Homozygosity for FARSB mutation leads to Phe-tRNA synthetase-related disease of growth restriction, brain calcification, and interstitial lung disease
Funding information:
This work was supported by His Majesty Trust Funds at the Sultan Qaboos University; study code: SR/MED/GENT/16/01.
Abstract
Aminoacyl-tRNA synthetases (ARSs) canonical function is to conjugate specific amino acids to cognate tRNA that are required for the first step of protein synthesis. Genetic mutations that cause dysfunction or absence of ARSs result in various neurodevelopmental disorders. The human phenylalanine-tRNA synthetase (PheRS) is a tetrameric protein made of two subunits coded by FARSA gene and two subunits coded by FARSB gene. We describe eight affected individuals from an extended family with a multisystemic recessive disease manifest as a significant growth restriction, brain calcifications, and interstitial lung disease. Genome-wide linkage analysis and whole exome sequencing identified homozygosity for a FARSB mutation (NM_005687.4:c.853G > A:p.Glu285Lys) that co-segregate with the disease and likely cause loss-of-function. This study further implicates FARSB mutations in a multisystem, recessive, neurodevelopmental phenotype that share clinical features with the previously known aminoacyl-tRNA synthetase-related diseases.
Aminoacyl-tRNA synthetases (ARSs) are cytoplasmic and mitochondrial enzymes that are ubiquitously expressed. ARSs canonical function is to conjugate specific amino acids to cognate tRNA that are required for the first step of protein synthesis. These enzymes are also involved in cellular signaling and metabolism which are linked to the survival of the cell (Kim et al., 2003; Wakasugi & Schimmel, 1999). Therefore, dysfunction or absence of ARSs results in severe or lethal conditions in multiple species (Berg, Rogers, Muralla, & Meinke, 2005; Ni & Luo, 2018; Wang et al., 2016). Mutations in ARS genes implicated in a wide array of monogenic diseases in human with autosomal recessive and dominant inheritance (Oprescu, Griffin, Beg, & Antonellis, 2017).
Super-specificity of some of ARSs is mediated by their proofreading activity in which tRNA identity is facilitated by the productive interaction of tRNA with specific ARS (McClain, 1993). Major nucleotides in tRNA that are essential in tRNA identity lie in the acceptor end and anticodon, and variable pocket (McClain, 1993). In humans, all tRNAs are charged by 37 ARSs: 16 in the cytoplasm, 17 in mitochondria and 3 in both (Antonellis & Green, 2008). ARSs are categorized into two major classes: class I has two conserved motifs and usually monomeric or dimeric (Jakó et al., 2007; Moras, 1992). Class II ARSs including phenylalanine-tRNA synthetase (PheRS) contains three highly conserved motifs and usually are dimeric or tetrameric. The human PheRS is a tetrameric protein made of two subunits coded by FARSA gene and two subunits coded by FARSB gene (Finarov, Moor, Kessler, Klipcan, & Safro, 2010).
We describe eight members of a large consanguineous family with an autosomal recessive multisystem condition associated with growth restrictions, brain calcifications, and interstitial lung disease (ILD). Consent was obtained for this research, which was approved by the Medical Research Ethical Committee of the Sultan Qaboos University.
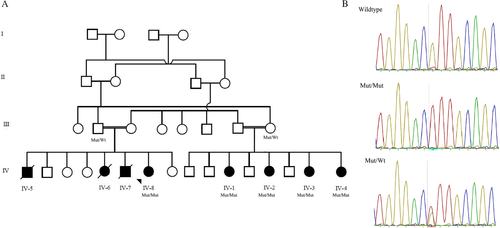
Previously, Rajab and colleagues reported the same consanguineous Omani family in 2009 with detailed clinical phenotype and linkage analysis (Rajab, Aldinger, El-Shirbini, Dobyns, & Ross, 2009). The clinical details and family pedigree updated for affected individuals (Supp. Table S1 and Figure 1A). In summary, the phenotype was consistent among all affected individuals and include no significant history of neonatal or perinatal complications. However, growth restriction was evident at birth with microcephaly, low birth weight, and short stature. Growth delay continues to be the main issue during early childhood. They all continue to have microcephaly, short stature, and reduced weight associated with thin build and reduced muscle mass. Latest growth parameters of five affected individuals assessed recently shown in Supp. Table 1.
Early childhood developmental delay and learning difficulties at the school were prominent, however, were not consistent among them. Patient IV-6 was the most severely affected where she was bedridden and suffered from severe spasticity. This is likely due to the sequel of post-meningitis and downward brain herniation during infancy; she died at the age of 20 years due to pneumonia and respiratory failure. Patient IV-4 was diagnosed with hydrocephalus at the age of 6 months, and she had had a ventriculoperitoneal (VP) shunt inserted at 8 months of age. At the age of 20 months, she developed seizures, which was controlled on antiepileptic drugs. She was seizure free then, and medications stopped at the age of 3 years. Despite poor school performance, all recently assessed (IV-1, IV-2, IV-3, IV-4, IV-8) patients completed the school with support and three of the family members (IV-8, IV-7, IV-3) admitted to university-level education. As adults, they were all able to perform activities of daily living independently and had normal social interactions.
Widespread brain calcifications were evident among all affected individuals; earliest report was at the age of 6 months on IV-4 detected by increased echogenicity at the basal ganglia by transcranial ultrasound. Seventeen years of age was the earliest age when brain computed tomography (CT) was performed on this cohort of patients, while the age of 28 years was the latest age. Brain CT showed similar features among all. It showed symmetrical extensive brain involvement with calcifications of bilateral subcortical white matter (U-fibers) and deep gray matter including cerebellar dentate nuclei and the basal ganglia; caudate, putamen, and thalamus. No significant brain atrophy was seen, and ventricular size appeared normal (Figure 2A and B). MRI brain on two affected individuals showed normal white matter and signal intensity. IV-1 started to develop extrapyramidal signs at the age of 27 years. Her symptoms include symmetrical choreoathetoid movements involving the upper limbs and head. The symptoms are slowly progressive and not associated with cerebellar signs, seizures, forgetfulness, or behavioral changes.
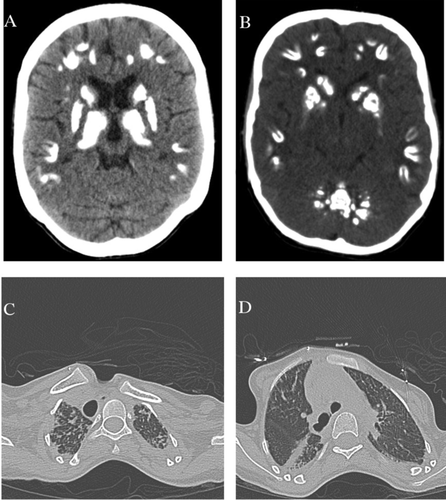
Respiratory failure and interstitial lung disease was a constant finding among affected individuals, with onset during the second decade of life and is the cause of death in IV-5, IV-6, and IV-7. Additionally, IV-8 and IV-4 started to show signs of respiratory failure with a chronic cough. The pulmonary phenotype manifests eventually as type 2 (hypercapnic) respiratory failure requiring home non-invasive ventilatory support and long-term oxygen supplement. Patients with milder pulmonary involvement had mild exertional dyspnea and occasional cough but developed episodes of acute hypercapnic respiratory failure during respiratory tract infections. High-resolution computed tomography showed bilateral pleuroparanchymal fibrotic changes involving both upper lobes and to a lesser degree, the superior segment of the lower lobes (Figure 2C and D). Pulmonary function test showed severe restriction with raised residual volume but no evidence of airflow limitation.
Otherwise, patients examined recently (IV-1, IV-2, IV-3, IV-4, IV-8) have had no dysmorphic features or skin changes. The neurological examination showed thin build and reduced muscle mass, otherwise normal power, tone, and deep tendon reflexes. There was no evidence of spasticity or cerebellar dysfunction, IV-1 showed extrapyramidal signs as described above. Repeated funduscopic examination at the age of 26 year-old for IV-8 was reported as normal consistent with the previously published eye exams for the other affected individuals, which showed normal retinas with no retinal exudates and normal retinal vessels. Echocardiograms were performed at least on two affected individuals and revealed normal cardiac anatomy and good systolic function. Abdominal ultrasound showed normal liver, spleen, and kidneys. Routine laboratory tests obtained in most of the affected individuals were normal. These included blood and platelet counts, serum electrolytes, alkaline phosphatase, corrected-calcium, magnesium, parathormone, thyroid function tests (TSH, T4), renal function tests (blood urea nitrogen, creatinine), and glucose. Recently repeated liver function tests on two affected individuals revealed hypoalbuminemia 29 (35–52 g/L) otherwise normal liver enzymes and coagulation profile. Complete blood count (CBC) showed hypochromic microcytic anemia with iron deficiency anemia and features of alpha-thalassemia trait in one affected individual (IV-8).
Genome-wide linkage analysis was completed as described previously (Rajab et al., 2009). It included four normal parents, four healthy siblings and seven affected individuals. Fine-mapping using polymorphic microsatellite markers between rs2373041 and rs10498202 on 2q35–q36.3 was performed, and confirmed a significant LOD score of 6.17 at D2S351 and D2S2390 on 2q36.2. To further explore the genetic cause of this phenotype in this family, we performed whole-exome sequencing (WES) analysis on DNA isolated from blood from the proband (IV-8). In brief, genomic DNA of the patient was isolated from peripheral blood using a DNeasy Blood and Tissue Kit (Qiagen, Courtaboeuf, France). DNA was barcoded and enriched for the coding exons of targeted genes using hybrid capture technology (Agilent SureSelect-V6-60 MB). Prepared DNA libraries were then sequenced using a Next Generation Sequencing (NGS) technology (Hiseq4000, 150 bp paired-end, at 200× coverage). Raw data was analyzed at Sultan Qaboos University, Department of Genetics using in-house annotation and analysis pipelines. The average throughput depth of target regions was 284 while the mean depth on target was 176. The percentage of coverage of target regions was 99% and 97.7% at 10× and 20×, respectively. Variant filtration was conducted to only keep novel or rare variants (≤1%). Publically available variant databases (1000 Genomes, Exome Variant Server, and gnomAD) were used to determine the frequency. Also, an in-house database of 284 exomes was used to filter out common variants specific to our population. Only coding/splicing variants considered. No mutations in known disease genes explaining the phenotype were identified. The following criteria were then used to prioritize variants; high impact or highly damaging missense, a CADD score ≥ 20 and a variant within autozygosity area. Thereafter two damaging rare homozygous mutations were prioritized (FARSB and ALDH16A1), of which one segregated completely with the phenotype.
The analysis revealed homozygosity for a missense mutation (NM_005687.4:c.853G > A p.Glu285Lys; rs767956337) that was confirmed by Sanger sequencing and co-segregate with the phenotype (Figure 1B). This variant is located within the linkage area 2q36.2 identified previously. It is absent from the gnomAD database (Lek et al., 2016) and predicted to be damaging/pathogenic by multiple in-silico prediction tools include DANN, GERP, LRT, MutationAssessor, MutationTaster, SIFT, and PROVEAN. This site is highly conserved among 100 vertebrate genomes (including human).
To address if this mutation causes unstable protein and downstream degradation, we performed western blot analysis on whole cell lysate proteins isolated from lymphocytes obtained from the peripheral blood of subjects IV-8, IV-1, IV-3, IV-4, and one control (i.e., from an individual without FARSB variants). Briefly, protein samples (60 μg per sample) were subjected to electrophoresis, transferred onto a PVDF membrane, and incubated with an anti-FARSB antibody (Invitrogen; PA5-30350) or an anti-GAPDH antibody (Invitrogen; PA1-16777) to control for protein loading. Our results revealed no significant reduction of FARSB protein levels in the tested lymphocytes cells (Supp. Figure S1). Primary fibroblasts from patients were not accessible to test FARSB protein levels.
We further did 3D molecular modeling using SWISS-MODEL and structural alignment using PyMOL; resolving the structure of human cytoplasmic phenylalanyl-tRNA synthetase revealed that glutamate 285 resides in the B strand between B3/4 and B5 domains (Supp. Figure S2). The p.Glu285Lys results in loss of the B strand in the region between B3/4 and B5. The B3/4 domain is involved in editing activity of the human enzyme (Finarov et al., 2010; Sasaki et al., 2006).
Initially, biallelic mutations of FARSB were reported on one patient. Antonellis et al. (2018) reported on a male proband born to a healthy nonconsanguineous couple. Growth restriction was evident at birth. Later, he developed developmental delay, and signs of liver cirrhosis, and interstitial lung disease. Extensive investigations were completed and compound heterozygous for FARSB variants: p.Thr256Met and p.His496Lysfs*14 were identified. Expression studies using fibroblasts isolated from the proband revealed a severe depletion of both FARSB and FARSA protein levels.
Recently, Xu et al. (2018) reported on five affected individuals from four families. The phenotype consists of multi-organ manifestations of interstitial lung disease with cholesterol pneumonitis, diffuse cerebral calcifications, hypotonia, and liver cirrhosis caused by biallelic mutations in FARSB. Other features include intracranial aneurysms, renal disease, and intestinal malrotation. The eldest living affected individual was 18 year-old, while three died between the age of 8 and 10 years. Both FARSB and FARSA protein levels were diminished on primary fibroblast from one affected individual. Although a slightly reduced aminoacylation rates were evident, but protein synthesis rate was normal.
A detailed comparison of the phenotype of our cohort of patients, the recently described patients and other ARS-associated recessive diseases (Meyer-Schuman & Antonellis, 2017), revealed a significant overlap. Our cohort of patients had a milder course of the disease but eventually manifested the common features seen in this group of conditions. The growth restriction was the major concern during early childhood in our cohort. This was reported as a significant sign of these disorders (Antonellis et al., 2018). The interstitial lung disease was the most troublesome in our patients and the cause of death in three affected individuals. CT scan chest indicated a condition that usually starts at the upper lobes. Hypoalbuminemia, was evident during the first decade of life, though yet none of our current patients developed signs of liver cirrhosis neither elevated liver enzymes. Extensive brain calcifications were evident from infancy. Cerebral and basal ganglia calcification is a cardinal sign of this syndrome, and it should be considered in the differential diagnosis of brain calcifications.
Aminoacyl-tRNA synthetases play a crucial role in the cellular translation process and ensure faithful translation of the genetic information into functional proteins. Translation fidelity is an essential quality step during translation, errors in aminoacylation can occur when synthetases encounter noncognate amino acids or analogs that are structurally similar to their cognate substrates. Several studies have suggested that increased translational error rates can slow growth in bacteria and cause neurodegeneration in mammals (Ling, Yadavalli, & Ibba, 2007). The heterotetrameric phenylalanyl-tRNA synthetase possess an editing domain, located at the B3/B4 domain of β subunit. While Phe is the cognate amino acid for PheRS, it can mischarge tRNAPhe with isosteric tyrosine (Tyr) causing more than usual tyrosyl-tRNAPhe and incorporation of tyrosine instead of phenylalanine during translation (Finarov et al., 2010). However, the exact mechanisms by which FARSB mutations lead to the described phenotype remain to be clarified and whether it is related to the non-canonical functions of FARSB.
In summary, we further describe eight affected individuals from a large extended family with a multisystemic recessive aminoacyl-tRNA synthetase-related disease. This report further expands Phe-tRNA synthetase-related phenotype and confirms FARSB mutations association with a multisystem, recessive, neurodevelopmental phenotype.
CONFLICTS OF INTEREST
The author declares no conflict of interest.