The novel p.Ser263Phe mutation in the human high-affinity choline transporter 1 (CHT1/SLC5A7) causes a lethal form of fetal akinesia syndrome
Abstract
A subset of a larger and heterogeneous class of disorders, the congenital myasthenic syndromes (CMS) are caused by pathogenic variants in genes encoding proteins that support the integrity and function of the neuromuscular junction (NMJ). A central component of the NMJ is the sodium-dependent high-affinity choline transporter 1 (CHT1), a solute carrier protein (gene symbol SLC5A7), responsible for the reuptake of choline into nerve termini has recently been implicated as one of several autosomal recessive causes of CMS. We report the identification and functional characterization of a novel pathogenic variant in SLC5A7, c.788C>T (p.Ser263Phe) in an El Salvadorian family with a lethal form of a congenital myasthenic syndrome characterized by fetal akinesia. This study expands the clinical phenotype and insight into a form of fetal akinesia related to CHT1 defects and proposes a genotype-phenotype correlation for the lethal form of SLC5A7-related disorder with potential implications for genetic counseling.
The neuromuscular junction (NMJ) is a complex structure that serves to efficiently communicate the electrical impulse from the motor neuron to the skeletal muscle to signal contraction (Hughes, Kusner, & Kaminski, 2006). With the advent of next-generation sequencing, several molecular causes of disorders of the NMJ have been deciphered over the past few years. Thirty genes acting at different levels in the NMJ define a growing class of disorders known as congenital myasthenic syndromes (CMS; McMacken, Abicht, Evangelista, Spendiff, & Lochmüller, 2017). In addition to genetic heterogeneity, wide clinical heterogeneity is also observed in CMS. At the most severe end of the spectrum are lethal forms of the condition characterized by reduced intrauterine movement, postnatal severe generalized weakness, inability to maintain airways independently, and a high rate of mortality.
Over the past few years, the discovery of a new autosomal recessive type 20 CMS (CMS20; OMIM #617143) due to pathogenic variants in the choline transporter 1 (CHT1) has included seven individuals (out of 10 families) that survived with a clinical presentation ranging from arthrogryposis and hypotonia to treatable neonatal CMS with occasional apnea (Bauché et al., 2016; Pardal-Fernández et al., 2018; Wang et al., 2017). In this group, two families presenting with a lethal phenotype were also described (Table S1; Bauché et al., 2016; Wang et al., 2017). An additional patient, who is possibly deceased, appears to have had a severe form of the disorder (Table S1; Pardal-Fernández et al., 2018). An autosomal dominant allelic form of CMS20 or distal hereditary motor neuropathy type VII (HMN-VII; OMIM 158580) with onset between the first and second decade of life was described in 2012 in an extensive pedigree (Barwick et al., 2012).
Homozygous Slc5a7 knockout mice displayed irregular breathing, became cyanotic and died within 1 hour of birth (Ferguson et al., 2004), a phenotype that closely resembles the lethal form of CHT1-related disorders previously reported in (Bauché et al., 2016; Pardal-Fernández et al., 2018; Wang et al., 2017).
CHT1 is a glycoprotein belonging to the family of sodium-dependent glucose transporters (Okuda et al., 2000). CHT1 is composed of 13 transmembrane helices, has an extracellular amino-terminus, cytosolic carboxy-terminus, and forms dimers/oligomers (Okuda et al., 2012). The pathogenic variants reported by Bauché et al. (2016) and Wang et al. (2017), show a near complete loss of CHT1 function in cell models, which aligned with abnormal synaptic maturation or maintenance of the NMJ.
This report describes a new and severe CMS phenotype associated with a novel homozygous missense variant in the SLC5A7 gene. This variant has only been reported to date in a heterozygous state in the gnomAD database (Lek et al., 2016). Our functional studies confirm that the variant c.788C>T, encoding the substitution p.Ser263Phe is associated with a total loss of CHT1 function, which explains the lethal phenotype observed in the patients. Furthermore, we review the clinical presentation of the families with a lethal phenotype due to recessive mutations in the SCL5A7 gene and propose a possible hypothesis for a phenotype-genotype correlation which will assist with genetic counseling.
A Canadian consanguineous family of El Salvadorian ethnicity was evaluated in the neonatal period with a CMS-like disorder in two of their children, as shown in the pedigree (Figure 1a). The first child, who had displayed reduced prenatal movements, was a girl born at term who required resuscitation and was intubated following the delivery. She displayed profound generalized hypotonia, was ventilator-dependent, did not show dysmorphic features, but had arthrogryposis in all four limbs with no pterygia. The diagnostic workup for hypotonia showed a normal female chromosomal complement, and normal Prader-Willi, spinal muscular atrophy, and myotonic dystrophy type 1 genetic testing. Nerve conduction studies/electromyography were suggestive of a myasthenia gravis-like pattern (decrement with slow repetitive nerve stimulation). Further clinical genetic testing detailed in the Supporting Information file was performed but as there was no clinical improvement with the use of pyridostigmine, 3,4-diaminopyridine, fluoxetine, and salbutamol, the family decided to withdraw care at 5 months of age. The second child had a neonatal presentation similar to his sister. Nerve conduction studies (Figure S1A) showed severe axonal motor and sensory polyneuropathy with NMJ dysfunction. A nerve biopsy showed primary axonopathy with giant axons and secondary myelin loss (Figure S1B). In the absence of a response to pyridostigmine and with similarity with the sister's clinical presentation, withdrawal of care was decided at 1.5 months of age. Parents, who are first cousins once removed, were reportedly healthy with no congenital anomalies, no neurodevelopmental disorders, or any motor restrictions. In the context of consanguinity with unaffected parents and two similarly affected children, the possibility of an autosomal recessive disorder was raised. A combination of SNP array based on identity by descent and exome sequencing of the two affected patients allowed the identification of a candidate gene, SLC5A7 (See Supporting Information data for more information).
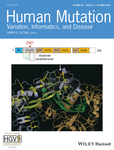
(a) Autosomal recessive pedigree of our study family with the corresponding Sanger sequencing electropherograms for the c.788C>T. Consanguineous parents are carriers. One male offspring is unaffected. One male and one female offspring were affected, required ventilation at birth and died at 2 and 5 months, respectively. Children displayed lack of prenatal movement, postnatal difficulties, breathing and sucking, and progressive muscle weakness. (b), SLC5A7 topological model and location of mutations reported to date. Red circles and arrows: all mutations in patients with a lethal form of the condition are located in the intracytoplasmic domain—see text for details; black circles: mutations in viable patients; branches represent predicted N-glycosylation sites. Note: There are viable patients who are combined heterozygous with one mutation in the intracellular domain and one in the transmembrane domain. The topology plot was generated using Protter 1.0 Software (Omasits, Ahrens, Müller, & Wollscheid, 2014). (c) Immunoblot of HEK293 cells stably expressing the empty vector (EV), CHT1-WT or CHT1-S263FSer263Phe. (c, d) Black circle indicates CHT1 carrying complex oligosaccharide, the white circle shows core glycosylated CHT1, arrowhead indicates unglycosylated CHT1. (d) Cell surface biotinylation of cells expressing the empty vector, CHT1-WT or -S263FSer263Phe. The membranes were blotted with anti-CHT1 (top blot), anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (middle blot), or anti-Na+/K+−ATPase (bottom blot) antibodies. Lack of GAPDH in the biotinylated fraction shows that the membrane impermeant biotin reagent did not leak intracellularly and the Na+/K+−ATPase was chosen as a positive control for the biotinylation. (e) Quantification of the total (input), unbound, and cell surface CHT1-S263FSer263Phe relative to CHT1-WT indicating that the mutant is less abundant at the plasma membrane, but not significantly different when corrected for input level. Error bars correspond to means ± SD, n = 3. **p < .01, ***p < .001 versus “WT” condition using one-way ANOVA with Dunett's Multiple Comparisons post hoc test. ANOVA, analysis of variance; CHT1, choline transporter 1
A homozygous variant was identified in SLC5A7 (NM_021815.4, g. 108622551, c.788C>T, and p.Ser263Phe; Figure 1a). This transition from C to T in exon 7 substitutes a highly conserved nucleotide (phyloP 6.18 [−14.1; 6.4]) and amino acid up to C. elegans (Figure S2). In silico analysis of c.788C>T, p.Ser263Phe using Alamut visual version 2.7, April 2015 (Interactive Biosoftware, Rouen, France) indicated this mutation to be probably damaging (Polyphen2, score 1.00, both HumVar and HumDiv), deleterious (score 0, SIFT), and disease-causing (Align GVGD, classC65; score GV: 0.00, GD:154.81). This variant is present in gnomAD with an overall frequency of 0.00041% and 0.0030% in Latinos. Ser263 is predicted to be in the fourth cytosolic loop of CHT1 (Figure 1b). The variant reported here has been submitted to the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/; submission ID: 5111505).
The single nucleotide variant c.788C>T (p.Ser263Phe) was generated in the human CHT1-WT complementary DNA (gift from Randy Blakely [plasmid #15766; Addgene]; Apparsundaram, Ferguson, George, & Blakely, 2000) by site-directed mutagenesis as detailed in the Supporting Information File. HEK293 cells stably expressing either empty vector, CHT1-WT or CHT1-Ser263Phe, were generated through the selection of cells in hygromycin after a lentiviral infection process, as further detailed in the Supporting Information File. To begin the characterization of CHT1-Ser263Phe and compare it with CHT1-WT, cell lysates from these cell lines at confluency were subjected to immunoblotting with anti-CHT1 rabbit polyclonal antibody (Supporting Information File for further details). CHT1-WT and CHT1-Ser263Phe protein mobilities were compared. Figure 1c shows that in contrast to cells containing the empty vector, lysates expressing CHT1-WT displayed one band at around 70 kDa (black circle), an intermediate second band at a molecular weight consistent with the predicted 63 kDa (white circle), and a diffuse band with a slightly lower molecular weight (arrowhead) corresponding to 8 ± 4% of the total CHT1 protein (n = 3, ±SD). Interestingly, we observed that total CHT1-Ser263Phe (a) was significantly less abundant than total WT-CHT1 (68 ± 7% of WT-CHT1, n = 5, ±SD), and (b) displayed the same three bands as the WT-CHT1, but the intensity of the top band (black circle) significantly decreased from 46 ± 1% in CHT1-WT to 33 ± 1% in CHT1-Ser263Phe mutant (n = 3, ±SD).
The detection of multiple bands suggests that CHT1 is posttranslationally modified possibly by carrying various forms of oligosaccharides, as suggested previously (Haga, 2014). To determine if N-glycosylation was the reason for the different band mobilities, an enzymatic N-deglycosylation of the proteins in the lysate was performed with peptide-N-glycanase F (Figure S3A). Compared with mock digested samples (lane “−”), we observed a shift of the top bands to a lower molecular weight after digestion (arrowhead, lane “+”), supporting that CHT1 is N-glycosylated. The same pattern was observed for both CHT1-WT and CHT1-Ser263Phe, indicating that the glycosylation pattern is similar.
An alteration of the glycosylated/deglycosylated ratio as seen in Figure 1c might indicate abnormal processing of the CHT1-Ser263Phe compared with CHT1-WT. To address this possibility, HEK293 cells expressing CHT1-WT, CHT1-Ser63Phe, or empty vector were immunostained with rabbit anti-CHT1 polyclonal antibody (Supporting Information File for further details). CHT1-WT and CHT1-Ser263Phe were both detected in their respective cells and predominantly showed perinuclear staining (Figure S3B), indicative of intracellular localization. No obvious difference in localization was observed between the two proteins.
To further determine whether there was a difference in plasma membrane targeting, we conducted cell surface biotinylation experiments using a membrane impermeant biotinylation reagent (Figure 1d). This was done as described previously (Banerjee et al., 2016), with minor modifications as detailed in the Supporting Information File. Despite loading the same amount of total protein per lane (2.5 μg) and consistent with Figure 1c and Figure S3A, CHT1-Ser263Phe displayed less intense bands, with a more intense low molecular weight band (white circle) compared with CHT1-WT. The unbound and biotinylated fractions of the mutant also displayed bands for the mutant that corresponded to 57 ± 4% and 36 ± 19% of the WT protein (n = 3, ±SD), respectively (Figure 1e). These results indicate that although the mutant was less abundantly expressed in HEK293 cells, it reached the plasma membrane to the same extent as WT-CHT1 when corrected for protein level, providing further evidence that plasma membrane targeting of CHT1 is not affected by the p.Ser263Phe alteration.
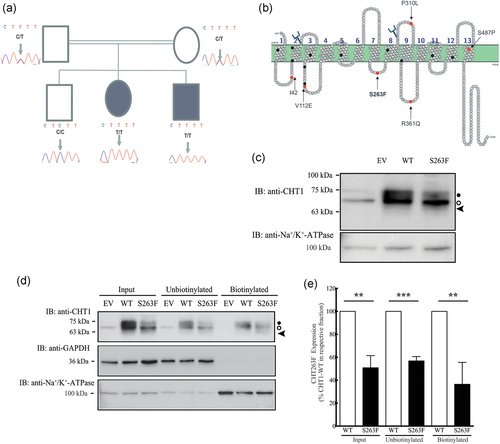
As CHT1-Ser263Phe is able to reach the plasma membrane, we measured its functional activity using a [3H]-choline uptake assay, based on a previously described method (Okuda & Haga, 2003) with modifications as detailed in the Supporting Information File. We compared the ability of HEK293 cells expressing either the empty vector, CHT1-WT or CHT1-Ser263Phe to transport [3H]-choline (0.1 µM, 100 nCi) for up to 20 min (Figure 2a, left panel), and the transport was quantified as described in the Supporting Information File. In contrast with the CHT1-WT protein, which accumulated [3H]-choline over the time course (linear up to 10 min), the mutant displayed a low transport activity that was similar to cells expressing the empty vector. This result indicates that CHT1-Ser263Phe is not able to transport choline at this single concentration. To further assess the function of the mutant, we measured its transport activity over multiple concentrations of [3H]-choline. As shown in Figure 2a (right panel), in contrast with the CHT1-WT that transported significantly more [3H]-choline with increasing concentrations than cells with the empty vector, CHT1-Ser263Phe had a transport activity that did not differ from empty vector cells. Therefore, despite being present at the cell surface, CHT1-Ser263Phe transport of [3H]-choline was not detected (Figure 2a).
(a) Transport of [3H]-choline by HEK293 cells stably expressing CHT1-WT or CHT1-Ser263Phe. Left panel, single concentration [3H]-choline (0.1 µM) transport assay over 20 min; right panel, multiple concentrations of [3H]-choline (0.1, 2.5, and 10.0 µM) and a single time point of 5 min. Error bars correspond to means ± standard deviation, n = 3. **p < .01, ***p < .001 versus “empty vector” condition using one-way analysis of variance (ANOVA) with Tukey's Multiple Comparisons post hoc test. Results from cells expressing CHT1-Ser263Phe were not significantly different from empty vector expressing cells. (b) Immunoblot from HEK293 cells expressing CHT1-WT or CHT1-Ser263Phe either untreated or after incubation with various amounts of chemical chaperones (C3, VX809) or the proteasome inhibitor MG132 for 24 hr. Numbers below the blot indicate the percentage of mutant protein expression relative to untreated condition. Black circle indicates CHT1 carrying complex oligosaccharides, the white circle shows core glycosylated CHT1, arrowhead indicates unglycosylated CHT1, # signs show a band nonspecifically recognized by the antibody. (c) Cell surface biotinylation of HEK293 cells expressing either WT- or Ser263Phe-CHT1 and untreated or incubated with 1% DMSO for 24 hr. Black circle indicates CHT1 carrying complex oligosaccharides, the white circle shows core glycosylated CHT1, arrowhead indicates unglycosylated CHT1. (d) Quantification of the total, unbiotinylated and biotinylated WT- or Ser263Phe- CHT1 with and without DMSO treatment. Error bars correspond to means ± SD, n = 3. *p < .05, **p < .01 versus “untreated CHT1-WT” condition using one-way ANOVA with Tukey's Multiple Comparisons post hoc test. (e) Choline transport measured from HEK293 cells expressing empty vector, WT- or Ser263Phe-CHT1 and either kept untreated or incubated with 1% DMSO for 24 hr. Error bars correspond to means ± SD, n = 3. *p < .05 versus “vector” condition using one-way ANOVA with Tukey's Multiple Comparisons post hoc test. CHT1, choline transporter 1
We next determined whether we could restore the activity of the mutant protein at the plasma membrane. We incubated confluent HEK293 cells stably expressing either empty vector, CHT1-WT or CHT1-Ser263Phe with various chemical chaperones (C3 [kind gift from Dr. Gergely Lukacs, McGill University], VX809 [Selleckchem] or dimethyl sulfoxide (DMSO); Brown, Hong-Brown, Biwersi, Verkman, & Welch, 1996; Goor et al., 2006, 2011) or the proteasome inhibitor MG132 for 24 hr, at the indicated concentrations. CHT1 expression, cell surface abundance, and activity were then examined. Chemical chaperones such as C3 and VX809 did not greatly increase the total abundance of CHT1-Ser263Phe although they increased CHT1-WT abundance (Figure 2b), therefore these chaperones were not further examined. The MG132 incubation stabilized the unglycosylated form (arrowhead) and high molecular weight aggregates of the mutant (star) but did not increase the abundance of complex-glycosylated protein. DMSO treatment significantly increased the total abundance of both CHT1-WT and CHT1-Ser263Phe by 49% and 42%, respectively, compared with the untreated samples (Figure 2c, lanes 1–4 & 2d). Further, the cell surface abundance of CHT1-Ser263Phe was no longer significantly different from untreated CHT1-WT samples, indicating that DMSO treatment rescued both the abundance and cell surface expression of the mutant. The rescuing effect of DMSO on CHT1-Ser263Phe transport activity was assessed next. As shown in Figure 2e, despite the increased level of CHT1-Ser263Phe at the cell surface, the transport activity was not rescued by 1% DMSO incubation as it remained similar to cells expressing the empty vector. These results show that although DMSO increased the total and cell surface expression of the mutant, it did not improve the functional defect induced by the substitution of serine 263 to phenylalanine.
In this manuscript, we report the characterization of a novel missense substitution within the CHT1 protein that caused a lethal form of fetal akinesia syndrome. The family studied presented at the most severe end of the spectrum seen in congenital myasthenic syndrome type 20 due to impaired cholinergic signaling (OMIM 617143). The p.Ser263Phe missense variant involves a highly conserved residue of the CHT1 protein in the fourth cytoplasmic loop, and all in silico analyses deemed the variant pathogenic or disease-causing. Stable expression of CHT1-WT or CHT1-Ser263Phe in HEK293 cells allowed us to characterize the behavior of this mutant. The mutant was found to migrate on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel as three main bands, however, the different ratio of complex glycosylated/high mannose glycosylated/deglycosylated protein seen in WT and mutant CHT1 suggests different intracellular processing for the two proteins. Indirect immunofluorescence showed similar localization for both the WT and Ser263Phe-CHT1 mutant, with predominant intracellular localization and minimal staining at the plasma membrane (Figure S3B). Our WT-CHT1 staining differs from that shown by Bauché et al. (2016), but is however consistent with previous findings supporting the presence of CHT1 in early and recycling endosomes with 90% of the protein residing in intracellular vesicles at the NMJ (Nakata, Okuda, & Misawa, 2004; Ribeiro et al., 2006, 2007). Nevertheless, the transport results confirmed that CHT1-WT was present at the plasma membrane and transported significant amounts of choline (Figure 2a). In contrast, CHT1-Ser263Phe did not have detectable choline uptake activity, despite its presence at the cell surface (Figures 1d,e and 2a). The increased expression and cell surface trafficking of CHT1-Ser263Phe variant in the presence of DMSO (Figure 2c–e) indicate that some chemical chaperones may potentially be used to rescue low expressing but functional CHT1 mutants. Interestingly, Choudhary et al. (2017) recently examined a library of 2,753 small molecules from Pfizer Chemogenomic Library and 880 additional compounds for their potential beneficial effect on CHT1 biology. These authors identified four compounds that acted as positive modulators of CHT1. Some of these compounds may have a rescuing effect on the inactive CHT1-Ser263Phe and/or other naturally occurring CHT1 variants.
The loss of function of CHT1 due to a substitution of serine at codon 263 with phenylalanine could have multiple explanations. The hydroxyl group of the serine could be important for CHT1 function or the bulky and hydrophobic phenylalanine could disrupt function. The cytosolic Ser263 may be phosphorylated, which could, in turn, affect the transport activity of CHT1. Previous studies have shown that activating protein kinase C or inhibiting protein phosphatases 1/2A affects the cell surface abundance and consequently the function of CHT1 in mouse and rat striatal and hippocampal synaptosomes, and that CHT1 is phosphorylated (Black; Ribeiro, Ferguson, & Rylett, 2010; Cooke & Rylett, 1997; Gates, Ferguson, Blakely, & Apparsundaram, 2004; Issa, Gauthier, & Collier, 1996). More recently, the CXCL12 chemokine was shown to upregulate CHT1 in an Akt-mediated process in rat pheochromocytoma PC-12 cells and rat primary neuronal cultures (Yan, Zhao, Guo, Han, & Feng, 2016). However, the phosphorylation site(s) on CHT1 responsible for this has (have) not been identified.
Our family is the fourth reported in the literature with a lethal phenotype of myasthenic syndrome suggesting an overlap with fetal akinesia syndromes. Three of the families with the lethal presentation, including the one described by us, have had two affected offspring each that have demonstrated high penetrance and homogeneity of the clinical condition (Table S1). While 15 missense mutations have been described to date (Bauché et al., 2016; Pardal-Fernández et al., 2018; Wang et al., 2017), the four families with a lethal phenotype are either homozygous for a missense mutation (Family 2 in Wang et al., 2017, patient described by Pardal-Fernandez et al., 2018 and our family) or have a combination of a missense and a nonsense mutation (Family 2 in Bauché et al., 2016). We observed that location of the mutations leading to lethal outcomes, in our family as well as Family 2 in Wang et al., 2017 and Family 2 in Bauché et al., 2016 are consistently located in the cytosolic domain of CHT1 (Figure 1b). However, the patient described by Pardal-Fernandez et al. (2018) and assumed to be demised based on their description, carried two missense mutations: one in transmembrane domain 13, and a second in the fifth extracellular loop (Figure 1b). The CHT1 amino acid mutated in our family, serine 263, is located at the beginning of the fourth cytosolic loop between transmembrane domains 7 and 8 according to the topological model from Okuda & Haga, (2003; Figure 1b). Based on the information to date, it is difficult to infer a genotype-phenotype correlation for the patients presenting with lethality compared with patients that survived and responded to treatment and this remains an important question to be answered by future studies. All the cases surviving beyond the neonatal or early childhood timeframe were compound heterozygous for missense variants with at least one variant in the transmembrane domain. Three of the families with a lethal phenotype, described by Bauché et al. (2016), Wang et al. (2017), and our family, have pathogenic substitutions in the cytosolic portion of the protein and demonstrate a loss of function despite significant proper plasma membrane targeting. Possible explanations for the phenotype in our family in relation to a cytosolic residue interfering with choline transport include an impairment of: (a) choline release into the cytosol, (b) posttranslational modifications (including potential phosphorylation), (c) regulatory protein:protein interactions, and (d) additive effects of multiple polymorphisms present in genes involved in NMJ function. However, these suggestions need further study and the topological model requires further validation.
An explanation for the recessive nature of the CHT1-Ser263Phe and thus the absence of symptoms in the parents of the families described with CMS20 remains speculative. As CHT1 is known to form homodimers (Okuda et al., 2012), it is likely that heterodimers formed of one WT allele and one mutated allele are less functional, but enough to maintain sufficient choline transport at the NMJ which is supported by the lack of reported motor issues by both parents of our family who are in their forties. C-terminal truncating mutations of SLC5A7 are known to cause late-onset dominant hereditary motor neuropathies, through a dominant negative effect of the truncated CHT1 on WT-CHT1 levels and targeting to the cell surface (Barwick et al., 2012; Salter et al., 2018). In Family 2 from Bauché et al. (2016), one parent is heterozygous for a nonsense mutation in the N-terminal part of the protein, I42*, expected to produce a truncated form of the protein possibly followed by nonsense-mediated decay rather than a dominant negative effect on the WT allele. No comments are made on a possible phenotypic effect on any of the parents of the autosomal recessive cases described to date. However, why certain CHT1 mutations have dominant versus recessive effects requires further investigation. Understanding a genotype-phenotype correlation between the clinical presentation and the underlying pathophysiologic mechanism could have a direct impact on counseling of families with similar pathogenic variants in relation to prognosis and anticipatory management, especially for cases diagnosed in the prenatal or neonatal time.
Interestingly, in our family, the nerve biopsy from one patient (Patient 2) indicates the presence of enlarged axons and reduced myelin (Figure S1B). Myelination is a process that continues after birth, however, this reduced level of myelin was unexpected. Cholesterol is an important constituent of myelin (Deber & Reynolds, 1991). Evidence suggests a critical and direct relation between the integrity of lipid rafts in the plasma membrane and CHT1 function and localization (Cuddy, Winick-Ng, & Rylett, 2014). It would be interesting to understand if this observation of reduced myelination in our patients with homozygous CHT1 pathogenic variants is supported in additional families. A nerve biopsy was not available for comparison between the two allelic disorders CMS20 and HMN7A (Barwick et al., 2012). Our observation regarding defective myelination in the recessive form of the SLC5A7-related disorder could suggest that pathogenicity of CHT1 variants is not limited strictly to the transport of choline but may also influence myelination with a combined functional effect.
Interestingly, the c.788C>T substitution is reported in a SLC5A7 carrier of Hispanic descent in the gnomAD database, suggesting that the C788T mutation may be a recurrent variation found in a subpopulation of individuals of this ethnic background. This finding underlines the importance of functional characterization of DNA variants for recessive traits present in databases of typical individuals.
ACKNOWLEDGMENTS
We thank the family for their invaluable participation to this study. This project was supported by the British Columbia Rare Disease Foundation (OC), Women's and Children's Health Research Institute at the University of Alberta Innovation (RES0018693; OC) and seed grant (RES0027086; OC, EC, EL, MB), and Branch Out Neurology Foundation (CJF). MB was partially supported by an Alberta Cancer Foundation Cancer Research Postdoctoral Fellowship award and Women's and Children's Health Research Institute at the University of Alberta seed grant. EML was an Alberta Innovates Health Solutions Scholar. Canadian Institutes of Health Research operating grants supported DA (MOP 142251) and MB (MOP 272075).