NF1 Molecular Characterization and Neurofibromatosis Type I Genotype–Phenotype Correlation: The French Experience
Contract grant sponsors: Association Neurofibromatoses et Recklinghausen; Ligue Française Contre les Neurofibromatoses; the French Clinical Research program (PHRC, 2002); INSERM (Nf1GeneModif project); Ministère de l'Enseignement Supérieur et de la Recherche.
Communicated by Finlay A. Macrae
ABSTRACT
Neurofibromatosis type 1 (NF1) affects about one in 3,500 people in all ethnic groups. Most NF1 patients have private loss-of-function mutations scattered along the NF1 gene. Here, we present an original NF1 investigation strategy and report a comprehensive mutation analysis of 565 unrelated patients from the NF-France Network. A NF1 mutation was identified in 546 of the 565 patients, giving a mutation detection rate of 97%. The combined cDNA/DNA approach showed that a significant proportion of NF1 missense mutations (30%) were deleterious by affecting pre-mRNA splicing. Multiplex ligation-dependent probe amplification allowed the identification of restricted rearrangements that would have been missed if only sequencing or microsatellite analysis had been performed. In four unrelated families, we identified two distinct NF1 mutations within the same family. This fortuitous association points out the need to perform an exhaustive NF1 screening in the case of molecular discordant-related patients. A genotype–phenotype study was performed in patients harboring a truncating (N = 368), in-frame splicing (N = 36), or missense (N = 35) mutation. The association analysis of these mutation types with 12 common NF1 clinical features confirmed a weak contribution of the allelic heterogeneity of the NF1 mutation to the NF1 variable expressivity.
Introduction
Neurofibromatosis type 1 (NF1; MIM #162200) is an autosomal disorder with an estimated birth incidence of one in 3,500 [Carey et al., 1986]. The main features of NF1 are multiple neurofibromas, café-au-lait (CAL) spots, axillary freckling, Lisch nodules, tibial pseudarthrosis, and a predisposition to develop benign and malignant nervous system tumors [Ferner et al., 2007]. This complete penetrant Mendelian disorder is characterized by a highly inter- and intrafamilial variable clinical expressivity in both the number of major features and the occurrence of complications [Easton et al., 1993; Sabbagh et al., 2009; Szudek et al., 2002]. The mechanisms underlying NF1 clinical variability remain poorly understood, probably because of the involvement of complex physiopathology and multiple factors.
NF1 is caused by dominant loss-of-function mutations of the tumor suppressor NF1 (Neurofibromin 1; MIM #613113), located at 17q11.2 and containing 60 translated exons over ∼280 kb. Almost half of all NF1 cases are caused by de novo sporadic mutations. A huge number of different pathogenic NF1 mutations have been reported [Ars et al., 2003; Fahsold et al., 2000; Mattocks et al., 2004; Messiaen et al., 2000]. Among them, 5%–10% are large 17q11.2 deletions encompassing the entire NF1 locus and neighboring genes [Mautner et al., 2010; Pasmant et al., 2010]. These recurrent large NF1 deletions have been associated with more severe and atypical manifestations, the so-called “NF1 microdeletion syndrome” with learning disabilities and facial dysmorphism and a tendency of childhood overgrowth and malignant peripheral nerve sheath tumors (MPNSTs) increased susceptibility [De Raedt et al., 2003; Mautner et al., 2010; Pasmant et al., 2010].
For patients with intragenic NF1 mutations (more than 90% of all NF1 cases), no clear-cut allele–phenotype correlations have been established so far [Castle et al., 2003; Easton et al., 1993; Sabbagh et al., 2009] with the exception of a 3-bp inframe deletion (c.2970–2972 delAAT) in exon 17 of the NF1 gene that has been associated with the absence of cutaneous neurofibromas [Upadhyaya et al., 2007].
The aim of the present study was to first characterize constitutional NF1 gene mutations, through a comprehensive mutation screening, in 565 well-phenotyped index cases with typical NF1 enrolled in a French clinical research program. We then statistically evaluated the genotype–phenotype correlation in 439 of these patients harboring a truncating (N = 368), in-frame splicing (N = 36), or missense (N = 35) NF1 mutation to provide further insights into the contribution of intragenic NF1 mutations to the variable expressivity of NF1.
Materials and Methods
Patients
Families for this study were enrolled between 2002 and 2005 in a project of the French clinical research program entitled “Study of expressivity of NF1: constitution of a phenotype–genotype database” (French clinical research programme PHRC NF1, coordinator: Prof. Pierre Wolkenstein, Henri Mondor Hospital, Creteil, France). The study was approved by the local ethical committee and all participants gave their written informed consent.
The French NF1 database constituted through this program included a collection of 565 families, consisting of 1,697 individuals, among whom 1,083 fulfilled NIH (National Institutes of Health) diagnostic criteria for NF1 [Ferner et al., 2007; NIH Consensus Conference, 1988]. For each patient, the full phenotypic information was recorded in a standardized way by using a standardized case report file (available upon request).
A comprehensive NF1 mutation screening was performed in 565 well-phenotyped sporadic or familial index cases. For multiplex families, the first case of the second generation was considered as the index case and was first tested for molecular investigation. When nucleic acids were available from other affected/nonaffected members of the same family, segregation analysis were performed. When nucleic acids were available from only one affected member of a NF1 family, this patient was considered as the index case.
DNA and RNA Extraction
DNA was isolated from peripheral blood leucocytes using standard proteinase K digestion followed by phenol–chloroform extraction. RNA was directly extracted from 5 ml of PAXgene™whole blood samples (Becton Dickinson, Rungis, France) with the PAXgene™Blood RNA System (Qiagen, Courtaboeuf, France) according to the manufacturer's instruction.
Microsatellites Genotyping
Four NF1 intragenic polymorphic microsatellites (D17S1307, D17S2163, D17S1166, and IVS38-GT53.0) were typed. Samples with a unique haplotype were tested for large NF1 deletion analysis using real-time PCR-based gene dosage. Four additional NF1 extragenic polymorphic microsatellites (D17S841, D17S635, D17S1800, and D17S798) were also studied for segregation analysis. Primer sequences and PCR conditions have been previously described [Pasmant et al., 2008].
Real-Time PCR-Based Gene Dosage
We quantified NF1 exon targets by determining the threshold cycle (Ct) number at which the increase in the signal associated with exponential growth of PCR products begins. The primer oligonucleotide sequences for real-time PCR-based gene dosage are given in Supp. Table S1. We also quantified the ALB gene (encoding albumin and mapping to chromosome region 4q11–q13) as an endogenous DNA control, and each sample was normalized on the basis of its ALB content. The relative copy number of the NF1 exon targets was also normalized to a calibrator, consisting of genomic DNA from a normal subject. Final results, expressed as N-fold differences in the NF1 exon targets copy number relative to the ALB gene, and the calibrator were determined as follows: N-fold = 2(ΔCt sample – ΔCt calibrator), where ΔCt values of the sample and calibrator are determined by subtracting the average Ct value of the NF1 exon target from the average Ct value of the ALB gene. Given the NF1 exon target, samples with N-fold values of <0.7, 1.0, and >1.3 were considered deleted, normal, or duplicated, respectively. All PCRs were performed on a LightCycler® 480 with the LightCycler® 480 SYBR Green I Master kit (Roche Diagnostics, Meylan, France). The thermal cycling conditions comprised an initial denaturation step at 95°C for 10 min and 50 cycles at 95°C for 15 sec and 65°C for 1 min. Experiments were done with triplicates for each data point.
Multiplex Ligation-Dependent Probe Amplification Analysis
Single- and multi-exon deletions/duplications screening was performed by multiplex ligation-dependent probe amplification (MLPA) analysis using the SALSA MLPA kits P081/P082 NF1 as recommended in the manufacturer's protocol (MRC Holland, Amsterdam, The Netherlands). The two probe mixes included in this MLPA kit contain probes for all constitutive NF1 exons except in exons 5, 7, 17, 18, 45, and 47. One probe is also present in NF1 promoter and in intron 1. In addition, two probes are present in the OMG gene (located within NF1 intron 27b). Briefly, four control samples and each NF1 patient samples (each containing 100 ng of genomic DNA) were used for overnight hybridization with the probe mixes. After ligation and amplification performed with FAM-labeled primers, PCR products were analyzed on an ABI Prism 3130 automatic DNA sequencer (Life Technologies, Saint Aubin, France). Peak areas for each separated fragment were measured using Genemapper software v4.0 (Life Technologies). Normalization of peak areas of a sample was performed by dividing each peak area (NF1-specific probes and reference probes) by the total of reference probes peak areas of the sample. Normalized peak areas obtained for each probe of a sample were then divided by the average normalized peak areas of four control samples for the probe. Ratios of <0.7, 1.0, and >1.3 were considered deleted, normal, and duplicated, respectively. DNA samples with NF1 copy-number variation were submitted to real-time PCR-based gene dosage for confirmation.
NF1 Mutation Analysis by cDNA Sequencing Approach
RNA samples (2 μg) were reverse transcribed using 50 units of Superscript II RNase H- reverse transcriptase (Life Technologies, Saint Aubin, France), 10 units of RNasinTM Ribonuclease inhibitor (Promega, Madison, WI, USA), 10 mM dithiothreitol, and 1.5 mM pd(N)6 Random hexamer 5′phosphate (Amersham, GE Healthcare Biosciences, Pittsburgh, PA, USA). The samples were incubated at 20°C for 10 min and 42°C for 30 min. Reverse transcriptase was then inactivated by heating at 99°C for 5 min and cooling at 5°C for 5 min. The full cDNA was then amplified in eight overlapping fragments ranging from 1024 to 1755 bp, using Expand Long Template PCR System (Roche). Sequencing analysis was then performed on purified RT-PCR fragments using RT-PCR and internal primers with the ABI BigDye terminator sequencing kit v1.1 (Life Technologies) on an ABI Prism 3130 automatic DNA sequencer (Life Technologies). Sequences were aligned with Seqscape analysis software v2.5 (Life Technologies) and were compared with the corresponding cDNA reference sequence NM_000267.3. The primer oligonucleotide sequences for cDNA sequencing are given in Supp. Table S2. The first nucleotide of the first methionine codon is denoted position +1 according to the NF1 mRNA sequence NM_000267.3. Exons are named according to the nomenclature used in the NF1 field (exons numbered 1 to 49).
NF1 Mutation Analysis by DNA Approach
Mutational screening was performed using bidirectional DNA sequencing of the purified PCR of the NF1 60 exons, including alternative exon 23a, [Barron and Lou, 2012] and 500 bp of the proximal promoter region defined by functional analysis [Horan et al., 2004; Zou et al., 2004] as described above. The primer oligonucleotide sequences for DNA sequencing are given in Supp. Table S3. Sequences were aligned with Seqscape analysis software v2.5 (Life Technologies) and were compared with the corresponding genomic DNA reference sequence NC_000017.10 (nt 29,422,328 to 29,701,173 on Human, Feb. 2009 [GRCh37/hg19] Assembly). Mutations identified by the cDNA approach were confirmed using DNA sequencing analysis performed on the corresponding exon only.
Genotype–Phenotype Correlation Analyses
Genotype–phenotype correlation analyses were performed only on those patients with an identified constitutional NF1 mutation that fell into one of the three following categories: truncating mutations (including nonsense and frameshift mutations), missense mutations, and splice site mutations that produce an in-frame protein. Six mutations in our database, which do not affect splice sites, produce an in-frame protein. Since they result in the deletion of only one or two amino acids in the NF1 protein, it is unclear whether they can be considered as pathogenic mutations. They were thus not included in the “in-frame” category, which was composed of only splice mutations. Patients with large deletions encompassing the entire NF1 gene (N = 24) were excluded from analyses because this type of alteration causes a specific phenotype [Mautner et al., 2010; Pasmant et al., 2010]. Patients harboring splice site mutations producing more than one transcript (N = 12) were also excluded since the different transcripts may have different effects at the protein level. We did not include the three patients carrying a missense mutation predicted as “benign” by PolyPhen (c.787A>G, c.4879A>C, and c.6341C>T) as well as the three patients carrying the p.Met1? mutation whose impact on NF1 protein is unclear. As the subset of patients with de novo mutations may include some uncovered cases of somatic mosaicism as compared with patients with inherited mutations, we hypothesized that mutation inheritance may explain some of the clinical variability of NF1. Therefore, this variable was included in all regression models used in genotype–phenotype correlation analyses, along with sex and age at examination, to control for potential confounding. Patients with no available data on the inherited nature (de novo vs. inherited) of their constitutional NF1 mutation were thus excluded from analyses (N = 39). The final study sample included 439 unrelated NF1 patients: 368, 35, and 36 carrying truncating, missense, and in-frame splice mutations, respectively.
Twelve major clinical features of NF1 were selected for this study. Four were quantitative traits: number of CAL spots (as a continuous variable), number of plexiform neurofibromas (as a continuous variable), and number of cutaneous and subcutaneous neurofibromas (each classified into one of four semiquantitative categories based on the number of neurofibromas: 0, 1–9, 10–99, and ≥100). The other eight clinical features were coded as binary variables: presence/absence of skin-fold freckling, blue-red macules, Lisch nodules, macrocephaly, facial dysmorphism, scoliosis, optic gliomas, and MPNSTs development. Patients with missing data for a particular feature were coded as “unknown,” and were not then considered in models involving that feature. Most features were identified by physical examination, with Lisch nodules being diagnosed, or excluded, by slit-lamp examination; individuals not given a slit lamp examination were coded as “unknown.” The presence or absence of optic gliomas was determined by cranial magnetic resonance imaging (MRI) or computed tomography (CT) examination with individuals not given cranial imaging being coded as “unknown.” Macrocephaly was coded as “present” if the subject's head circumference was two or more standard deviations above the age- and sex-matched population mean. A facial dysmorphism was diagnosed if two or more of the following signs were observed: coarse face, flat occiput/brachycephaly, facial asymmetry, prominent forehead, frontal bossing, ptosis, downslanting deep set eyes, eversion of the lateral eyelid, epicanthic folds, high and broad nasal bridge, bulbous nasal tip, large- and low-set ears, malar hypoplasia, wide and prominent philtrum, micrognathia, small pointed chin, and low posterior hairline. For scoliosis, only scoliotic curves of >10° were taken into account in our analysis. The prevalence of many other clinical abnormalities (epilepsy, hydrocephalus, medullary compression by neurofibroma, cerebrovascular complications, renal artery stenosis, pseudoarthrosis, congenital pseudarthrosis of the tibia, dysplastic vertebrae, sphenoid wing dysplasia, and xanthogranuloma) was too low (≤5%) for meaningful statistical analysis.
The association of the type of NF1 mutation with each of the 12 clinical features individually was investigated through multiple regression analysis. Logistic regression analysis was used to calculate odds ratios (OR) and 95% confidence intervals for binary traits, whereas linear regression analysis was used for continuous traits. Patients with truncating mutations were the reference group in comparisons between different types of NF1 mutations; the mutation variable was then coded as a binary variable (missense and in-frame splice mutations, each compared with truncating mutations). All regression models included age at examination (as a continuous variable), sex, and mutation inheritance (de novo vs. inherited) as possible explanatory variables to control for potential confounding. The Bonferroni correction, by which the nominal alpha is adjusted upon the number of tests performed, was applied to account for multiple testing, resulting in a robust Bonferroni-corrected significance threshold of 0.002 (0.05 divided by 24 for 12 traits tested individually and two between-group comparisons). All statistical analyses were performed using StatView 5.0 software (Cary, NC).
Results
A comprehensive mutation screening of the NF1 gene was performed for probants and their relatives through intragenic NF1 microsatellites analysis, real-time PCR-based gene-dosage, and NF1 sequencing at both cDNA and DNA levels (Fig. 1). MLPA, and segregation analysis in familial cases, were also performed for NF1 negative patients.
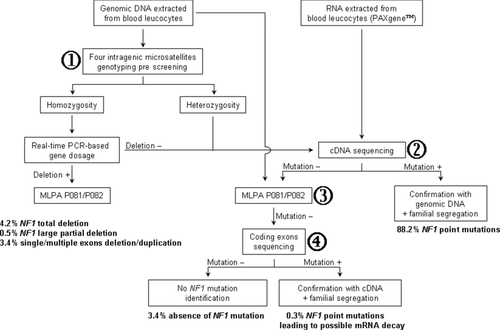
The molecular diagnostic strategy used in this study is presented in Figure 1. This strategy enabled the identification of an NF1 mutation in 546 out of 565 (96.6%) index cases.
Large NF1 Complete or Partial Deletion Identification by Intragenic Microsatellite Genotyping (Prescreening), Real-Time PCR Gene Dosage (Confirmation)
Analysis of four NF1 intragenic microsatellite markers (D17S1307 in intron 1, D17S2163 and D17S1166 in intron 27b, and IVS38-GT53.0 in intron 38) revealed a homozygous haplotype in 10% of cases (57/565), suggesting a potential NF1 deletion. Among 47% of them, real-time PCR gene dosage confirmed a complete (N = 24) or partial deletion (N = 3) of the NF1 locus. Patients with deletion involving the entire NF1 locus account for 4.3% (N = 24) of the French NF1 cohort [Pasmant et al., 2010]. The three other patients presented large (>three exons) partial NF1 deletions encompassing exons 3–39, exons 8–49, and exons 7–42, respectively.
NF1 Mutation Screening by Sequencing at Both cDNA and DNA Levels
NF1 point mutation screening, performed at both cDNA and DNA levels, identified a NF1 alteration in 507 out of 565 index cases. Table 1 summarizes the distribution of the different NF1 mutation types identified in the French cohort. About one-third were small insertions and/or deletions (N = 170), most of them with frameshift consequences (N = 164). Splice alterations accounted for 26% (N = 148), with frameshift (N = 111) or in-frame (N = 37) consequences. Splice alterations are detailed in Figure 2. Nonsense mutations corresponded to ∼21% of the mutations identified in our study (N = 118), whereas missense mutations accounted for 7.5% (N = 42). Deletion or duplication of one, two, or three exons accounted for less than 2% (N = 7). Other mutation types accounted for ∼4% (N = 22) and corresponded to rare complex mutations leading to the initiation codon modification (N = 3), two different abnormal transcripts (N = 15), instable transcripts (N = 2), or with multiple nucleotide substitutions (N = 2).
| NF1 mutation type | Number (total: 565) | Distribution among index cases |
|---|---|---|
| Missense | 42 | 7.5 |
| Nonsense | 118 | 20.9 |
| Frameshift short deletion and/or insertion | 164 | 29 |
| In-frame short deletion and/or insertion | 6 | 1.1 |
| Splice frameshift | 111 | 19.6 |
| Splice in-frame | 37 | 6.5 |
| Deletion or duplication of single/multiple exonsa | 22 | 3.9 |
| Complete NF1 gene deletion | 24 | 4.2 |
| Complex mutationsb | 22 | 3.9 |
| No mutation | 19 | 3.4 |
- Distribution of the different NF1 mutation types was performed according to the observed effects of the mutations at the mRNA level.
- a NF1 single- and multiexon deletions/duplications were screened by MLPA analysis. Large partial (>three exons) NF1 deletions were also identified by microsatellites prescreening and real-time PCR before any sequencing investigation (N = 3). One, two, or three exon deletions/duplications were also identified by cDNA sequencing approach (N = 7); the remaining 12 deletions/duplications were identified only by MLPA analysis.
- b Were considered “complex” those mutations leading to the initiation codon modification (p.Met1?; N = 3), two different abnormal transcripts (N = 15), instable transcript (N = 2), or multiple nucleotide substitutions (N = 2).
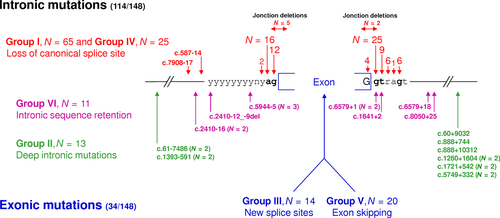
Missense variants accounted for 7.5% (N = 42). Among these 42 missense variants, 39 of them were classified as deleterious mutation by following all the four next arguments: (1) the variant was unique in the coding sequence; (2) Polyphen-2 Software predicted a “possibly damaging” or a “probably damaging” impact of the variant on the structure and function of the protein (http://genetics.bwh.harvard.edu/pph2/); (3) the variant was not referred as a polymorphism of the human genome in the dbSNP database; and (4) the variant was absent of exome sequencing of 4300 European American and 2202 African American controls (http://evs.gs.washington.edu/EVS/). Segregation analysis is an important criterion to assign pathogenecity to the variants; also, cosegregation investigations have been performed in familial cases, and de novo event have been verified for sporadic cases when both parents DNA were available. Variants reported by other groups in NF1 patients were also considered. These points were summarized in Supp. Table S4.
Mutations distribution across the NF1 gene did not exhibit any hot-spot domain (Supp. Fig. S1). The most recurrent mutation (c.499_502delTGTT) has been identified in 12 unrelated patients (only 2% of the 546 mutations). This mutation is localized in exon 4b and corresponds to a tetranucleotide deletion of a tandem repeat. The 363 distinct point mutations (Supp. Table S5) were reported according to the standard human genome variation society nomenclature (http://www.hgvs.or/mutnomen/). These mutations have been deposited in the Leiden Open Variation Database (https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=NF1). Among the 363 distinct point mutations, only 62 were recurrent.
NF1 Mutation Screening by MLPA Analysis
NF1 single- and multiexon deletions/duplications screening, performed by MLPA analysis, identified NF1 partial deletions/duplications in 22 out of 565 index cases (∼4%). Among these 22 deletions/duplications, three were also identified by microsatellites prescreening and real-time PCR before any sequencing investigation and seven were also detected by the cDNA analysis (cf above). The 12 remaining deletions/duplications were only detected by MLPA analysis.
Genotype–Phenotype Correlations
Genotype–phenotype correlations were statistically evaluated in 439 unrelated NF1 patients harboring either a truncating (N = 368), missense (N = 35), or in-frame splice (N = 36) NF1 mutation (Table 2). The study cohort consisted of 206 men (47%) and 233 women (53%) with a mean age at examination of 29.9 ± 16.7 years. Around 30% (130/439) of patients had de novo mutations, adjudged on the basis of the absence of a clinically affected parent. This prevalence is quite low compared with the usually reported proportions (∼50%) in the literature for the general NF1 population. This discrepancy may be explained by the preferential recruitment of familial cases, in the constitution of the French NF1 cohort. There were no significant differences in age, sex, and proportion of de novo mutations (P > 0.05) between the different groups of patients classified according to the NF1 mutation type (truncating mutations, missense mutations, and in-frame splice mutations). The clinical characteristics of the study population are provided in Table 3.
| Patients with truncating mutations | Patients with missense mutations | Patients with in-frame splice mutations | Total | |
|---|---|---|---|---|
| Number | 368 | 35 | 36 | 439 |
| Mean age at examination in years (±SD) | 29.3 (±16.7) | 32.2 (±18.2) | 33.7 (±15.5) | 29.9 (±16.7) |
| Gender | ||||
| Men | 168 (45.7%) | 16 (45.7%) | 22 (61.1%) | 206 (46.9%) |
| Women | 200 (54.3%) | 19 (54.3%) | 14 (38.9%) | 233 (53.1%) |
| Mutation inheritance | ||||
| De novo | 106 (28.8%) | 13 (37.1%) | 11 (30.6%) | 130 (29.6%) |
| Inherited | 262 (71.2%) | 22 (62.9%) | 25 (69.4%) | 309 (70.4%) |
| Clinical feature | Study sample (N = 439) |
|---|---|
| Cutaneous neurofibromas | |
| 0 | 32.6% |
| 1–9 | 21.2% |
| 10–99 | 27.3% |
| ≥100 | 18.9% |
| Subcutaneous neurofibromas | |
| 0 | 43.8% |
| 1–9 | 42.9% |
| 10–99 | 10.7% |
| ≥100 | 2.5% |
| Number of plexiform neurofibromasa | 0.7 ± 1.0 |
| Number of CAL spotsa | 22.8 ± 15.2 |
| Skin-fold freckling | 89.5% |
| Blue-red macules | 14.8% |
| Lisch nodules | 62.2% |
| Macrocephaly | 13.4% |
| Facial dysmorphism | 11.7% |
| Scoliosis | 38.3% |
| Optic gliomas | 16.1% |
| MPNSTs | 2.7% |
- a Data are expressed as mean ± standard deviation.
- CAL, café-au-lait; MPNSTs, malignant peripheral nerve sheath tumors.
Multiple regression analysis, in which age, sex, and mutation inheritance were included as covariates along with the type of NF1 mutation, revealed a significant effect of age at examination, measured as a quantitative variable, for Lisch nodules (P < 0.0001), scoliosis (P = 0.0007), cutaneous (P < 0.0001), subcutaneous (P < 0.0001), and plexiform neurofibromas (P < 0.0001), with an increasing prevalence or number of these features with increasing age. By contrast, the prevalence of optic gliomas and the number of CAL spots were found to significantly decrease with age (P = 0.0003 and P = 0.005, respectively). These findings are consistent with previous observations made on independent cohorts of NF1 patients [Castle et al., 2003; Easton et al., 1993; Szudek et al., 2002]. No significant effect of sex was observed for any clinical feature, except for macrocephaly, which was more prevalent in men compared to women (17% vs. 10%; P = 0.03). Finally, the inherited nature of the NF1 constitutional mutation was found to significantly impact the prevalence of facial dysmorphism (7% in familial cases vs. 23% in sporadic cases, P < 0.0001), MPNSTs (1% vs. 6%, P = 0.009), and scoliosis (35% vs. 46%, P = 0.004) in our study population. Two clinical features, Lisch nodules and CAL spots, exhibited a significant association with the type of constitutional NF1 mutation at a 0.05 significance threshold, after adjusting for age at examination, sex, and mutation inheritance. NF1 patients with truncating mutations had a higher prevalence of Lisch nodules when compared with NF1 patients with missense mutations (63.2% vs. 45.8%, P = 0.023, OR = 2.9 [1.2–7.3]). Similarly, a higher number of CAL spots were observed in patients with truncating mutations compared with patients with missense mutations (23.5 ± 15.6 vs. 16.6 ± 10.3, P = 0.016). However, these associations were no longer statistically significant after applying the Bonferroni correction for multiple comparisons. No significant associations were observed for any other clinical features.
Discussion
Here, we report our French experience of the NF1 gene mutation screening in a large cohort of NF1 patients. In this study, 565 typical NF1 index cases were analyzed using a cascade of complementary techniques and a NF1 alteration was identified in 546 of 565 index cases (96.6%). Hence, high mutation detection sensitivity can be achieved if well-phenotyped NF1 patients are studied with multiple complementary and optimized techniques (Fig. 1). The first step of the molecular diagnostic strategy is the study of four intragenic microsatellites located in introns 1, 27 (N = 2), and 38. When homozygous haplotypes suggest a NF1 large deletion, gene dosage by real-time quantitative PCR of exons 6, 21, 28, and 48 is performed to identify NF1 large deletion. When a large NF1 deletion is excluded (with at least one heterozygous microsatellite), the second step is the study of the NF1 transcript. After reverse transcription, eight overlapping PCR fragments are generated. At this step, abnormal polyacrylamide electrophoretic profiles can be observed (∼33%). Sequence analysis of cDNA is performed on PCR products using a panel of 25 sequencing primers covering the entire 8,520-bp NF1 coding sequence. When a molecular abnormality is identified at the cDNA level, a confirmation is made at the genomic DNA level (Fig. 1). When a molecular abnormality is not identified at the cDNA level, exons deletion/duplication screening is performed using MLPA. In case of a negative result, mutation screening is performed by sequencing the 60 coding exons at the DNA level.
The combined cDNA/DNA sequencing can increase mutation detection sensitivity. A total of 507 alterations were identified at both cDNA and DNA levels. Among these 507 alterations, only 487 would be identified if the single DNA sequencing approach were used. The 4% of mutations missed by the single DNA sequencing approach are due to the presence of deep intronic mutations (N = 13) and exons deletions (N = 7). Among these 507 alterations, only 505 would be identified if the single cDNA sequencing approach were used. The 0.4% of mutations missed by the single cDNA sequencing approach is potentially due to mRNA decay.
cDNA sequencing was performed using RNA extracted from PAXgene™Blood RNA System (Qiagen) allowing stabilization of RNA at the time of sample collection, and avoiding artifacts introduced during sample handling. This combined approach allowed to assess the impact of DNA mutations on NF1 mRNA. In the present study, a single DNA sequencing approach would have identified 138 NF1 nonsense mutations. The complementary cDNA sequencing approach showed that 19 mutations out of these 138 (13.7%) actually disturbed NF1 transcript splicing. Similarly, a single DNA sequencing approach would have identified 61 NF1 missense mutations. The complementary cDNA sequencing approach showed that 19 mutations out of these 61 (31.1%) actually disturbed NF1 transcript splicing. It thus appears that a significant proportion of NF1 missense mutations are deleterious by affecting normal pre-mRNA splicing. As previously described, the characterization of mutations at both cDNA and DNA levels enables to detect a broader spectrum of mutations than any single level approach, and provides a greater understanding of their molecular pathogenesis [Messiaen et al., 2000; Messiaen and Wimmer, 2008; Pros et al., 2008; Valero et al., 2011; Wimmer et al., 2011].
Deletion or duplication of single or multiple exons accounted for ∼4% (N = 22) of all NF1 alterations, in accordance with literature data [Wimmer et al., 2006]. MLPA allowed the identification of such restricted rearrangements that would have been missed by cDNA/DNA sequencing or microsatellite analysis.
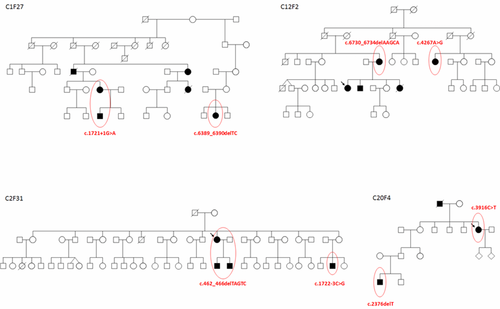
In four unrelated families, NF1 molecular investigation allowed us to identify the fortuitous association of two distinct NF1 mutations in the same family (Fig. 3). Because NF1 is a relatively common autosomal dominant disorder and because the mutation rate in NF1 is among the highest known for human disorders [Clementi et al., 1990], it is therefore possible to observe two distinct mutations arising through independent mutational events within a single family. The occurrence of two different mutations of the NF1 gene in one family has indeed been previously described, due to the coincidence of two de novo mutations in NF1 [Klose et al., 1999]. Our observation emphasizes the need to perform an exhaustive NF1 screening in molecular discordant cases within a family.
Our molecular diagnostic strategy did not allow detection of any NF1 molecular alteration in 19 out of the 565 index cases with a clinical NF1 diagnosis. Among them, two out of the 19 index cases were previously found to carry a SPRED1 mutation [Pasmant et al., 2009]. The 17 out of the 565 (3%) remaining negative index cases (nine sporadic cases and eight familial cases) could be analyzed with a whole-exome sequencing approach, using next-generation sequencing technologies, to search for causal mutations in other loci in the genome. However, the presence of an unknown mutation alteration of the NF1 locus in these patients cannot be fully excluded. The haplotype phase was determined in five out of the eight negative families. A segregation analysis did not allow excluding the NF1 locus (Supp. Fig. S2), probably due to the small size of the families that hampers a meaningful Lod-score analysis. This observation also points out the need for a more comprehensive NF1 molecular diagnostic strategy. For example, a cytogenetic analysis (karyotype or fluorescence in situ hybridization) should be performed in negative patients, to exclude potential genomic rearrangements that would have been missed by molecular screening. The precise localization of NF1 to 17q11.2 was historically characterized by the identification of a balanced translocation [Ledbetter et al., 1989]. Another explanation for the negative NF1 screening could be mosaicism. When possible, the first case of the second generation in negative multiplex families was considered as the index case and was first tested for molecular investigation. When DNA was available from different affected/nonaffected members in the same family, segregation analyses were performed and did not reveal any discrepancies. Other groups have described a different mutation detection rate in sporadic cases compared with familial cases, probably due to a mosaicism in the first case of NF1 in a family [Kehrer-Sawatzki and Cooper, 2008; Maertens et al., 2007].
NF1 is characterized by marked inter- and intra-familial variable expressivity [Easton et al., 1993; Sabbagh et al., 2009; Szudek et al., 2002]. The high allelic heterogeneity of the constitutional NF1 mutation may explain some of this variability. A more severe phenotype has indeed been described in patients with large deletions of the NF1 gene region, particularly in respect to the presence of learning disabilities, facial dysmorphic features, and cardiovascular malformations, as compared with patients carrying intragenic NF1 mutations [Mautner et al., 2010; Pasmant et al., 2010; Venturin et al., 2004]. However, in the case of intragenic NF1 mutations, no clear-cut allele–phenotype correlations have been established so far, with the exception of a 3-bp in-frame deletion (c.2970–2972 delAAT) in exon 17 of the NF1 gene, which was associated with a particular clinical phenotype characterized by the absence of cutaneous neurofibromas [Upadhyaya et al., 2007]. We were not able to verify this association in our own cohort since this mutation was not found in any of the NF1 patients investigated. But, we carefully examined the clinical characteristics of groups of patients harboring the same NF1 detrimental mutation so as to highlight potential associations between a specific mutation and a particular phenotype. By considering the five most recurring mutations in the French NF1 cohort (i.e., those that occurred in more than 10 patients in at least five independent families), namely c.910C>T, c.4537C>T, c.6709C>T, c.6792C>A, and c.499_502delTGTT, we did not observe any differences in the prevalence and number of the twelve clinical features examined between each of the five groups of patients (ranging from 11 to 24 subjects) and the overall study sample (all P > 0.10).
As the great majority of NF1 alterations are private mutations, unique to a single individual or kindred, genotype–phenotype correlations in NF1 can be more readily examined by categorizing NF1 mutations on the basis of the type of molecular defect. To our knowledge, only two previous studies have investigated the influence of the type of constitutional NF1 mutation on the disease phenotypic variability. Castle et al. (2003) statistically evaluated genotype–phenotype correlations in 110 NF1 patients by considering three main types of NF1 mutations (missense and splice site mutations, each compared with truncating mutations). De Luca et al. (2004) examined possible correlations between mutation type and various clinical signs in a sample of 51 unrelated Italian NF1 patients, considering four main mutation groups (nonsense, frameshift, splicing, and missense mutations). However, in both studies, the small sample size strongly limited the power to detect an association between a NF1 mutation type and distinct clinical features (particularly in the case of missense mutations that account for less than 10% of all mutations), and no associations were found at the 0.05 significance threshold (uncorrected for multiple testing). The strength of the present study is to provide a large sample of NF1 patients (N = 439) fully characterized for both constitutional NF1 gene mutations and clinical data, thereby allowing a thorough investigation of genotype–phenotype correlations in NF1 disease. This is the largest sample ever studied in that respect. We reported two significant genotype–phenotype correlations at the uncorrected 0.05 threshold. Our association findings suggest a tendency for truncating mutations to be associated with (1) a greater incidence of Lisch nodules (P = 0.023) and (2) a larger number of CAL spots (P = 0.016) as compared with missense mutations. However, since these results were no longer significant after applying the Bonferroni correction for multiple comparisons, they should be considered as principally negative and replications in independent cohorts are needed to confirm these associations. It is nevertheless interesting to note that the suggestive association of missense mutations with a lower frequency of Lisch nodules is consistent with the exploratory finding of Castle et al. (2003) who found a relative risk of Lisch nodules less than one, and of borderline statistical significance (P = 0.06), in NF1 patients with missense mutations as compared with patients with truncating mutations (including nonsense and frameshift mutations), after adjusting for age at examination [Castle et al., 2003]. Therefore, our result can be seen as a replication of the association found by Castle et al. (2003) in an independent population of NF1 patients. This strengthens the significance of the finding that is more likely to represent a true causal relationship rather than a spurious finding. Nevertheless, this association will be of limited predictive utility in clinical practice as it will be impossible to predict the development of Lisch nodules on the sole basis of the presence of missense mutations in the NF1 gene. Moreover, since no clear genotype–phenotype correlations have been found for the most clinically significant disease features of NF1, such as the different types of neurofibromas and cancers, the knowledge of the NF1 mutation type is not expected to provide any valuable prognostic information to patients and clinicians in the future.
However, it is important to point out the several limitations of the present study that may explain the failure to find any statistically significant association of NF1 clinical features with mutation type. First, despite the large sample size, the number of patients with missense and in-frame splicing mutations (N = 35 and N = 36, respectively) are still limited and provide low statistical power to detect significant associations, in particular given the multiple tests performed. Second, the classification of mutations into three main groups is maybe too crude and may need to be refined. The previous two examples of genotype–phenotype correlation recognized in NF1 indeed concerned patients with a single recurrent mutation (c.2970–2972 delAAT) or a deletion of the entire NF1 gene, which represent much more homogeneous groups. Even by considering only a few main groups of mutations, the choice of the most relevant categories to consider is questionable. This is particularly true for splicing mutations, which can have different consequences at the protein level, although they all impact mRNA processing. We decided to split these mutations according to their predicted effect on the NF1 protein, splicing mutations leading to premature stop codons being pooled with the other truncating mutations. However, one could have chosen to consider this group as a whole, as Castle et al. (2003) and De Luca et al. (2004) did in their studies. It is reassuring to see that when we considered these mutations (including both in-frame and frameshift splice site mutations) as a separate group, the suggestive associations of truncating mutations (N = 265) with a greater incidence of Lisch nodules and a larger number of CAL spots as compared with missense mutations (N = 35) were still found (P = 0.035 and P = 0.011, respectively). The different ways to group splicing mutations thus do not change the major findings of the genotype–phenotype correlation study.
These results are consistent with our previous findings showing a limited contribution of the allelic heterogeneity of the constitutional NF1 mutation to the variable expressivity of the disease [Sabbagh et al., 2009], with the exception of those patients with the NF1 microdeletion syndrome. In particular, the variance component attributable to the nature of the NF1 mutation was estimated to be low for CAL spots and null for the different types of neurofibromas. These observations suggested that genetic modifiers, unlinked to the NF1 gene, contribute to the variable expression of NF1 [Pasmant et al., 2011; Pasmant et al., 2012].




