Telomere Phenotypes in Females with Heterozygous Mutations in the Dyskeratosis Congenita 1 (DKC1) Gene
Contract grant sponsors: United States National Institutes of Health (NIH) (RO1CA160433, K99HL113105); Doris Duke Charitable Foundation; NIH (P30CA006973).
Communicated by Dominique Stoppa-Lyonnet
ABSTRACT
Dyskeratosis congenita (DC) is a telomere-mediated syndrome defined by mucocutaneous features. The X-linked mode of inheritance accounts for half the cases, and is thought to predominantly manifest in childhood as bone marrow failure. We identified two male probands who presented in the fifth decade with idiopathic pulmonary fibrosis and cancer. Their pedigrees displayed consecutively affected generations. Five of six females (83%) manifested mucocutaneous features of DC, and two had wound-healing complications. No mutations in autosomal dominant telomere genes were present, but exome sequencing revealed novel variants in the X-chromosome DKC1 gene that predicted missense mutations in conserved residues, p.Thr49Ser and p.Pro409Arg. Variants segregated with the telomere phenotype, and affected females were heterozygotes, showing skewed X-inactivation. Telomerase RNA levels were compromised in cells from DKC1 mutation carriers, consistent with their pathogenic role. These findings indicate that females with heterozygous DKC1 mutations may be at increased risk for developing penetrant telomere phenotypes that, at times, may be associated with clinical morbidity.
Dyskeratosis congenita (DC) is a syndrome of telomere shortening [Armanios and Blackburn, 2012]. It has been historically defined by a triad of mucocutaneous features in male children who have reticular skin hyperpigmentation, oral leukoplakia, and nail dystrophy [Dokal, 2000]. DC patients suffer premature morbidity most commonly from bone marrow failure that affects 85% of cases before the age of 20 years [Dokal, 2000]. Pulmonary fibrosis and cancer account for the majority of the remaining life-threatening complications [Dokal, 2000; Parry et al., 2011aa]. DC falls on the severe end of a spectrum of syndromes characterized by inherited defects in telomere maintenance [Armanios and Blackburn, 2012]. In contrast to affected children, older adults with telomere-mediated disease usually manifest with pulmonary fibrosis, and in the absence of the classic DC features [Armanios et al., 2007]. Although DC was initially recognized as an X-linked disorder [Devriendt et al., 1997; Knight et al., 1998; Vulliamy et al., 1997], it is appreciated now that autosomal dominant telomere syndromes are most prevalent [Armanios and Blackburn, 2012]. Loss-of-function mutations in the telomerase holoenzyme genes account for the majority of telomere syndrome cases. Mutations in the telomerase reverse transcriptase, TERT (MIM #187270), and the telomerase RNA, TR (also TERC; MIM #187270), cause haploinsufficiency and manifest in an autosomal dominant telomere syndromes [Armanios et al., 2005; Vulliamy et al., 2001]. Mutations in the DKC1 gene (MIM #300126), which encodes the telomerase holoenzyme component dyskerin, cause X-linked disease [Heiss et al., 1998; Mitchell et al., 1999]. Dyskerin functions to stabilize TR, and interacts with TR through an essential Box H/ACA RNA domain in its 3′end [Alder et al., 2011b; Mitchell et al., 1999]. Approximately one-third of DC cases remain genetically uncharacterized.
Although DC and telomere syndromes follow Mendelian patterns of inheritance, several unique features distinguish their genetics. Disease phenotypes show variable penetrance that is age-dependent, and autosomal dominant families display genetic anticipation, an earlier and more severe onset in successive generation [Armanios and Blackburn, 2012]. These features can confound recognizing the hereditary nature as well as the mode of inheritance in a given family [Armanios and Blackburn, 2012]. Moreover, autosomal dominant families display gender differences in disease penetrance with males developing complications at a younger age than their female siblings [Basel-Vanagaite et al., 2008]. We sought here to characterize the genetic basis of telomere phenotypes in two unrelated families that had consecutively affected generations. The details of the subject recruitment, consent, and the methods for the molecular studies are included in the Supporting Information's Methods Section.
Family 1
The first proband was evaluated as part of the Johns Hopkins Telomere Syndrome Registry [Jonassaint et al., 2013]. He was a previously healthy male who was diagnosed with idiopathic pulmonary fibrosis at the age of 46. He showed no features of DC. His family history was significant for a daughter with premature hair graying since the age of 20 and who had a history of wound dehiscence after abdominal surgery. During the proband's pulmonary fibrosis treatment course on a clinical trial with pirfenidone, he developed progressive thrombocytopenia, and hematologic evaluation revealed aplastic anemia. The hematologic abnormalities persisted despite discontinuation of the drug, and he became transfusion dependent by the age of 48. He was subsequently diagnosed with myelodysplastic syndrome with complex cytogenetics including deletions of chromosomes 5q, 7, 17, and 20. He was treated with a demethylating agent, but continued to have progressive dyspnea and died at the age of 49 from end-stage lung disease and marrow failure. Because the co-occurrence of pulmonary fibrosis and bone marrow failure is specific for telomere syndromes [Parry et al., 2011b], telomere length was measured and confirmed a very short age-adjusted length in lymphocytes, below the first percentile. His daughter's telomere length was near the fifth percentile (Fig. 1B). The clinical history is summarized in Figure 1A and B.
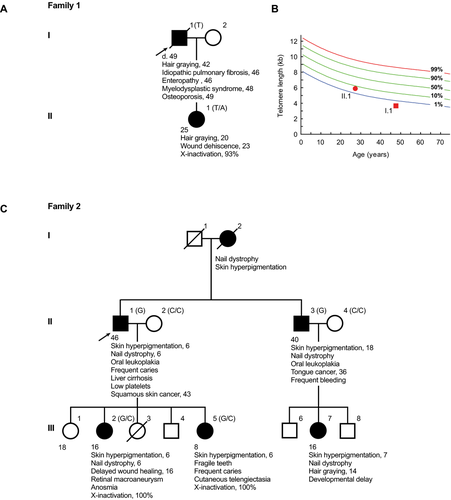
Family 2
Proband 2 and his family were evaluated at Memorial Sloan Kettering Hospital for a genomic instability syndrome in 1979 (Fig. 1C). Based on the clinical phenotype, the family was enrolled in the International Fanconi Anemia Registry [Auerbach et al., 1979]. The proband had the mucocutaneous triad of DC and reported a history of skin cancer as well as liver cirrhosis. His family history was remarkable for three consecutive generations with classic DC features that were documented in his mother, a brother, two daughters, and a niece (Fig. 1C). Female relatives had a history of delayed wound healing and brittle teeth, both complications reported in DC, and one daughter was found to have anosmia, a loss of the sense of smell (Fig. 1C). Phytohemagglutinin stimulation of peripheral blood did not show spontaneous chromosome instability, and challenge with diepoxybutane, an alkyling agent, showed no abnormal breaks or sister chromatid exchange events relative to controls, and in contrast to Fanconi anemia cells [Auerbach, 2009].
Genetic and Molecular Studies
Given the recent progress in the genetics of human telomere-mediated disease, we sought to identify the responsible gene(s) in these families. Several factors led us to prefer an autosomal dominant model. The probands had adult-onset disease, in contrast to nearly 90% of reported X-linked DC cases [Dokal, 2000], and the first presenting symptoms in both individuals were not bone marrow related. Furthermore, the high penetrance of disease in females (five of six affected) made X-linked inheritance less likely, given the reported low estimate of penetrance in DC females [Knight et al., 1998]. Sequencing of TERT and TR revealed no variants, and genotyping of flanking microsatellite ruled out the involvement of these loci in Family 2. We thus performed exome sequencing on the two probands using genomic DNA extracted from blood or fibroblasts. We used the SureSelect Human Exome 38-Mb Kit (Agilent, Santa Clara, CA), and the ABI SOLiD sequencing platform (Applied Biosystems, Carlsbad, CA). Manual inspection of candidate telomere gene sequences using the Integrative Genome Viewer identified a novel DKC1 exon 3 variant in proband 1: c.145A>T that predicted a missense substitution p.Thr49Ser near the nuclear localization signal of dyskerin (Fig. 2A). This variant was initially missed by the calling software because of low depth of coverage (2×; Fig. 2B). In the second proband, an exon 12 variant, c.1226C>G, was called and predicted a p.Pro409Arg substitution distal to the pseudouridine synthase domain of dyskerin (Fig. 2A and B). The single-nucleotide variants identified in the probands segregated with the telomere phenotypes, with affected males showing hemizygous status; and females were heterozygous, showing extremely skewed X-inactivation patterns (Fig. 1A and C). These data suggested that females with DKC1 mutations can show highly penetrant telomere phenotypes.
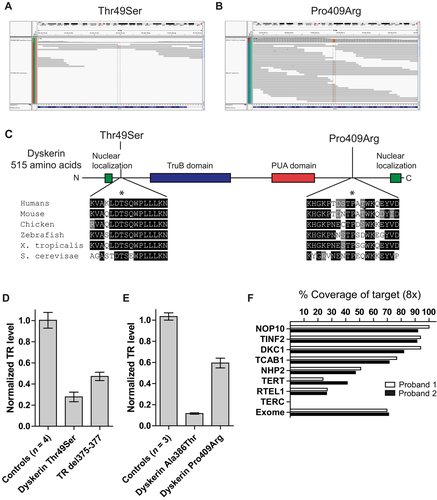
To test the functional significance of the dyskerin variants, we examined their evolutionary conservation and found the mutated amino acids fell in highly invariant residues in vertebrate dyskerins as well as in the yeast homolog NAP57 (Fig. 2C). Alternate substitutions at both of these residues have been described previously in DC (Thr49Met and Pro409Leu) [Ding et al., 2004; Knight et al., 1999], suggesting that mutations in these amino acids enrich for telomere phenotypes. Cells derived from the probands showed severely compromised TR levels (20% and 50% of control levels in families 1 and 2, respectively; Fig. 2D and E). TR levels were similarly decreased in cells from known DKC1 mutation carriers, as well as deleterious TR mutations in the Box H motif (Fig. 2D). These data established that the identified dyskerin missense mutations are functionally deleterious.
We report here that mutations in the telomerase holoenzyme component DKC1 can cause telomere-related phenotypes in heterozygous females. Five of six females examined (83%) displayed at least two telomere phenotypes in this study. There was morbidity associated with this heterozygous state evidenced by postoperative wound dehiscence. Females also had nonclassic DC features including fragile teeth, and one had anosmia. The olfactory bulb has a high turnover rate and telomerase null mice have been suggested to have olfaction defects [Jaskelioff et al., 2011]. The anosmia in this female may thus be telomere-mediated and may represent a first association in humans. The high degree of penetrance seen in our families (83%) lies in contrast to a prior estimate of 15% [Knight et al., 1998], which predated elucidation of the genetic basis of DC and appreciation for its full phenotypic spectrum. Vigilance for telomere-related complications in heterozygous females with DKC1 mutations may therefore be warranted in certain clinical settings, and the possibility of telomere-dependent phenotypes should inform genetic counseling discussions. X-linked inheritance should in turn be considered in individuals with adult-onset telomere presentations, and in families with consecutively affected generations where male-to-male transmission is not evident.
Two mechanisms may contribute to telomere-mediated disease in female carriers. Telomere length is heritable, and X-linked DC females have shorter telomere lengths relative to age-matched controls [Mitchell et al., 1999; Parry et al., 2011a]. In the families we studied, the male-to-female pattern of transmission of the mutated gene, therefore, may have contributed to the penetrance of the telomere phenotypes in female carriers in contrast to families where female-to-female inheritance predominates. Short telomere length is sufficient to cause degenerative phenotypes in wild-type mice that inherit their telomere defects from telomerase mutant parents [Armanios et al., 2009; Hao et al., 2005]. Similarly, in autosomal dominant families, telomerase wild-type individuals of telomerase mutation carriers have short telomere length and lung disease has been described in some of these individuals, heretofore exclusively in the setting of a cigarette smoke exposure history [Alder et al., 2011a; Diaz de Leon et al., 2011]. It may also be that the dyskerin mutant cells in females contribute directly to disease risk in parenchymal organs. Although bone marrow-derived cells from heterozygous DKC1 mutation carriers show skewed X-inactivation patterns consistent with an advantage for cells that have intact telomerase levels, such a competitive advantage is not possible in parenchymal organs. It is therefore possible that female carriers of DKC1 mutations may be at risk for developing life-threatening telomere-mediated organ failure especially when they are exposed certain environmental toxins, albeit at low penetrance. In support of this model, none of the female carriers from X-linked families who have been studied in the Johns Hopkins Telomere Syndrome Registry have had evidence of hematologic abnormalities to date (zero of 10 total). However, one female smoker developed symptomatic lung disease by 45 years of age, and was diagnosed with emphysema. It is therefore possible that DKC1 heterozygous mutation carriers may be at risk for developing degenerative parenchymal disease, such as emphysema, fibrosis, or cancer, especially in the setting of certain environmental insults.
Our study highlights some of the limitations of current exome-sequencing technology for clinical use. Although the variant calling software detected one of the mutated variants, the other was missed due to low coverage and it was only detected after manual inspection of the candidate gene and confirmation by direct Sanger sequencing. To date, nine genes have been linked to the Mendelian telomere syndrome phenotypes [Armanios and Blackburn, 2012; Ballew et al., 2013; Le Guen et al., 2013; Walne et al., 2013], and of these, several had extremely poor coverage including TERT (24%–41% at 8× coverage) and TR (0% at 8×) relative to the entire exome (mean 70%; Fig. 2F). Although exome capture and sequencing technologies continue to rapidly evolve, this caveat (i.e., missed calls in low-coverage regions) may present one limitation of current platforms for clinical use because the coverage depth at individual genes is not yet routinely assessed or reported. These limitations should be considered in ongoing efforts to integrate new exome technology in clinical practice. Advances in capture and sequencing technologies along with whole-genome approaches may minimize these limitations in the future.
In summary, mosaic females with DKC1 mutations may be at risk for developing cutaneous and parenchymal telomere phenotypes. Longitudinal studies of carrier females will be important to fully define disease penetrance in these females.
Acknowledgments
We are grateful to the subjects who participated in these studies and to their families. We acknowledge Jennifer Meyers and Timothy Mosbruger for their assistance with exome sequencing and data analysis. Dr. Alder is a Parker B. Francis Foundation Fellow.
Disclosure statement: The authors declare no conflict of interest.




