); and provided higher sensitivity, allowing the detection of minor transcripts with splicing fractions <5% (e.g., see BRCA1 c.302-1G>A and c.4675+1>C variants in Figure 2b,c respectively). Capillary electrophoresis of fluorescent amplicons also allowed the estimation of known BRCA1/2 alternative splicing events occurring in peripheral blood samples from our control group. Such events (BRCA1 Δ1q, Δ5q, Δ5, Δ8p, Δ9,10, Δ13p, Δ14p and Δ15; and BRCA2 Δ6q,7, Δ9,10, Δ17,18, Δ18, Δ19, ▼20A and Δ23p) were detected at similar levels in lymphoblastoid cell lines using qualitative and quantitative approaches (Table S5; Colombo et al., 2014; Davy et al., 2017; Fackenthal et al., 2016; Lattimore et al., 2018), indicating that CE provides concordant data.
The BRCA1/2 alternative splicing landscape comprises a broad spectrum of minor and predominant splicing events that add complexity to variant interpretation. Some of these isoforms maintain the open reading frame and could potentially generate BRCA proteins with complete or partial tumor suppressor activity (Wang et al., 2016). In fact, a rescue model was proposed for BRCA1 Δ9,10 isoform (la Hoya et al., 2016). Authors found that although BRCA1 c.(594–2A>C; 641A>G) alleles did not contribute to full-length transcript expression and produced a major frameshift event lacking exon 10, an estimated proportion of 30% of Δ9,10 isoform was sufficient to confer tumor suppressor capacity in variant carriers (la Hoya et al., 2016). Another example illustrating the complexity of splicing interpretation is BRCA2 c.68–7T>A variant, for which authors concluded that BRCA2 alleles producing up to approximately 20% exon 3 skipping were not associated with cancer risk (Colombo et al., 2018). Here, we describe three variants producing “leaky” splicing effects (see BRCA1 c.135–18 T>G, BRCA2 c.9501+3A>T and BRCA2 c.7447A>G variants in Figures 2a, and 3e,f, respectively), and the methodology used in this study is limited for the clinical interpretation of this type of variants. Such cases stress the need for establishing thresholds of exon exclusion tolerance to accurately assign cancer estimation risks.
We also compared our experimental data with the probabilities of pathogenicity obtained from the Breast Cancer Genes Prior Probabilities tool, developed with the aim of incorporating prior probabilities into the integrated evaluation of BRCA1/2 unclassified variants (Vallée et al., 2016; Table 2). In brief, nine variants scored a high (0.97) or capped (0.5) probability of pathogenicity due to splice site damage that we confirmed experimentally, classifying them in Class 4 or Class 5 categories, with the exception of BRCA2 c.8488–20T>G (false positive) that showed normal splicing patterns. Seven variants scored a moderate (0.34) probability of pathogenicity, with five showing normal splicing profiles and two producing “leaky” splicing effects that we classified in Class 3. Nine variants scored a low/improved (0.04) or weak/null (0.02) probability of pathogenicity that we confirmed experimentally, with the exception of BRCA1 c.4484+5G>C (false negative) which was found to generate a major exon skipping. For six variants, splicing scores were not available (Table 2). As mentioned in Vallée et al. (2016), the majority of splicing assays published to date are rarely quantitative, and the lack of aberrant or alternate transcripts measurements may overestimate the rates of splicing alterations, posing a limitation when calibrating in silico tools and defining grades of variant pathogenicity. In this regard, semi-quantitative data reported in this study may help to calibrate in silico probabilities of pathogenicity for their incorporation into the integrated evaluation of BRCA1/2 unclassified variants.
In summary, the appropriate use of BRCA1/2 genetic testing in routine clinical practice requires the use of robust in silico and in vitro approaches, as well as the use of accurate classification systems such as the ENIGMA BRCA1/2 variant classification criteria. Refinement of the multifactorial likelihood model for the interpretation of BRCA1/2 variants is an ongoing process (Easton et al., 2007; Goldgar et al., 2004; Spurdle et al., 2008; Tavtigian et al., 2008), and the application of quantitative assays for the study of splicing alterations will be crucial to incorporate calibrated experimental measures against cancer estimation risks (Spurdle, Couch, Hogervorst, Radice, & Sinilnikova, 2008; Vallée et al., 2016; Walker et al., 2013). Our study remarks the need for applying semi-quantitative or quantitative assays to better understand the clinical relevance of splicing occurring in hereditary breast/ovarian cancer alleles, and supports the incorporation of quantitative splicing data in current variant classification guidelines to improve variant interpretation.
ACKNOWLEDGMENTS
The authors acknowledge the Cellex Foundation for providing research facilities and equipment and Leo Judkins for English language editing.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
FUNDING
This work was supported by Spanish Instituto de Salud Carlos III funding an initiative of the Spanish Ministry of Economy and Innovation partially supported by the European Regional Development FEDER Funds, Grant/Award Numbers: PI13/01711, PI15/00355, PI16/01218, Miguel Servet Progam CPII16/00034. S. B. is recipient of an “Asociación Española Contra el Cáncer” (AECC) fellowship.




