Position of Glycine Substitutions in the Triple Helix of COL6A1, COL6A2, and COL6A3 is Correlated with Severity and Mode of Inheritance in Collagen VI Myopathies
Contract grant sponsors: MDA Clinical Research Training Grant (RJB); Primary Children's Medical Center Foundation (RJB); Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institutes of Health (5K12HD001410–10) (RJB); Muscular Dystrophy Campaign (ARF); NIH/NINDS Intramural Research Funds (CGB).
Communicated by Madhuri Hegde
ABSTRACT
Glycine substitutions in the conserved Gly-X-Y motif in the triple helical (TH) domain of collagen VI are the most commonly identified mutations in the collagen VI myopathies including Ullrich congenital muscular dystrophy, Bethlem myopathy, and intermediate (INT) phenotypes. We describe clinical and genetic characteristics of 97 individuals with glycine substitutions in the TH domain of COL6A1, COL6A2, or COL6A3 and add a review of 97 published cases, for a total of 194 cases. Clinical findings include severe, INT, and mild phenotypes even from patients with identical mutations. INT phenotypes were most common, accounting for almost half of patients, emphasizing the importance of INT phenotypes to the overall phenotypic spectrum. Glycine substitutions in the TH domain are heavily clustered in a short segment N-terminal to the 17th Gly-X-Y triplet, where they are acting as dominants. The most severe cases are clustered in an even smaller region including Gly-X-Y triplets 10–15, accounting for only 5% of the TH domain. Our findings suggest that clustering of glycine substitutions in the N-terminal region of collagen VI is not based on features of the primary sequence. We hypothesize that this region may represent a functional domain within the triple helix.
Introduction
The collagen VI myopathies, Ullrich congenital muscular dystrophy (UCMD) and Bethlem myopathy (BM) are among the most common congenital muscular dystrophies and are characterized by distal joint laxity and a combination of distal and proximal joint contractures [Clement et al., 2012; Okada et al., 2007]. In UCMD (MIM #254090), progressive weakness is manifest in the neonatal period or early childhood, frequently resulting in early loss of ambulation [Ullrich, 1930]. In BM (MIM #158810), weakness begins mid-childhood or early adolescence, but progression is slow and ambulation is retained into adulthood [Jobsis et al., 1996]. UCMD and BM had been considered distinct clinical and genetic entities until both were linked to mutations in the genes encoding collagen VI (COL6A1, MIM #120220; COL6A2, MIM #120240; COL6A3, MIM #120250) [Camacho Vanegas et al., 2001; Jobsis et al., 1996]. UCMD and BM phenotypes are thought to represent the ends of a clinical spectrum that includes intermediate (INT) phenotypes of variable severity. A myosclerosis myopathy phenotype [Merlini et al., 2008] (MIM #255600) and a limb-girdle muscular dystrophy phenotype [Scacheri et al., 2002] have also been described.
Collagen VI is a ubiquitous nonfibrillar collagen composed of three chains, α1(VI), α2(VI), and α3(VI) organized into a network of microfibrils important in anchoring the basement membrane to the extracellular matrix (ECM) [Kuo et al., 1997]. Each chain contains a comparatively short triple helical (TH) domain with repeating Gly-X-Y subunits flanked by large globular von Willebrand factor type A domains (Fig. 1) [Chu et al., 1989; Chu et al., 1990a]. Cultured skin fibroblasts from UCMD and BM patients have decreased matrix deposition and poor localization of collagen VI to the basement membrane [Hicks et al., 2008].

Domain structure and assembly of collagen VI. A: Domain structure of collagen VI α1(VI), α2(VI), and α3(VI) chains. N-terminal von Willebrand factor A domains are light gray and C-terminal von Willebrand factor A domains are dark gray. Cysteine residues in the 30th Gly-X-Y triplet of COL6A1 and COL6A2 and 17th triplet of COL6A3 important for higher order assembly are labeled “S.” The 17th Gly-X-Y triplet (delineated with a dashed line) is an important landmark with 89% of glycine substitutions N-terminal this site. A cysteine residue at this site in the α3(VI) chain in this is important for disulfide binding of tetramers. B: Assembly of collagen VI highlighting, first, association of α1(VI), α2(VI), and α3(VI) chains to form the monomer subunit, second, antiparallel association of monomers stabilized by disulfide bonds between cysteine residues in the 30th Gly-X-Y triplet of α1(VI) and α2(VI) chains with the C-terminal globular domains of the adjacent monomer, and third, parallel association of dimers stabilized by disulfide bonds formed between cysteine residues in the 17th triplet of adjacent α3(VI) chains. Final assembly into beaded microfibrils occurs by extracellular association of tetramers in end-to-end fashion [Chu et al., 1990b; Pace et al., 2008]. Size of the globular N- and C-terminal domains and the length of the TH domain are proportional to size estimations proposed by Beecher et al. (2011).
Genotype/phenotype associations in the collagen VI myopathies have been difficult to assess due to the considerable clinical and genetic heterogeneity, including presentations of UCMD, BM, and INT phenotypes in patients with similar mutations [Baker et al., 2007; 2005; Brinas et al., 2010; Lampe et al., 2005; Reed et al., 2005]. Missense mutations involving the conserved glycine residue in the repeated Gly-X-Y motif in the TH domain are a common pathogenic mutation in both collagen VI myopathies and in disorders of other collagens. In the collagen VI genes, such glycine substitutions account for 30% of known pathogenic alleles [Brinas et al., 2010; Lampe and Bushby, 2005]. In contrast to glycine substitutions in the TH domain of other collagens [Dang and Murrell, 2008; Marini et al., 2007; Pescucci et al., 2003], glycine substitutions in collagen VI appear to cluster in the N-terminal end of the TH domain [Pace et al., 2008]. Substitution of a glycine residue or exon skipping in the N-terminal end of the TH domain does not appear to disrupt the formation of TH monomers, but it does disrupt higher order assembly, thus resulting in a dominant negative mode of action [Lamande et al., 2002; Pace et al., 2008]. It has been suggested that glycine substitutions in a critical region including Gly-X-Y triplets 10–15 in the N-terminal triple helix are correlated with a more severe disruption of assembly and with increased clinical severity [Pace et al., 2008].
Here, we describe clinical and genetic characteristics of 97 individuals with glycine substitutions in the TH domain of the collagen VI α1(VI), α2(VI), and α3(VI) chains, and add a review of 97 published cases, for a total of 194 cases. Clinical findings in these patients include severe UCMD, INT, and mild BM phenotypes. Identical glycine substitutions are associated with both severe and mild phenotypes. In all three chains, glycine substitutions are heavily clustered in a short segment of the TH domain N-terminal to the 17th Gly-X-Y triplet (TH17). The most severe cases are clustered in the critical tetramer assembly region including Gly-X-Y triplets 10–15 accounting for only 5% of the length of the TH domain.
Subjects and Methods
Patients
Ninety-seven individuals from 83 families with missense mutations resulting in glycine substitutions in the TH domain of the COL6A1, COL6A3, and COL6A3 genes were identified from patients undergoing clinical genetic testing performed at the University of Utah (Salt Lake City, UT) and Prevention Genetics (Marshfield, WI), or who were seen clinically at the University of Utah (RB), Children's Hospital of Philadelphia (ARF and CGB), or National Institutes of Health, Neuromuscular and Neurogenetic Disorders of Childhood Section (JD and CGB). Patients were enrolled in our collaborative collagen VI myopathy project and written informed consent was obtained according to the ethics committees of the participating institutions. Phenotype data were collected from medical records and patient questionnaires with an emphasis on major motor events such as initiation and loss of ambulation [Nadeau et al., 2009]. We collected data on pulmonary function when it was available, but due to the heterogeneity of the patient population, we did not have sufficient data for correlation with respiratory outcomes. We also conducted an extensive literature search and identified 97 additional patients from 65 families with published glycine substitutions in the TH domain. Phenotype of individuals in published reports was collected with a similar focus on motor function and in particular ambulation.
Phenotype
Patients were divided into groups based on clinical severity and progression. The early severe (ES)-UCMD category includes patients who never achieved independent ambulation [Brinas et al., 2010]. The typical UCMD category includes patients who achieved independent ambulation but subsequently lose ambulation by 12 (an average age of 10), or who remain ambulatory indoors only. This “typical” UCMD group is comparable to the moderate progressive UCMD patients reported by Brinas et al, (2010). Patients under 12 years of age at the time of evaluation were classified as UCMD if their first wheelchair use was before the age of 8 years or they started nighttime noninvasive ventilation before the age of 10 years. The INT-UCMD category includes patients who have remained ambulant beyond 12 years of age but have significant motor impairment with loss of independent ambulation by adulthood. Patients under 12 years of age were classified as INT if they had modest gait impairment and infrequent use of assistive devices in childhood. BM patients demonstrated independent ambulation into adulthood without significant gait impairment. Patients less than 18 years of age were classified as BM if they had joint contractures and minimal motor impairment or if a BM phenotype was predicted by family history. We were able to determine a phenotype classification in 51 of 97 cases in our cohort and in 52 of 97 published cases based on clinical data presented in the respective publications (Supp. Tables S1 and S2).
Mutation Analysis
Sequence analysis of the COL6A1, COL6A2, and COL6A3 genes was performed from genomic DNA as a clinical genetic test in CLIA-certified laboratories (University of Utah, Salt Lake City, UT and Prevention Genetics, Marshfield, WI) by Sanger sequencing methods, including SCAIP sequencing [Lampe et al., 2005]. In one case, the mutation was identified by exome sequencing and confirmed by Sanger sequencing. Variants are numbered according to RefSeq transcripts NM_001848.2 for COL6A1, NM_001849.3 for COL6A2, and NM_004369.3 for COL6A3. To examine the diversity of all variants seen in the TH domain of COL6A1, COL6A2, and COL6A3, we compared all coding variants in the TH domain of 417 individuals referred for clinical genetic testing at the University of Utah with annotated variants in dbSNP, build 135 [Sherry et al., 2001]. For variant analyses, familial alleles were considered only once per family. Variants reported here have been submitted to the Leiden Muscular Dystrophy Pages for COL6A1 (http://www.LOVD.nl/COL6A1), COL6A2 (http://www.LOVD.nl/COL6A2), and COL6A3 (http://www.LOVD.nl/COL6A3).
To determine whether the observed clustering of glycine substitutions was predicted by factors in the primary sequence, we developed two different simulations generating glycine substitutions in the TH domain based on the distribution of glycine codons in the primary sequence and on the neighbor-dependent predicted mutation rates for each glycine codon [Hess et al., 1994]. First, to test the hypothesis that the observed N-terminal clustering of glycine substitutions in each of the three genes was not predicted by the primary sequence, we simulated glycine substitution in each gene based on the total number of observed glycine substitutions for that gene (93 for COL6A1, 33 for COL6A2, and 22 for COL6A3). In each round of the simulation, the number of glycine substitutions N-terminal to TH17 was counted for each gene. After 50,000 rounds, an empiric distribution of the count of glycine mutations N-terminal to TH17 was determined for each gene and compared with the observed count for that gene. Mean and 95% confidence intervals were determined based on the empiric distribution. Second, to test the hypothesis that the observed clustering of glycine mutations in the α1(VI) chain versus the α2(VI) and α3(VI) chains is not dependent on the primary sequence, we focused our simulation on the region N-terminal to TH17, and simulated 133 glycine substitutions on α1(VI), α2(VI), or α3(VI), counting the number of glycine substitutions per gene for each of 10,000 cycles. We determined a mean and 95% confidence interval for the number of glycine mutations in each gene based on the empiric distribution and compared with the observed count.
Mutations in glycine codons (GGA, GGC, GGG, GGT) can result in substitution of eight different amino acids or one stop codon. To test whether glycine mutations in our cohort were enriched for any of the possible amino-acid substitutions, we calculated expected values for each substitution based on a total of 148 glycine substitutions in the TH domain using neighbor-dependent predicted mutation rates [Hess et al., 1994; Persikov et al., 2004]. Expected values for each potential substitution and nonsense mutation were compared with the observed glycine substitutions including our cohort and published cases using a χ2 test under the null hypothesis that observed glycine substitutions occur at expected rates given neighbor-dependent substitution rates and distribution of glycine codons. Deviations from the observed to expected glycine substitutions were correlated with the published destabilization scale for glycine substitutions in the triple helix (Ala<Ser<Cys<Arg<Val<Glu<Asp<Trp) [Persikov et al., 2004].
Immunofluorescence Analysis of Collagen VI in Muscle and Cultured Fibroblasts
Muscle biopsy and cultured skin fibroblast samples were obtained from existing clinical samples. Dual labeling of 9 μm frozen muscle sections was performed for colocalization of collagen VI with collagen IV, a marker for the basement membrane. Collagen VI labeling was performed using a rabbit anticollagen VI polyclonal antibody (a gift from Dr. Rupert Timpl) at 1:3,000 dilution and Alexa Fluor 488-conjugated goat antimouse immunoglobulin (Molecular Probes, Eugene, OR) at 1:500 dilution. Basement membrane labeling was performed using a monoclonal mouse anticollagen IV (Chemicon, Temecula, CA) at 1:1,500 dilution, and Alexa Fluor 568-congugated goat antirabbit (Molecular Probes, Eugene, OR) at 1:500 dilution. Images were obtained using a Leica SP5 confocal microscope. Immunofluorescence analysis of cultured fibroblasts was performed as previously described [Lampe et al., 2008]. Briefly, fibroblasts were grown to 90% confluence and then treated with l-ascorbic acid (50 ng/μl) for 5 days and then fixed in 4% paraformaldehyde and blocked with 10% fetal bovine serum albumin with or without 0.1% TritonX-100. Staining for collagen VI was performed using anticollagen VI monoclonal antibody MAB3303 (Chemicon) at 1:2,500 dilution, and Alexa Fluor 568-conjugated goat antimouse immunoglobulin (Molecular Probes) at 1:500 dilution. Images were obtained using a Nikon Eclipse Ti microscope.
Results
Patients and Clinical Phenotype
We identified 194 individuals with mutations resulting in glycine substitutions in the TH domain of COL6A1, COL6A2, and COL6A3. Included are 97 newly reported cases from 83 different families and 97 published cases from 65 different families (Fig. 2). Phenotype data sufficient to classify individuals as ES-UCMD, typical UCMD, INT-UCMD, or BM was available for 51 newly reported cases and 52 published cases, for a total of 103 cases (Supp. Tables S1 and S2). Clinical characteristics of the newly reported cases are summarized in Table 1. Pace et al. (2008) have suggested that patients with glycine substitutions in a critical region including Gly-X-Y triplets 10–15 have a more severe disruption of collagen VI assembly and a more severe clinical phenotype. In our cohort, patients with mutations inside the critical region tend to a more severe phenotype with 48% ES-UCMD or typical UCMD compared with 23% ES-UCMD or typical UCMD in cases with mutations outside the critical region. Patients with glycine substitutions outside the critical region tended toward a milder phenotype with 40% BM compared with 7% BM in patients with mutations inside the critical region (Fig. 3, Table 2).
| Clinical phenotype | Number | Agea | CKb | Age walked (mo) | Avg. first wheelchair (yr) | Avg. full wheelchair (yr) | FTTc | Respiratory compromised |
|---|---|---|---|---|---|---|---|---|
| ES-UCMD | 2 (4%) | 4.5 (3–6) | 214 | – | – | – | 1 (50%) | 1 (50%) |
| UCMD | 10 (20%) | 11.7 (5–19) | 245 (116–395) | 19.8 | 7.8 | 9.2 | 4 (40%) | 8 (80%) |
| INT | 24 (44%) | 11.6 (4–41) | 302 (193–470) | 16.8 | 10.8 | 15.0 | 11 (46%) | 5 (21%) |
| BM | 11 (20%) | 28.0 (8–50) | 1,000 (500–1,500) | 14.8 | – | – | 0 | 1 (9%) |
| RECESSIVE | 2 (2%) | 7.5 (5–10) | – | 18.0 | – | 10.0 | 2 (100%) | 1 (50%) |
| UNAFe | 2 (4%) | 49.5 (38–61) | – | – | – | – | – | – |
- a Age at last clinical evaluation.
- b CK = mean and range of creatine kinase in patients from which it was available.
- c FTT = failure to thrive, defined as weight under 5th centile for age.
- d Forced expiratory volume (FEV1) <60% predicted for age, or noninvasive ventilation, or tracheotomy.
- e Clinically unaffected patient with glycine substitution.
| Substitution in critical regiona | ES-UCMD | UCMD | INT | BM | UNAFb | Total |
|---|---|---|---|---|---|---|
| No | 6 | 12 | 14 | 1 | 33 | |
| Yes | 9 | 24 | 29 | 5 | 1 | 68 |
| 9 | 30 | 41 | 19 | 2 | 101 |
- a Critical region as defined by Pace et al. (2008) from Gly-X-Y triplets 10–15.
- b Clinically unaffected patient with glycine substitution.

Summary of known cases with glycine substitutions in the TH domain of α1(VI), α2(VI), and α3(VI) chains (194 cases). Triangles above the axis represent glycine substitutions identified in our cohort and below the axis represent glycine substitutions identified in published cases. Related individuals are linked by a bracket. Position of cysteine residues important for dimerization (α1(VI), α2(VI)), and tetramerization (α3(VI)) are marked with a red box. The critical region important for assembly proposed by Pace et al. (2008) is marked with a horizontal orange box. * indicates both alleles in one patient with two different glycine substitutions. + represents patients homozygous glycine substitutions. # indicates alleles known/suspected to be acting in recessive fashion. TH domain exons are indicated by alternating dark and light grey rectangles.
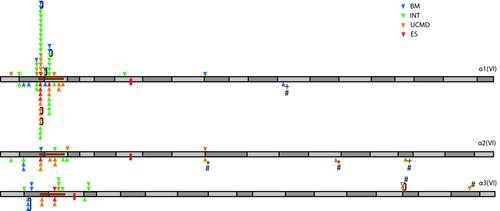
We identified two unaffected carriers that were identified after the mutation was first identified in an affected child. In one case, the mutation was demonstrated to be acting recessively in combination with a nonsense mutation (patient 61, Supp. Table S1). In the second case, the unaffected father (patient 29) of a patient with an INT-UCMD phenotype (patient 28) carried the same mutation on COL6A1 (c.859G>A; p.G287R) that was seen in his son. Peak height ratios from sequence trace data suggest that this mutation may be mosaic in the father (Supp. Fig. S1).
Variant Analysis
Glycine substitutions in COL6A1, COL6A2, and COL6A3 are a common mutation type in our clinical sequencing effort. Of 604 patients sequenced at the University of Utah and Prevention Genetics clinical genetic testing services, 166 have known pathogenic mutations, and 39% (64/166) of these are glycine substitutions in the TH domain. In the 194 cases presented here, glycine substitutions occurred at 44 of the 332 potential sites in the TH domain, 28 of which are newly reported. In 148 unrelated cases (83 from our cohort and 65 from published cases), 89% (131/148) were clustered N-terminal to TH17 (Fig. 4A). We considered whether nonglycine coding variants in the TH domain identified in our clinical sequencing effort or in dbSNP build 135 were also clustered on the N-terminal to TH17. In both cases, nonglycine variants were relatively evenly distributed between the three genes and within the TH domain (Fig. 4B). Ten variants resulting in glycine substitutions in the TH domain are reported in dbSNP 135 including five on COL6A1, two on COL6A2, and three on COL6A3 (Supp. Table S3). Five of these variants originate from published pathogenic variants reported in OMIM and also reported here. The variant rs11701912 on COL6A1 (p.G332S) is pathogenic and dominant, but was reported without allele frequency data or other information on phenotype. The remaining variants were reported in exome sequencing projects and are in the C-terminal end of the TH domain where they are expected to act recessively.

To determine whether the clustering of glycine substitutions in the TH domain is determined by factors in the primary sequence, we simulated glycine substitutions in α1(VI), α2(VI), and α3(VI) chains based on neighbor-dependent mutation rates [Hess et al., 1994], the distribution of glycine codons in each gene, and the observed number of glycine substitutions in each chain. We performed two simulations, first to address the clustering of substitutions within each chain N-terminal to TH17, and second to address the clustering of substitutions on the α1(VI) chain versus the α2(VI) and α3(VI) chains. In the first simulation, the observed clustering of the glycine substitutions N-terminal to TH17 was highly significant for each gene, with the observed number of glycine substitutions in this region exceeding even the maximum number seen in 50,000 simulations (Table 3). In the second simulation, the observed glycine substitutions in the α1(VI) chain were significantly more than the expected (86 observed vs. 44 expected; Table 4). The α2(VI) chain had approximately the expected number of glycine substitutions (28 observed vs. 33 expected), whereas the α3(VI) chain showed a paucity of glycine substitutions (17 observed vs. 54 expected).
| Simulated substitutions N-terminal to TH17a | |||||
|---|---|---|---|---|---|
| Observed glycine substitutions in TH domain | Observed glycine substitutions N-terminal to TH17 | Avg ± 2SDb | Max | Min | |
| α1(VI) | 93 | 86 | 17.5 (10–25) | 34 | 3 |
| α2(VI) | 33 | 28 | 4.2 (0.3–8) | 13 | 0 |
| α3(VI) | 22 | 17 | 4.6 (0.8–8.4) | 13 | 0 |
- a 50,000 cycles per gene, each cycle placing the observed number of glycine substitutions from the entire TH domain and counting the number of substitutions in the region N-terminal to TH17.
- b Average count of glycine substitutions N-terminal to TH17 in 50,000 simulations for each gene. 95% confidence interval in parenthesis.
- c Max and min indicate the maximum and minimum counts for the number of substitutions N-terminal to TH17 for each chain from all 50,000 cycles.
| Simulated substitutionsa | ||||
|---|---|---|---|---|
| Observed substitutions | Avg ± 2SDb | Maxc | Min | |
| α1(VI) | 86 | 43.9 (33.1–54.7) | 69 | 23 |
| α2(VI) | 28 | 33.0 (23.1–42.9) | 59 | 13 |
| α3(VI) | 17 | 54.1 (42.8–65.4) | 77 | 32 |
- a 10,000 cycles with each cycle placing 131 glycine substitutions in the region N-terminal to TH17 on any of the three chains with count of the number of substitutions for each chain in each cycle.
- b Average count of glycine substitutions in each chain in 10,000 simulations. 95% confidence interval in parenthesis.
- c Max and min indicate the maximum and minimum counts for each chain from the 10,000 cycles.
We next scrutinized whether any of the eight potential amino-acid substitutions or one potential nonsense mutation were occurring more frequently than expected given the sequence context of the glycine codons, and whether the substituted amino acid is associated with severity. In the 148 observed, unrelated cases, there was a significant overabundance of Gly>Arg and Gly>Asp substitutions and a significant underrepresentation of Gly>Ala and Gly>Ser substitutions compared with predictions based on the sequence context (Fig. 5), χ2 = 70.9; P = 3.3 × 10−12. A spectrum of clinical phenotypes was seen for all glycine substitutions (Table 5). While the number of cases is too small to make definite conclusions, the three cases with Ala or Ser substitutions were all relatively mild: two presented with BM phenotypes and one with INT. These substitutions are the least disruptive to the structure of the TH. It has been suggested in other collagen disorders that these milder substitutions may have a milder phenotype and may even remain unascertained [Persikov et al., 2004]. With the exception of the underrepresented Ala and Ser substitutions, there are no obvious differences in severity based on the substituted amino acid or the gene containing the substitution (Table 6).
| Substitutiona | ES-UCMD | UCMD | INT | BM | UNAFb | Total |
|---|---|---|---|---|---|---|
| A | 1 | 1 | 2 | |||
| S | 1 | 1 | ||||
| C | 2 | 2 | 2 | 6 | ||
| R | 7 | 19 | 28 | 10 | 2 | 66 |
| V | 2 | 2 | 3 | 7 | ||
| E | 3 | 4 | 7 | |||
| D | 4 | 7 | 2 | 13 | ||
| W | ||||||
| X | 1 | 1 | ||||
| Total | 9 | 31 | 42 | 19 | 2 | 103 |
- a Amino-acid substitutions are arranged in increasing disruption to triple helix [Persikov et al., 2004].
- b Clinically unaffected patient with glycine substitution.
| Gene | ES-UCMD | UCMD | INT | BM | UNAFa | Total |
|---|---|---|---|---|---|---|
| COL6A1 | 6 | 17 | 30 | 10 | 1 | 64 |
| COL6A2 | 1 | 9 | 6 | 2 | – | 18 |
| COL6A3 | 2 | 6 | 5 | 7 | 1 | 21 |
| Total | 9 | 32 | 41 | 19 | 2 | 103 |
- a Clinically unaffected patient with glycine substitution.

Clinical Variation in Patients with Identical Mutations
The most commonly observed mutation in our cohort is the p.G284R substitution in the α1(VI) chain due to the c.850G>A mutation in COL6A1. This mutation is seen in the context of a CpG, which may provide an explanation for its frequent occurrence. While most individuals with the p.G284R mutation had typical UCMD or INT-UCMD phenotypes, patients with both mild (BM) and severe (ES-UCMD) phenotypes were also seen. The c.850G>C mutation also resulting in p.G284R was seen in two related individuals (twins), both with INT-UCMD phenotypes. Twenty-eight individuals with the p.G284R substitution have clinical information sufficient to classify their phenotype including: five ES-UCMD, nine typical UCMD, 12 INT-UCMD, and two BM. The second most common substitution, p.G290R on the α1(VI) chain, was seen in 18 cases due to two different mutations (16 cases from c.868G>A and two cases from c.868G>C), whereas two cases showed the c.869G>A mutation, resulting in a p.G290E substitution. Phenotype data were available in nine of these cases with one ES-UCMD, two typical UCMD, and six INT-UCMD. Substitutions at p.G281 of the α1(VI) chain showed the most diversity with three different substitutions in seven cases (four p.G281R; two p.G281E and 1 p.G281A).
Inheritance
Glycine substitutions in the TH domain are dominantly acting in 96% (186/194) of cases in the combined cohort. Three cases of recessively acting glycine substitutions have been previously reported, all with UCMD phenotypes (Supp. Table S2). In all three cases, the glycine mutations identified were outside the critical N-terminal end of the TH domain (triplets 47, 65, and 92). In two of these cases, PL6 [Jimenez-Mallebrera et al., 2006] and PL45 [Brinas et al., 2010], the glycine substitution was homozygous. In the third case, PL51 [Brinas et al., 2010], the patient was compound heterozygous for two glycine substitutions in the α2(VI) chain, one at the 47th Gly-X-Y triplet, and another at the 77th Gly-X-Y triplet. Family data were not reported. The more N-terminal of these two glycine substitutions (p.G394E, Gly-X-Y triplet 47) is situated outside the critical region and was also seen in patient 49 in our cohort (Supp. Table S1). Although a dominant mechanism is likely, this patient has a second allele on the COLA3 gene that disrupts in the X-position of the 13th Gly-X-Y triplet in the critical region of COL6A3. This variant has been seen in the dbSNP135 (rs113331139; minor allele frequency, 1/4,545 = 0.02%), but pathogenicity has not been documented. Lending support to the notion that a glycine substitution at the 47th Gly-X-Y triplet of the α2(VI) chain is dominant, a glycine substitution in patient 63 on the 47th triplet of the α1(VI) chain (c.1184G>T; p.G395V) also appears to be acting in a dominant fashion.
We report two new recessive cases. Patient 61 (Supp. Table S1) has a recessive glycine substitution in the 91st Gly-X-Y triplet of α3(VI) (c.6931G>A, p.G2311R). The second allele is a nonsense mutation in the Y position of the 8th Gly-X-Y triplet (c.6181C>T, p.R2061X). While both mutations are apparent on sequencing from genomic DNA, sequencing of the fibroblast-derived cDNA from this patient does not show the nonsense allele, consistent with nonsense-mediated decay of this allele (data not shown). The nonsense mutation in this case is de novo, whereas the glycine substitution is inherited from the clinically unaffected mother. Patient 77 has a recessive nonsense mutation (c.7066G>T; p.G2356X) in the glycine position of the 106th Gly-X-Y triplet of COL6A3. This is the only known nonsense mutation of a glycine in the TH domain. The second allele (c.5044delC; p.Q1682Sfs12X) introduces a frameshift in the N2 domain. Parental DNA was not available for further analysis of these alleles. In both cases, the patient had a typical UCMD phenotype.
In addition to the glycine substitution, we identified rare variants not annotated in dbSNP 135 in 10 cases in our cohort (Supp. Table S1). In most cases, these are likely rare polymorphisms. In one case (patient 75, Supp. Table S1), we questioned whether the second variant is acting as a modifier. This patient was compound heterozygous for two variants on COL6A1, a glycine substitution (c.1022G>A; p.G341D) and a novel second allele (c.1763C>T; p.P588L). The glycine substitution is dominant and has been previously reported in a family with a limb-girdle muscular dystrophy phenotype (Supp. Table S2, PL12) [Scacheri et al., 2002]. Another substitution (p.G341V) at the same codon has also been reported in patients with BM (Supp. Table S2, PL4 and PL16) [Lampe et al., 2005; Lucioli et al., 2005]. The potential modifying allele results in a substitution of leucine for proline (p.P588L) in the X position in the second to last triplet (Gly-Pro-Pro) in the TH domain. Gly-Pro-Pro triplets in this region of other collagens are thought to be important for nucleation of the triple helix [Hyde et al., 2006] and a mutation here may be acting as a modifier.
Immunofluorescence of Collagen VI in Muscle and Cultured Skin Fibroblasts
Muscle biopsies from existing clinical samples were available for our analysis from 10 patients, all with dominantly acting glycine mutations. Findings were typical of other collagen VI myopathy patients including presence of collagen VI immunofluorescence in the ECM, but loss of colocalization to the basement membrane (Fig. 6A). Collagen VI immunofluorescence in cultured fibroblasts was available for 26 patients. Findings were similar in patients with BM, INT-UCMD, typical UCMD, or ES-UCMD phenotypes and include an overall decrease of collagen VI in the ECM, often with a knot-like appearance, and marked intracellular retention (Fig. 6B).
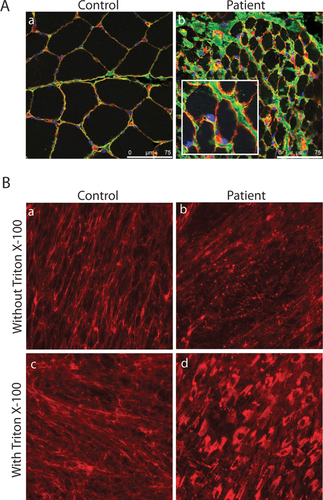
Immunofluorescent staining of muscle (A) and cultured fibroblasts (B) from a patient with c.812G>A; p.G271D mutation in COL6A2. Dual labeling of collagen VI (green) and collagen IV (red) in muscle shows presence of collagen VI in the ECM, but poor colocalization with the basement membrane (yellow). Scale bar is 75 μm. Inset shows higher magnification for patient muscle (A; b). Staining for collagen VI in cultured fibroblasts from the same patient shows decreased deposition and speckled appearance of collage VI in the ECM of patient fibroblasts compared with control (B; a and b). Collagen VI staining in the presence of Triton X-100 to permeabilize the cell membrane demonstrates intracellular retention of collagen VI in patient fibroblasts (B; c and d).
Discussion
Glycine substitutions in the conserved Gly-X-Y motif in the TH domain of collagen VI are the most commonly identified mutations in the collagen VI myopathies, accounting for almost 1/3 of all pathogenic mutations in COL6A1, COL6A2, and COL6A3. Here, we present the hitherto largest clinical and genetic study of patients with glycine substitutions in the TH domain of collagen VI α1(VI), α2(VI), and α3(VI), including 97 newly identified patients and 97 patients identified from review of the literature. INT phenotypes were the most common presentation, accounting for almost half of patients, emphasizing the importance of INT phenotypes to the overall phenotypic spectrum of collagen VI myopathy patients. The p.G284R mutation in the α1(VI) chain was the single most commonly observed mutation with 28 known cases. While both extremes of the clinical spectrum (ES-UCMD and BM) were seen in patients with this mutation, half had an INT phenotype, mirroring the cohort as a whole. The broad range in severity for this and other frequently occurring mutations suggests the possibility for modifying genes as has been recently reported in Duchenne muscular dystrophy [Flanigan et al., 2013]. It is also possible that variants within the genes encoding collagen VI are modifiers of severity of clinical symptoms. In most cases, these likely represent rare polymorphisms as we have reported them here (Supp. Tables S1 and S2). In at least one case (Supp. Table S1, patient 75), the second allele, a substitution of leucine for proline in the penultimate Gly-X-Y triplet in COL6A1 is suspicious for acting as a modifier. We did not observe a correlation between clinical severity and which of the three chains was mutated nor were we able to demonstrate a clear correlation of severity with the substituting amino acid, although a significant underrepresentation of Gly>Ser and Gly>Ala mutations was observed.
The majority of glycine substitutions in the TH domain are de novo dominantly acting mutations. Rare recessive cases have been reported and were also seen in our cohort. While dominant cases are clustered in the N-terminal end of the TH domain, mutations in recessive cases are near the C-terminal end of the TH domain. It has been suggested that the dominant negative mechanism in collagen VI myopathies reflects the ability of mutant chains to be incorporated into the tetramer subunits [Lampe et al., 2008]. The presence of recessive cases, all with mutations in the C-terminal end of the TH domain, suggests that such positional factors within the TH domain are important in the initial formation of the TH monomer and incorporation versus exclusion of mutant chains in the assembly process. It is not entirely clear whether the recessive glycine substitutions in the C-terminal TH domain are so severely disruptive to the triple helix assembly that they are not incorporated into higher order structures (similar to in frame deletions in the region [Lampe et al., 2008]) or whether the mutant chains are simply less disruptive and thus better tolerated, although the former seems more likely.
The most striking feature of the glycine substitutions in collagen VI is the N-terminal clustering, with 89% of these mutations N-terminal to the 17th Gly-X-Y triplet (TH17). This important landmark is delineated by cysteine residues in the α3(VI) chain, which form disulfide bonds stabilizing tetramers (Fig. 1). Of the 148 unrelated cases, 89% (131/148) are in this N-terminal segment of the TH domain, accounting for only 15% of the length of the TH domain. N-terminal clustering of glycine substitutions in the TH domain was prominent for all three chains, but the α1(VI) chain showed significantly more glycine substitutions than α2(VI) or α3(VI). The three most commonly observed mutations (p.G284R, p.G290R, and p.G293R) are all on the α1 (VI) chain and account for 35% of all glycine known substitutions in the TH domain. Mutations at these sites are all associated with a CpG motif; however, factors in the primary sequence such as CpG motifs do not predict the magnitude of the observed excess of mutations on COL6A1, nor do they predict the clustering of these mutations on COL6A2 or COL6A3. We considered whether the TH domain N-terminal to TH17 was more prone to variation generally; however, rare variants, including both those reported in dbSNP 135 and those seen in our clinical sequencing effort, do not show clustering N-terminal to TH17. The excess of glycine substitutions in this region generally, and on the α1(VI) chain specifically may reflect a region of local hypermutability beyond that predicted solely by the local sequence. Alternatively, mutations in this region may be more severely disruptive to the assembly and function of collagen VI and thus more likely to result in a clinical phenotype.
Structural analyses have shown that the C-terminal segments of the TH domain are closely associated with each other in the dimer forming an antiparallel 75 nm supercoiled helix, and leaving a 30 nm segment of the N-terminal TH that is not overlapped with other chains (Fig. 1) [Baldock et al., 2003; Knupp and Squire, 2001]. Within this relatively unencumbered segment, Pace et al. (2008) proposed a critical region including Gly-X-Y triplets 10–15 that is important in microfibril formation and influences severity of disease. Patients with more severe clinical symptoms had glycine mutations in the critical region, secreted nondisulfide bonded collagen VI tetramers, and had a severe compromise of microfibril formation. In contrast, patients with mutations outside this region were more mildly affected, secreted disulfide-bonded collagen VI tetramers, and had less severe compromise of microfibril assembly. In our cohort, 59% (88/148) of glycine substitutions occur in this critical region, accounting for only 5% of the length of the TH domain. Consistent with the assertion that this region is critical to assembly and function of collagen VI, patients with mutations in this region in our cohort tend toward a more severe phenotype. Almost half (33/68) of patients with mutations in this region have severe (ES-UCMD or typical UCMD) phenotypes compared with 22% (8/35) of patients with mutations outside the critical region.
Taken together, our findings suggest that clustering of glycine substitutions in the N-terminal region of collagen VI is not based on features of the primary sequence. We hypothesize that this region may represent a functional domain within the triple helix. It has been proposed that the 30 nm segment at the N-terminal end of TH domain containing the critical region forms a loop around the N-terminal globular domain of the adjacent tetramer during microfibril formation [Beecher et al., 2011]. A glycine substitution in this region may result in mutant tetramers that are unable to form normal associations with adjacent N-terminal globular domains. It remains to be seen whether disruptions of the TH in this region are steric, limiting association of tetramers to form microfibrils, or whether glycine mutations in this region also affect the overall binding associations of collagen VI with other ECM components.
In summary, we report a large cohort of patient with glycine substitutions in the Gly-X-Y motif in the TH domain of collagen VI. Glycine substitutions in the TH domain are a common mutational mechanism in collagen VI myopathies and have complex inheritance patterns and molecular pathogenesis. Similar to exon skipping mutations in the TH domain [Lampe et al., 2008], glycine substitutions cluster N-terminal to the 17th Gly-X-Y triplet and are associated with a spectrum of clinical severity. While not fully predictive, glycine substitutions in the critical region from Gly-X-Y triplets 10–15 tend to result in more severe clinical phenotypes. This dissection of the most prevalent mutational mechanism of collagen VI significantly advances our understanding of the molecular genetic mechanisms underlying these disorders and has obvious implication for the development of molecular treatment approaches, such as the allele specific knockdown approaches recently proposed [Gualandi et al., 2012]. Appropriate anticipatory care and genetic counseling in collagen VI myopathy patients should include an assessment of mode of inheritance and clinical phenotype, which is at least in part predicted by the position of the glycine substitution in the TH domain.
Acknowledgments
We are grateful to the patients and families for their participation in the study. We also extend our thanks to the clinician who have referred these patients for sequencing or otherwise brought patients to our attention: Amy Harper, Anne Connolly, Anne Rutkowski, Anthony Amato, Antigone Papavasiliou, Brenda Banwell, Brenda Wong, Bruce Cohen, Christoffer Jonsrud, Claudia Castiglione, Craig McDonald, Daniel P. Judge, Diana Escolar, Edward Elmendorff, Edward Leung, Eva Rudd, Gihan Tennekoon, Gyula Acsadi, Hugh McMillan, J. Edward Spence, Jack Faircloth, James Collins, James Reggin, Jan Kirschner, Janice McAllister, Jennifer Semel, Jerry Mendell, John Bodensteiner, Joline Dalton, Julie S. Cohen, Justin Kwan, Katherine Mathews, Kathryn North, Kathryn Wagner, Kim Ramme, Kristina Karraman, Livija Medne, Maria Soller, Mark Tarnopolsky, Martha Walker, Michele Yang, Monica Troncoso, Muneera Al Husain, Neil Friedman, Nizar Chahin, Patrick Ferreira, Perry B. Shieh, Peter Karachunski, Peter T. Heydemann, Pierre Fequiere, Randall Richardson, Richard Finkel, Robin Clark, Sidney Gospe, Spencer G. Weig, Stanley Johnsen, Sumit Parikh, Susan Iannaccone, William B. Burnette, and Yadollah Harati. We also thank laboratory personnel involved in the clinical genetic testing at the University of Utah: Brett Duval, Cindy Hamil and Maha Mahmood.
Disclosure statement: The authors declare no conflict of interests.




