De novo mutations in FLNC leading to early-onset restrictive cardiomyopathy and congenital myopathy
Contract grant sponsors: Government of Russian Federation (074-U01); ALF Funding (20140240).
Communicated by Nancy B. Spinner
Abstract
Mutations in FLNC for a long time are known in connection to neuromuscular disorders and only recently were described in association with various cardiomyopathies. Here, we report a new clinical phenotype of filaminopathy in four unrelated patients with early-onset restrictive cardiomyopathy (RCM) in combination with congenital myopathy due to FLNC mutations (NM_001458.4:c.3557C>T, p.A1186V, rs1114167361 in three probands and c.[3547G>C; 3548C>T], p.A1183L, rs1131692185 in one proband). In all cases, concurrent myopathy was confirmed by neurological examination, electromyography, and morphological studies. Three of the patients also presented with arthrogryposis. The pathogenicity of the described missense variants was verified by cellular and morphological studies and by in vivo modeling in zebrafish. Combination of in silico and experimental approaches revealed that FLNC missense variants localized in Ig-loop segments often lead to development of RCM. The described FLNC mutations associated with early-onset RCMP extend cardiac spectrum of filaminopathies and facilitate the differential diagnosis of restrictive cardiac phenotype associated with neuromuscular involvement in children.
1 INTRODUCTION
Restrictive cardiomyopathy (RCM) is one of the most severe inherited cardiac disorders with very limited number of treatment possibilities and poor prognosis (Muchtar, Blauwet, & Gertz, 2017; Richardson et al., 1996). In most RCM cases, the hemodynamic properties of the restrictive cardiac physiology make it impossible to use cardiac assist devices, and heart transplantation is the only remaining treatment option (Lee et al., 2017; Patel et al., 2017). Furthermore, a large subgroup of RCM cases manifesting in childhood have an extremely poor prognosis due to underlying metabolic defects (Lee et al., 2017; Papadopoulou-Legbelou, Gogou, & Evangeliou, 2017; Russo & Webber, 2005). Recent advances in genetic sequencing technology have facilitated the identification of mutations causing early-onset RCM and highlighted the involvement of genes encoding contractile and structural proteins (Gallego-Delgado et al., 2016; Kaski et al., 2008; Kostareva et al., 2016).
Notably, RCM myocardial dysfunction, as well as other types of cardiomyopathies, can manifest concomitant with skeletal muscle system pathology, highlighting the involvement of genes expressed both in cardiac and skeletal muscle (Janin et al., 2017; Konersman et al., 2015; Kostareva, Sejersen, & Sjoberg, 2013). One such gene recently associated with several types of cardiomyopathies is FLNC (MIM# 102565) (Begay et al., 2016; Brodehl et al., 2016; Gomez et al., 2017; Ortiz-Genga et al., 2016; Reinstein et al., 2016; Valdes-Mas et al., 2014). FLNC encodes filamin C, an actin cross-linking protein abundant both in cardiomyocytes and skeletal muscle cells. Apart from a structural role in muscle cell integrity and Z-line organization, filamin C also has an important role in protein degradation system and autophagy control (Kley et al., 2012; Leber et al., 2016; Ruparelia, Oorschot, Ramm, & Bryson-Richardson, 2016). In the past decade, FLNC mutations have been described in connection to several different inherited neuromuscular disorders such as myofibrillar myopathy and distal myopathy, and recently also as a frequent cause of cardiomyopathy (Begay et al., 2016; Brodehl et al., 2016; Gomez et al., 2017; Ortiz-Genga et al., 2016; Reinstein et al., 2016; Valdes-Mas et al., 2014). However, even though FLNC mutations can be a frequent cause of hypertrophic (HCM), dilated (DCM), and arrhythmogenic cardiomyopathy (ACM), little is still known about the association of FLNC with RCM (Brodehl et al., 2016; Tucker et al., 2017). Importantly, among all the reported FLNC-caused cardiomyopathies there are only a few cases with a combined cardiac and muscle phenotype, none of which included arthrogryposis (Gomez et al., 2017; Ortiz-Genga et al., 2016).
Here, we present four unrelated cases showing early-onset RCM in combination with congenital myopathy due to similar FLNC mutations. We further confirm pathogenicity of the described missense variants using cellular and morphological studies as well as an in vivo modeling in zebrafish. Combined experimental and in silico approaches reveal that FLNC missense variants localized in Ig-loop segments lead to development of RCM and HCM independently of intracellular protein aggregate formation.
2 MATERIALS AND METHODS
2.1 Patient cohort
The study cohort included 24 previously published RCM cases (Kostareva et al., 2016) and an additional RCM case not previously reported elsewhere. The study was performed according to the Declaration of Helsinki, and approval was obtained from Karolinska Institute Ethical Review Board and Almazov National Medical Research Centre Ethical Committee. Written informed consent was obtained from all subject representatives prior to investigation. The diagnosis of RCM was based on the WHO/International Society and Federation of Cardiology Task Force clinical criteria and classified according to the European Society of Cardiology classification of cardiomyopathies (Richardson et al., 1996). In pediatric patients the diagnosis was based on the following echocardiography features: atrial dilatation in combination with normal or nearly normal left ventricular size and preserved or nearly preserved systolic function (left ventricular end diastolic dimension z-score ≤3, left ventricular wall thickness z-score ≤3 and fractional shortening ≥0.25).
2.2 Genetic investigation
Sequencing of full exome libraries was performed using SureSelect Human All Exon V6 r2 (60Mbp) target enrichment kit (Agilent Technologies, Santa Clara, CA, USA) with Illumina HiSeq instrument and SBSv4 chemistry (Illumina, San Diego, USA). Alignment (BWA-MEM-0.7.1), data processing (Picard 2.9), and variant calling (GATK-3.5.0) were performed according to GATK BestPractice recommendation (Broad institute), using hg19 human genome reference, within script described earlier (Kostareva et al., 2016). Variant annotation was performed using Annovar (Wang, Li, & Hakonarson, 2010). Average target region coverage was ∼×150, 95% of all target regions were covered at least ×20. Next, identified variants with a maximum frequency of 0.01% in several normal population variant databases (1000G, ESP, ExAc0.2, and gnomAD) and deep intronic variants were filtered out. Finally, variants in genes with a known expression in cardiac and skeletal muscle (based on GTEx and GSE71613 datasets) were considered clinically relevant and confirmed by Sanger sequencing. The paternity was proved using STR analysis (Loci D2S1360, D7S1517, D8S1132, D12S1064, SE-33) according to previously published protocol (Lion et al., 2012). Newly described FLNC variants (GenBank entry NM_001458.4) were submitted to ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/, Variation ID 427928, 430733).
2.3 Bioinformatics approach to predict damaging effect of the missense mutation and variant classification
Pathogenicity of A1186V was assessed based on the MetaSVM predictions obtained from the dbNSFP database version 3.4 (Dong et al. 2015; Liu et al. 2013) according to algorithm previously described (Tarnovskaya, Kiselev, Kostareva, & Frishman, 2017). Substitution A1183L is produced by a multi nucleotide variant (i.e., clustered mutations of two neighboring nucleotides) and it could not be found in dbNSFP database as single-nucleotide variant. Therefore, to predict deleteriousness of A1183L substitution we used all listed above methods separately. Domain organization of a filamin-C protein sequence was performed using Pfam database (Finn et al., 2014). Each domain in the protein was numbered according to its order of appearance in the protein structure.
Related protein sequences from other organisms for 10th Ig-like domain of filamin-C were identified by BLASTP (Altschul, Gish, Miller, Myers, & Lipman, 1990) searches against the Uniref90 database using the E-value threshold of 0.001. Multiple sequence alignment for Ig-like domain was calculated by Clustal Omega (Sievers et al., 2011) and visualized in Jalview 2.8.2 (Waterhouse, Procter, Martin, Clamp, & Barton, 2009). For each column in the alignment Jalview calculates a conservation score which varies between 0 (no conservation) and 10 (high conservation). All identified genetic variants were classified according to American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015).
2.4 Structure analysis of proteins: homology modeling and structure comparison
Solution structure of the Ig-like domain of human filamin C (PDB code: ID 2D7M) was extracted from the Protein Data Bank (Berman et al., 2000) based on BLASTP hits with an E-value below 0.001 and an alignment coverage of the query sequence of at least 80% (Camacho et al., 2009). Disease-related mutations of various filamin domains were systematically mapped onto this structural model. In silico visualization was conducted using the PyMol software.
2.5 Plasmid generation
Wild-type (FLNCWT) and mutant (FLNCp.A1183L and FLNCp.A1186V) coding sequences of short FLNC isoform (NM_001127487.1) tagged C-terminally with EGFP (enhanced green fluorescent protein) were synthesized in vitro and verified by Sanger sequencing (GeneScript, Piscataway, NJ, USA). Described genetic variants are localized at similar positions in short (NM_001127487.1) and full (NM_001127487.4) isoforms, therefore sharing the mutation nomenclature. Constructs were inserted into the pCS2+ plasmid for in vitro transcription of capped mRNA using the SP6 mMessage mMachine transcription kit (Ambion, Foster City, CA, USA). Transcribed RNA was run in an agarose gel for quality check (Supp. Figure S1) and concentration was determined using a Nanodrop, then stored at −80°C.
2.6 Zebrafish maintenance and overexpression studies
Adult zebrafish were maintained on a 14 hr light/ 10 hr dark cycle at the Karolinska Institutet zebrafish core facility. Embryos were produced via light-induced spawning and raised at 28°C. All procedures were performed in accordance with standard operating procedures approved by the Stockholm Ethical Board for Animal Experiments (permit number 13063-2017).
Overexpression of FLNCwt/p.A1183L/p.A1186V-EGFP in zebrafish was achieved by injecting the synthesized mRNAs at 2,000 ng/μl with phenol red at 0.1 μg/μl (concentration in injection solution) into 1-cell stage zebrafish embryos. Embryos expressing EGFP at similar levels were positively selected at 24 hr post fertilization and maintained at 28°C until used.
2.7 Histology and confocal imaging
Tissue samples from the heart and skeletal muscle, collected during the autopsy (patient 25) were mounted in Tissue-Tek and immediately frozen in liquid nitrogen. Cryosections of 10 nm tick were air-dried, blocked with 15% fetal calf serum, and incubated overnight at 4°C with a primary anti-filamin C polyclonal antibody (NBP1 89300, NovusBio, Littleton, CO, USA) diluted 1:200 or anti-desmin monoclonal antibody (D33, Dako, Santa Clara, CA, USA) diluted 1:200. After being washed in phosphate-buffered saline (PBS), sections were incubated with an anti-rabbit Alexa Fluor 488-conjugated secondary antibody (Life Technologies, Waltham, MA, USA, Sigma–Aldrich, St. Louis, MO, USA), diluted 1:1,000 for 1 hr at room temperature. Sections were examined using Zeiss Axio Observer Z1 microscope with a 40× magnification and 1.6× tubelens (64× total). Cell studies were performed using C2C12 cell line (European Collection of Authenticated Cell Cultures, cat. number 91031101) according to standard culturing conditions.
Zebrafish embryos at the desired developmental stage were collected and fixed with 4% paraformaldehyde overnight, washed with 1x PBS supplemented with 0.1% Tween20 (PBST) and permeabilized with acetone for 45 min at −80°C. Embryos were then washed in water and PBST and blocked with a solution of 2% goat serum, 1% DMSO, 1% BSA, and 0.1% TritonX-100. Antibodies used were rabbit anti-GFP (ab290; AbCam, Cambridge, MA, USA), mouse anti-myosin (A4.1025, D.S.H.B., Iowa city, IA, USA), and anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 594-conjugated antibodies (Life Technologies, Waltham, MA, USA). Nuclear staining was achieved by incubating embryos with DAPI (4,6-diamidino-2-phenylindole) at a final concentration of 10 μg/ml. Embryos were mounted in Gelvatol and imaged using a Zeiss LSM 700 confocal microscope (minimum nine embryos per group). Confocal settings were set to acquire pixels of every grey intensity level (0–255 in an 8-bit scale). Z-step size at acquisition was approximately 1/3 the thickness of the optical section.
2.8 Statistics
Statistical analysis was performed using GraphPad Prism software to illustrate data in Supporting Materials. For determination of differences between groups t-test was used. P value < 0.01 was considered significant. Plots represent means ± standard deviations.
3 RESULTS
3.1 Detection of FLNC mutations and patient characteristics
Earlier, we reported the results of genetic screening in a group of 24 RCM patients using a target panel of 108 cardiomyopathy-associated genes (Kostareva et al., 2016). To further explore the genetic cause in genotype-negative patients, we applied whole exome sequencing approach in those four patients who had RCM and clinical signs of neuromuscular disorder and for whom an exact genetic cause was not established using target sequencing approach. Three of these patients also presented with arthrogryposis. Removal of variants with allele frequency > 0.01%, deep intronic variants and variants predicted to be benign and tolerated by all methods of in silico analysis resulted in a list of non-synonymous and potentially affecting protein function variants (missense, frameshifts, nonsense, and splicing) which were further analyzed (Supp. Table S1 and Supp. Figure S2). Analysis of cardiac and muscle specific expression in combination with in silico analysis using MetaSVM prediction tool resulted in identification of two damaging variants in FLNC in the four patients. The first variant (NM_001458.4:c. 3557C>T, p.A1186V) was identified in patients 16, 22, and 25 and the second variant (NM_001458.4:c.[3547G>C; 3548C>T], p.A1183L) was identified in patient 15 (Figure 1). Both identified variants were absent in ExAc0.2 and gnomAD data bases demonstrating that they do not represent rare polymorphisms (https://exac.broadinstitute.org, https://gnomad.broadinstitute.org).
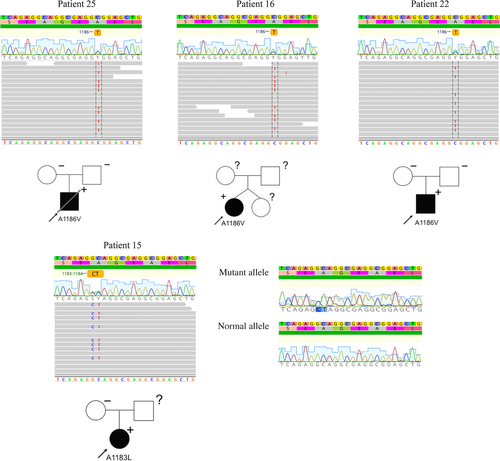
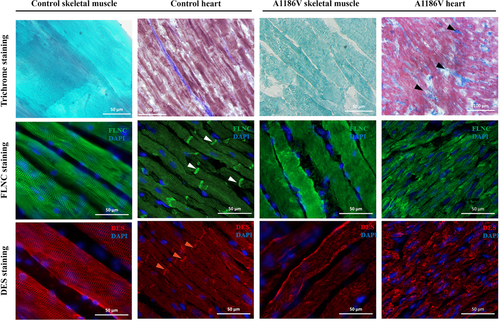
Patient 25, carrying a FLNC p.A1186V mutation (rs1114167361:C>T), was delivered by caesarean section at term, due to breech presentation, following a normal uncomplicated pregnancy (Table 1). At birth, he presented with arthrogryposis and hip dystopia followed by muscle hypotonia and muscle weakness during the first year of life. He exhibited slightly late motor development, kyphoscoliosis, hyperopia, and achieved independent sitting ability only after 12 months. During the second year of life he presented with the signs of heart failure. Echocardiography showed signs of RCM with markedly enlarged atria and no significant myocardial hypertrophy (Table 2). Biochemical work-up revealed CK, CK-MB, and LDH elevation in blood along with marked proBNP increase. Brain MRI revealed no focal abnormalities but signs of immature white matter. At 2.5 years of age, he still could not rise independently from lying to sitting position, showed axial weakness and could walk only with help on bent legs. He exhibited multiple joint contractions (elbow, knee, and ankle), rigid muscles, and umbilical hernia. Neurological examination revealed increased reflex response, upper limb myoclonus, bradykinesia, signs of pyramid insufficiency, clonic seizures, and focal epileptiform activity on EEG. At 2.5 years of age, he experienced a respiratory infection and died from an acute episode of heart failure. Morphological examination of myocardium revealed moderate fibrosis and hypertrophy (Figure 2). Immunostaining using anti-filamin C antibody did not reveal any obvious intracellular inclusions, but reduced filamin C staining in the areas of intercalated discs. Correspondingly, no clear desmin staining was detected, neither in the area of Z-lines nor in the intercalated discs. To exclude the possibility that negative intercalated disc staining resulted from a poor epitope availability in autopsy samples, rather than from filamin C derangements, we performed anti-cardiac actin immunostaining, which confirmed intact preservation of sarcomeric structures in myocardial samples (Supp. Figure S3). Morphological examination of skeletal muscle demonstrated variation in fiber size, fiber splitting, increased frequency of central nuclei and disrupted myofibrillar organization with no signs of degeneration or fat infiltration. No gross filamin C aggregated were seen, however weaker and less regular desmin staining was observed compared with control skeletal muscle. No family history of cardiomyopathy, myopathy or sudden cardiac death was reported. Neither of parents carry p.A1186V FLNC mutation, thus confirming its de novo nature.
| Sex | Mutation | Mutation status | Age of RCM presentation | Age of NM presentation | Signs of NM involvement | CK and serum markers | EMG | ECG | Outcome | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 25 | M | p.A1186V | De novo | 1.4 year | At birth |
|
|
|
|
Death at 2. years of age | ASD cox dystopia umbilical hernia kyphoscoliosis hyperopia |
| Patient 15 | F | p.A1183L | n/a | 6 months | At birth |
|
|
Diffuse myopathic pattern |
|
Listed for `Hx at 3 y.o. | ASD cox dystopia white line hernia microsomia |
| Patient 16 | F | p.A1186V | n/a | 3 years | During first year |
|
|
Diffuse myopathic pattern |
|
Alive, 7 y.o. | ASD cox dystopia white line hernia microsomia |
| Patient 22 | F | p.A1186V | De novo | 15 years of age | At birth |
|
|
Normal |
|
Hx at 19 y.o |
|
- CK, creatine kinase; CK-MB, creatine kinase myocardial fraction; LDH, lactate dehydrogenase; RBBB, right bundle branch block; SVT, supraventricular tachycardia; VT, ventricular tachycardia; ASD, atrial septal defect; AVB-I, atrioventricular block degree I; RAE, right atrial ectopies; LAE, left atrial ectopies; Hx, heart transplantation.
| LVEDD (mm) | LVESD (mm) | EF (%) | LVPWd (mm) | IVSd (mm) | LA (mm) | PAP (mmHg) | |
|---|---|---|---|---|---|---|---|
| Patient 25 | 33.1 (z-score −1.40) | 21.9 (z-score −1.64) | 72 | 5.4 (z-score 1.64) | 5.3 (z-score 0.68) | 37.1 (z-score 5.62) | 26 |
| Patient 15 | 25.0 (z-score −0.98) | 14.0 (z-score −1.56) | 63 | 8.0 (z-score 3.86) | 12.0 (z-score 4.60) | 34.7 (z-score 5.38) | 50 |
| Patient 16 | 27.1 (z-score −1.20) | 15.2 (z-score −1.72) | 77 | 6.0 (z-score 1.95) | 6.6 (z-score 1.54) | 35.0 (z-score 4.89) | 45 |
| Patient 22 | 47 (z-score 0.05) | 29 (z-score −0.25) | 65 | 6.0 (z-score −1.40) | 12 (z-score 3.33) | 42.0 (z-score 3.52) | 22 |
- IVSd, interventricular septum, diastolic dimension; LVPWd, left ventricular posterior wall thickness, diastolic dimension; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LA, left atrium dimension; EF, ejection fraction; PAP, pulmonary artery pressure (mean).
Patient 15, carrying a FLNC p.A1183L mutation (rs11316921851:GC>CT), was born at term following a complicated pregnancy with preeclampsia and signs of intrauterine growth retardation (Table 1). At birth, she presented with arthrogryposis and hip luxation, muscle weakness abdominal hernia. At the age of 6 months, she developed signs of heart failure and was diagnosed with RCM by echocardiography. During the next several years, RCM transformed to hypertrophic with prominent restrictive phenotype and preserved ejection fraction (Table 2). Skeletal muscle weakness and moderate joint contractions remained stable and did not progress. She achieved independent walking ability at 2 years of age, while at 7 years old she could still not sit independently without assistance or rise from squatting position and demonstrated axial weakness. Biochemical tests showed constant LDH and CK-MB elevation while total CK never exceeded upper limit. Neurological examination and brain MRI showed no signs of central nervous system involvement. A myopathic process was confirmed by electromyography. The family did not report any history of cardiac or muscle disorders. Genetic investigation revealed no substitution in codon 1183 in maternal DNA, while paternal DNA was not available for analysis.
Patient 16, carrying a FLNC p.A1186V mutation (rs1114167361:C>T), was born at term from a dizygotic twin pregnancy, and presented with muscle weakness and delayed motor development during the first year of life (Table 1). She started to walk independently at 2 years of age, and at 7 years was not able to rise from squatting position or to sit independently. She presented with signs of heart failure at 3 years of age when a first echocardiography examination was performed and revealed RCM with preserved cardiac contractility and no signs of hypertrophy (Table 2). Biochemical tests showed constant LDH and CK-MB elevation with mild intermittent total CK increase. A diffuse myopathic process was confirmed by electromyography, and no signs of central or peripheral nervous system involvement were observed. The family refused further genetic testing and no information is available regarding de novo mutation status or sibling clinical status and genotype.
Patient 22, carrying a FLNC p.A1186V mutation (rs1114167361:C>T), was born after a 42-week normal pregnancy by caesarean section due to insufficient rotation in the birth canal. He presented at birth with arthrogryposis with knee, hip, foot, and hand contractures that were normalized by 2 years of age (Table 1). At 4 years of age, he again showed problems of muscle stiffness, and restricted movements in shoulders, hips, and feet. Thus, he was diagnosed as having mild arthrogryposis multiplex congenita type I. Normal CK and moderately increased CK-MB was noted and electromyography was normal. Physiotherapy had no effect on the developed joint contractures. Over time scoliosis developed, and spinal surgery was performed at 13 years of age. Morphological examination of muscle tissue revealed disrupted myofibril organization and fiber splitting often in combination with necrotic fibers. Fibers contained rimmed vacuoles and central nuclei as well as elongated irregular structures, stained in red by hematoxylin–eosin with central lighter staining zone positive for Gomori trichrome. Immunostaining of the latter inclusions confirmed positive desmin staining on their periphery. At 15 years of age, an inappropriate pulse rise on a 6-min walk test lead to cardiac investigation and biventricular restrictive physiology, normal ejection fraction, and dilated atria were demonstrated (Table 2). Over time, heart failure progressed to NYHA class III, and he had episodes of atrial flutter and fibrillation requiring electroconversion. Due to pulmonary hypertension, levosimendan treatment was initiated and carried out for 6 months, and subsequently a heart transplantation was performed successfully at 19 years of age. Genetic investigation confirmed that none of the parents carried the A1186V FLNC mutation.
3.2 Structural and bioinformatic analysis of mutations
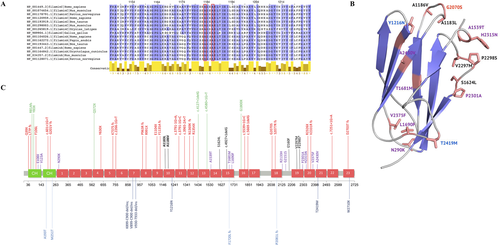
In silico analyses revealed that both 1,183 and 1,186 amino acids of filamin C are highly conserved throughout Ig domains (conservation score = 11, Figure 3A). Both of them are located in a loop likely involved in interdomain interactions with neighbor Ig-like domains in the segment of super-repeats of filamin C (Tarnovskaya et al., 2017). According to the MetaSVM prediction model, A1183L and A1186V substitutions appear to be highly pathogenic. However, transfection of C2C12 cells with either A1183L or A1186V mutations did not lead to alteration of intracellular filamin C network or protein aggregate formation (Supp. Figure S4).
3.3 Morphological and functional analysis of FLNC mutations in zebrafish
To uncover the impact of FLNC mutations identified in RCM patients, we used the zebrafish model. EGFP–tagged wild-type and mutant FLNC coding sequences were synthesized (GeneScript) and cloned into pCS2+ plasmid for mRNA in vitro synthesis and overexpression in zebrafish embryos. Injection of wild-type and mutant FLNC–EGFP in zebrafish embryos did not result in severe muscle disruption, as seen by myosin immunolabeling (Figure 4A–C). However, skeletal muscle overexpressing FLNCp.A1183L–EGFP displayed the presence of small EGFP-positive aggregates especially in the perinuclear region (Figure 4E, box). Aggregates were also found in myofibrils overexpressing FLNCp.A1186V–EGFP (Figure 4F), which were not detected in FLNCwt–EGFP mRNA-injected embryo myofibrils, including the perinuclear region (Figure 4D, box). Additionally, some myofibers of FLNCp.A1186V–EGFP-injected embryos were found to have a complete disruption of the Z-discs and therefore loss of the striated pattern (Figure 4F, arrow).
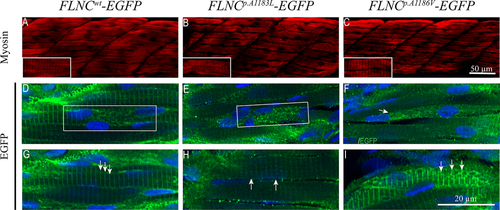
A more detailed observation of zebrafish embryos revealed Z-disc streaming along the myofibrils of FLNCp.A1183L–EGFP embryos (Figure 4H, arrows) or abnormal Z-disc patterning on FLNCp.A1186V–EGFP myofibrils (4I, arrows), while Z-discs of control myofibrils were aligned and with similar density throughout the fibril (4G, arrows). These results suggest that the overexpression of FLNCp.A1183L and FLNCp.A1186V are capable of disrupting the normal organization of FLNC in the myofibrils.
4 DISCUSSION
Technical achievements and dramatic progress associated with introducing next-generation sequencing technology into clinical practice has brought up on the stage genes that were previously underestimated as disease causing genes. One such gene is FLNC, known for a long time exclusively in association with distal and myofibrillar myopathies (Kley et al., 2012), and only more recently described in connection to cardiac phenotypes (Kley et al., 2012; Valdes-Mas et al., 2014; Vorgerd et al., 2005). After the first description of FLNC mutation in a familial case of HCM in 2014, the number of reports documenting its role in the development of cardiac disorders has rapidly grown, making FLNC one of the most common genes associated with cardiomyopathies causing about 10% of HCM and up to 5% of DCM (Begay et al., 2016; Brodehl et al., 2016; Dal Ferro et al., 2017; Gomez et al., 2017; Janin et al., 2017; Ortiz-Genga et al., 2016; Reinstein et al., 2016; Tucker et al., 2017; Valdes-Mas et al., 2014). Similar to titin, FLNC is linked to all types of cardiomyopathies, including arrhythmogenic cardiomyopathy. In the majority of FLNC–linked cases, cardiomyopathy is the predominant phenotype with subtle signs of muscular system involvement, more common among patients with HCM than DCM, and often accompanied by mild CK elevation (Gomez et al., 2017; Ortiz-Genga et al., 2016). Of note, the only patient with a pathogenic variant in FLNC and muscle system involvement reported in a series of cardiomyopathies last year, was a patient with RCM (Ortiz-Genga et al., 2016). Typically, when HCM and DCM are caused by dominant FLNC mutations, the disease presentation occurs during the third or fourth decade of life, in line with findings of a high proportion of familial cases (Gomez et al., 2017; Janin et al., 2017; Ortiz-Genga et al., 2016). For most of these cases sudden cardiac death and ventricular arrhythmias represent the most serious disease complications (Ortiz-Genga et al., 2016). Additionally, for FLNC-truncating variants a severe heart failure, an involvement of the right ventricle and transformation toward arrhythmogenic cardiomyopathy with high risk of ventricular arrhythmias and sudden cardiac death are also very typical clinical presentations. In this report, we describe a new clinical phenotype for a filaminopathy caused by FLNC mutations. All four cases have quite unique clinical phenotypes with a combination of severe early-onset RCM and myopathy, and in three out of four also arthrogryposis. In three out of four patients, the presentation of cardiomyopathy occurred within first 3 year of life and the median age of presentation was 2.2 years. In previously published families of FLNC-associated RCM, the mean age of presentation was 20.5±11.4 (range 2–40), including four patients presenting in childhood (Brodehl et al., 2016; Gomez et al., 2017; Ortiz-Genga et al., 2016). Altogether, this emphasizes an earlier presentation of FLNC-caused RCM phenotype compared with DCM and HCM (Supp. Figure S4). Importantly, two HCM patients, reported by Gomez et al. (2017), were subject to heart transplantation due to severe diastolic heart failure and restrictive filling patterns, further underlining the high association rate of point FLNC mutations with a restrictive cardiac phenotype.
In all four described cases, signs of myopathy were evident early in life and corresponded to a clinical diagnosis of congenital myopathy with proximal weakness. The morphological studies of skeletal muscles performed in two patients (patient 25 and patient 22) did not reveal typical signs of muscle dystrophy consistent with the mild CK elevation observed. The most typical presentation (three out of four) was arthrogryposis at birth with subsequent improvement over time. The prominent involvement of central nervous system in one of the patients is rather unusual for filaminopathies, nevertheless could take place in the light of a previously published case of FLNC-associated myopathy with cerebellar ataxia and a role of filamin C in development of frontotemporal dementia (Janssens et al., 2015; Tasca et al., 2012; Xie, Xu, Davie, & Chung, 1998). Altogether, our and previously reported data support the hypothesis that FLNC-caused RCM has an early, often childhood, presentation and could be combined with a congenital myopathy accompanied by mild CK elevation. Of note, the A1186V variant was previously reported in association with myofibrillar myopathy and presented in Leiden Muscular Dystrophy pages, however without detailed clinical information regarding cardiovascular system involvement and prognosis (Ghaoui et al., 2015). The possible association of cardiomyopathy with neuromuscular phenotype is a well-known feature for several types of cardiomyopathies, but most often reported in association with DCM (Janin et al., 2017; Kostareva et al., 2013). The present study together with available reports demonstrate that RCM caused by mutations in cytoskeletal and Z-disc protein encoding genes such as DES, CRYAB, BAG3, and FLNC and leading to intracellular protein aggregate formation often present with concomitant neuromuscular phenotype (Brodehl et al., 2016; Brodehl et al., 2017; Gudkova et al., 2013; Konersman et al., 2015; Kostera-Pruszczyk et al., 2015).
Interestingly, both in vitro and in vivo models used revealed some inconsistency in aggregate formation. While we were not able to detect any alteration of intracellular filamin C network or protein aggregates formation in C2C12 cells with either p.A1183L or p.A1186V mutations, overexpression of mutant FNLC in zebrafish revealed the presence of FNLC aggregates consistent with previously reported zebrafish models for FNLC-myopathies (Ruparelia et al., 2012; Begay et al., 2016). This, possibly, can be explained by the absence of a proper Z-line architecture in C2C12 cells and, thus, lack of a proper binding partners for FLNC triggering aggregate formation in an area of Z-line discs. This also suggests that the overexpression of mutant FLNC, despite the presence of endogenous protein, is still sufficient to elicit disruption of its normal localization at the Z-discs. The aggregate formation at the developmental stage studied, however, did not result in disruption of muscle force generation (data not shown), which may be due to the expression of variable amounts of the transgene in the skeletal muscle which, in a wild-type background, may not result in clear muscle function impairment. Given the presence of filamin C aggregates in patient 22, who was the oldest at the time of examination, and their absence in patient 25 despite the same genetic background, suggests that the FLNC aggregates might reflect the efficacy of the intracellular protein degradation system rather than an obligatory condition for clinical manifestation of filaminopathies (Kley et al., 2013; Ruparelia et al., 2016; Tucker et al., 2017). This is well in line with the fact that clinical presentation of filaminopathies does not always depend on the presence of intracellular aggregates (Janin et al., 2017; Ortiz-Genga et al., 2016; Reinstein et al., 2016; Tucker et al., 2017; van den Bogaart et al., 2017). Remarkably, one of the typical morphological features in patients with the A1186V mutation was the absence of filamin C regular staining in the areas of intercalated discs, a sign previously reported in association with FLNC-caused cardiomyopathies (Brodehl et al., 2016; Reinstein et al., 2016).
The spectrum of FLNC-associated phenotypes includes all four major types of cardiomyopathies, namely HCM, DCM, ACM, and RCM as well as isolated distal or myofibrillar myopathy. The distribution of all previously reported pathogenic variants associated with these types of cardiomyopathy does not allow to identify any gene domains, predominantly linked with a particular phenotype (Figure 3B). Instead, the combined data suggest that the type of variant rather than location within the gene predicts the specific form of cardiomyopathy and type of myocardial remodeling. Thus, almost all DCM/ACM cases are caused by loss of function genetic variants—nonsense, frameshift, or splicing mutations. The same is true for most of myopathy-associated FLNC mutations. In contrast, HCM/RCM mutations mainly represent missense variants and all but two are located within Ig-loops (Figure 3B). For example, V2297M, P2298S, and S1624L are known pathogenic variants previously associated with RCM and even though they localize far apart on the FLNC gene they fall into a similar region on the Ig structure, and, possibly, participate in the similar protein–protein interactions. The latter fact corresponds with the data demonstrating localization of most of RCM-associated genetic variants within disordered regions responsible for functional and structural protein–protein interactions (Tarnovskaya et al., 2017). In the four patents reported here, both identified FLNC variants localize in the same gene region suggesting that it might represent a gene area prone for mutation occurrence. However, it does not locate within a CpG island and the exact mechanism underlying these events remains unclear.
In conclusion, we describe a new clinical phenotype of filaminopathies presenting with severe early-onset RCM and congenital myopathy and arthrogryposis. The pathogenic effect of identified variants is confirmed by morphological examination and in vivo modeling in zebrafish. Finally, the combined clinical and experimental data support the early presentation and poor prognosis of filamin C-associated RCM which does not directly depend on intracellular aggregate formation and structural cellular abnormalities.
ACKNOWLEDGMENTS
Sequencing was performed at Research Resource Centre «Biobank» of Research park of St.Petersburg State University and Research Resource Center of Molecular and cell technologies of St.Petersburg State University. Special thanks to Dr. Olga A. Pavlova, specialist of Research Resource Center of Molecular and cell technologies SPbSU for great help in libraries preparation and Dr. Dmitrii E. Polev, leading specialist of Research Resource Center Biobank SPbSU, for great help and professionality in field of sequencing, and data handing. We thank investigators for the A4.1025 antibody (H.M. Blau), obtained from the Developmental Studies Hybridoma Bank (developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA).
DISCLOSURE STATEMENT
The authors declare no conflict of interest.