The TREAT-NMD Duchenne Muscular Dystrophy Registries: Conception, Design, and Utilization by Industry and Academia
Contract grant sponsor: European Commission and TREAT-NMD (FP6 LSHM-CT-2006–036825, 20123307 UNEW_FY2013, AFM (Association Francais contre les Myopathies) 16104).
Communicated by Mark H. Paalman
ABSTRACT
Duchenne muscular dystrophy (DMD) is an X-linked genetic disease, caused by the absence of the dystrophin protein. Although many novel therapies are under development for DMD, there is currently no cure and affected individuals are often confined to a wheelchair by their teens and die in their twenties/thirties. DMD is a rare disease (prevalence <5/10,000). Even the largest countries do not have enough affected patients to rigorously assess novel therapies, unravel genetic complexities, and determine patient outcomes. TREAT-NMD is a worldwide network for neuromuscular diseases that provides an infrastructure to support the delivery of promising new therapies for patients. The harmonized implementation of national and ultimately global patient registries has been central to the success of TREAT-NMD. For the DMD registries within TREAT-NMD, individual countries have chosen to collect patient information in the form of standardized patient registries to increase the overall patient population on which clinical outcomes and new technologies can be assessed. The registries comprise more than 13,500 patients from 31 different countries. Here, we describe how the TREAT-NMD national patient registries for DMD were established. We look at their continued growth and assess how successful they have been at fostering collaboration between academia, patient organizations, and industry.
Introduction
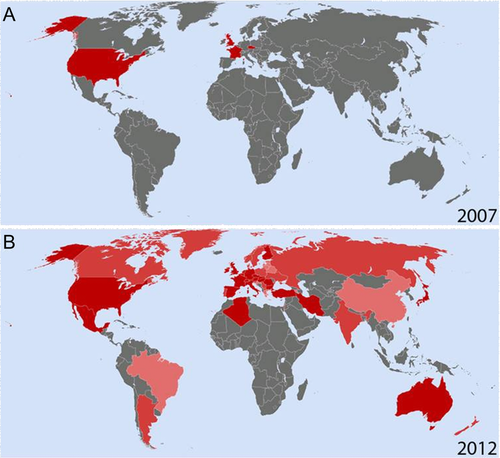
Duchenne muscular dystrophy (DMD; MIM #310200) is the most frequent pediatric muscle disorder [Ahn and Kunkel, 1993]. It is an X-linked genetic disease with an incidence of one in every 3,500 to 5,000 male births [Dooley et al., 2010; Emery, 1993]. DMD is caused by the absence of the protein dystrophin [Koenig et al., 1987] (DMD; MIM #300377). Affected boys become symptomatic at an early age (2–5 years of age) because of weakness in their proximal muscles, leading to an abnormal gait and frequent falls [Gowers, 1892]. DMD boys who are not steroid treated are in a wheelchair at a mean age of 9.5 years, and with steroids are often wheelchair bound by their teens [Humbertclaude et al., 2012]. Death occurs typically in the second or third decade due in part to respiratory failure though ventilation and other treatments are delaying death in many countries into the fourth or fifth decades [Baxter, 2006; Emery, 2002]. Currently there is no cure for DMD, but several novel treatment strategies are currently under evaluation in clinical trials including cell therapy, gene replacement, exon skipping, and the repurposing of existing drugs. Current care recommendations (such as the use of corticosteroids, cardiac medications, and assisted ventilation) improve outcomes and quality of life but do not modify the underlying progression of the disease [Hoffman et al., 2012; Sejerson et al., 2009]. Examples of more innovative treatment strategies include exon skipping, an RNA-based technology with the potential to target up to 83% of patients with deletions in the DMD gene [Aartsma-Rus et al., 2009; Beroud et al., 2007; van Ommen and Aartsma-Rus, 2013]. This and other DNA- or RNA-directed treatments require a greater understanding of the genetic basis and variability of a particular disease state. For the diagnosis of DMD, it is no longer standard of care to simply state that a patient is missing dystrophin in the muscle biopsy; a precise genetic diagnosis is required [Bushby et al., 2010; Sejerson et al., 2009]. Genetic diagnosis is confirmed by multiplex ligation-dependent probe amplification or associated sequencing of the dystrophin gene, reviewed in White and den Dunnen (2006). At the population level, DMD is a rare disease (prevalence <5/10,000). Individual countries have small pools of affected patients. Often the patient pool is too small for the initiation of clinical trials or for meaningful statistical analysis of current and historical research data [Brabec et al., 2009]. As with other rare diseases, individual groups have therefore opted to share patient information in the form of patient registries to increase the overall patient cohorts on which clinical outcomes and new technologies can be assessed [Sarkozy et al., 2008]. Before 2007, there were a number of independent registries already in existence for DMD (Fig. 1A). These included the French UMD Duchenne database (www.umd.be), the UK Parent Project (later Action Duchenne) Duchenne registry, the Czech National DMD/BMD registry, and the United Dystrophinopathy Project (UDP) in the USA. The total number of patients represented in these original registries was approximately 2,500. The information stored within these registries, while extremely important, was not freely available to the wider scientific community. There were also significant differences in the types of patient data collected and stored in each registry, and not all registries had consent forms, IRB approval, or data protection act approval, and so on. Differences also existed in the number of questions asked in these original registries, with some asking as few as 36, whereas others collected information on as many as 830, data fields. It was widely agreed that improvements could be made to patient registries if a more standardized and harmonized set of registries could be achieved. Discussions amongst patients, patient advocacy groups, and clinicians highlighted the need for a more harmonized approach to patient registries. This was further highlighted by discussions within the European Neuromuscular Centre (ENMC), where clinical and scientific experts outlined a framework for existing and emerging DMD registries [Mercuri et al., 2008]. What followed was the formation of a “charter” for DMD registries lead by TREAT-NMD. TREAT-NMD was initially established as an EU funded ``network of excellence” with the remit of “reshaping the research environment” in the neuromuscular field [http://www.treat-nmd.eu/, ; Bushby et al., 2009]. Initial TREAT-NMD milestones for disease-specific registries included: defining the data content for each disease/gene, defining the regulatory and ethical framework, identifying and analyzing existing national registries, and training curators for quality control of new and existing registries. The primary objective for a harmonized set of registries was to allow feasibility assessment, planning, and recruitment for clinical trials. Secondary objectives included collecting epidemiological data, establishing genotype–phenotype correlations, defining the natural history, and assessing treatment outcomes and standards of care. Ultimately, discussions under the auspices of TREAT-NMD decided upon a series of mandatory and highly encouraged items to be collected by each registry. TREAT-NMD registries also agreed to adhere to legal/ethical best practices allowing for patient feedback, possibility of data withdrawal from a registry, and encryption of data sets.
Methods and Results
TREAT-NMD was established in 2007 with the aim of “reshaping the research environment” in the neuromuscular field and to support translational research in NMD. This was achieved in part by the formation of a harmonized set of national DMD patient registries with a common data sharing philosophy, comprising both new registries set up to follow the TREAT-NMD guidelines and existing national registries who agreed to follow them. Information collected follows a mandatory or highly encouraged set of questions agreed on by the TREAT-NMD global database oversight committee. As a result, information can be shared and compared between the different national registries, with the ultimate goal of all national registries eventually linking into a centralized global DMD registry. We present here a systematic review of the content and activities of the DMD registry members in August 2012 collected via a comprehensive questionnaire (Supp. Fig. S1). All countries with active patient registries (31 countries representing 33 registries) replied to the questionnaire however there are still a number of countries (approximately 11) with DMD patient registries either planned or under construction at this time.
Purpose of the Registry
Registries reported multiple functions ranging from therapeutic intervention and scientific development to strategic planning (Fig. 2). The most prevalent use of registries included clinical research and recruitment of patients to clinical trials (94%), epidemiological research (83%), natural history surveys and disease surveillance (75%), genotype/phenotype analysis (66%), and mutation data collection (65%). Forty-nine percent of registries reported using the registry for social planning and healthcare services planning.
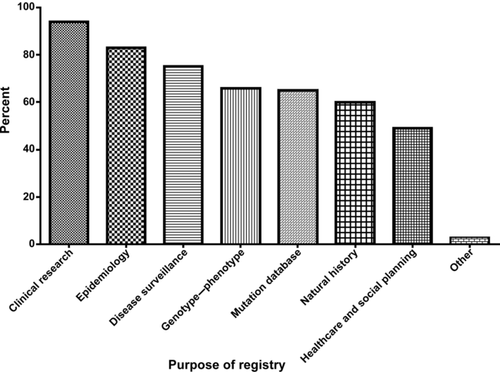
Characteristics of the Registries
Geographical patient demographics
The registries contained >13,500 patients from 31 different countries. The smallest registry consisted of 18 patients and the largest of 3337 patients (Fig. 3 and Supp. Fig. S2). Seventy-five percent of registries report geographical coverage at the national level, 20% at the regional level (a specific region within a country), and 5% either at the international or EU level. Coverage of the patient population in the different countries varies. For example, the registry from Denmark includes >95% of Danish DMD patients due to the infrastructure (all patients are recruited into a registry), and organization of their healthcare system (where clinicians enter registry data). A smaller percentage of patients are enrolled in those countries where patients or families have to initiate registry enrolment, but further work is needed to determine the completeness of the data sets for each country (i.e., the percentage of DMD patients from each country enrolled in their national registry). Three countries have more than one DMD registry (Spain, Italy, and the United States). A number of registries represent patients from more than one country; for example, the German registry also represents patients from Austria. Australia and New Zealand collaborated, with Australia providing logistic support and data storage for New Zealand's otherwise independent database [Rodrigues et al., 2012].

Funding sources for setting up registries
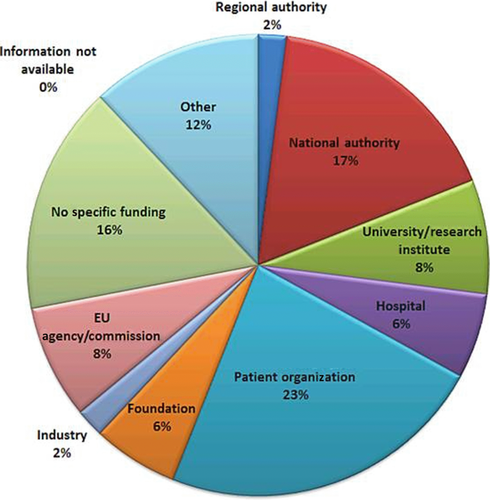
TREAT-NMD made a direct financial contribution to the funding of the registries. Funding from the original FP6 grant was 1,000,000€ in total (200,000€ per year over 5 years). TREAT-NMD partially funded national registries in four countries and contributed to the coordination efforts of the global registry. National registry responses to the TREAT-NMD questionnaire indicated that initial funding provided to set up the registries came from a variety of sources ranging from personal donations to designated national and regional funding (Fig. 4). 30% of registries were set up with funding from more than two sources; for example, Finland received funds from patient organizations (Lihastautiliitto), a university/research institute (The University of Helsinki, Turku University Central Hospital), the EU agency/commission (TREAT-NMD), and other funding sources (private donations). The amount of money used for initial funding of the registries was variable with some registries being set up with ≤3,000€, whereas others had funds in excess of 200,000€. The median amount of money invested to set up a registry was 27,435€. Australia was a slightly different case with its initial funding coming from the Department of Health at the Government of Western Australia, with guaranteed funding up until 2014. Generally, we found that there was a shift from national funding (e.g., local government initiatives) to university/research institute or private funding after the initial funding period expired. An example would be Iran, who had funding from the Molecular Medicine Network 2010–2011 but now are limited to funding acquired by the registry curator. Eight percent of registries had their funding reduced after the initial funding period expired (generally 1–3 years), whereas only 3% of registries saw an increase in funding.
Annual funding of the registries was highly variable with 28% of registries reporting no annual funding at all. Twenty-five percent were funded with 50,000€ or less, 19% with 51,000–100,000€, 5% with 101,000–200,000€, and 3% with 501,000–1M€. A further 5% reported their annual funding as coming from donations and benefactors. The total amount of annual funding for all of the registries for 1 year was in excess of 1,610,000€.
Provision and entry of data
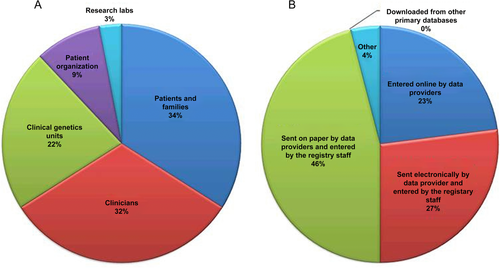
Most of the registries obtained data directly from the patients and their families (34%) or clinicians (32%) (Fig. 5A). Clinical genetics units provided 22% of the data reported. Patient organizations provided 9% of the data collected, whereas occasionally data were provided by research laboratories (3%). Data entry methods were also variable with the plurality of registries capturing data on paper for later entry by registry staff (46%), whereas 23% of data providers directly uploaded their data online (Fig. 5B). Data are updated annually in 88% of registries (29 of 33 that replied).
Mandatory and highly encouraged data requested by TREAT-NMD
TREAT-NMD suggested a series of mandatory and highly encouraged items to be collected by each registry including patient demographics; molecular diagnosis; clinical diagnosis (DMD versus Becker muscular dystrophy); motor function; use of steroids; history of scoliosis surgery; cardiac and respiratory function; clinical trial participation; muscle biopsy; and family history. Genetic diagnosis was collected by all registries. Consistent with previously published data and databases [Brabec et al., 2009; Cunniff et al., 2009; Nakamura et al., 2013; Tuffery-Giraud et al., 2009], DMD mutations observed were mainly large deletions and duplications of one or more exons, with a smaller proportion of point mutations or more complex rearrangements. These questions were chosen (after consultation with TREAT-NMD partners) to maximize the versatility and functionality of the registries for clinical, academic, and industrial applications. Thirty-eight percent (12 of 33) of registries collected data on all of the required fields, 78% (26 of 33) reported on more than five of the required fields, whereas 30% (10 of 33) reported less than five of the required fields, focusing on molecular data and family history. Fifty-two percent (17 of 33) of registries included data over and above that required from TREAT-NMD including natural history, CK values for female carrier, medications, vitamins/supplements, gastrointestinal complications, and cognitive status.
All of the registries questioned reported that their registry had been established following TREAT-NMD guidelines, either as part of a research project or following autonomous initiatives (clinician or patient driven). Only 11% (four registries) reported a legal obligation to set up a registry or to comply with regulatory requirements.
In cases where the registry would be terminated, 28% of registries reported that they would either archive the registry for an undetermined amount of time or reported having no policy in place for termination. Three percent had a policy in place to destroy the registry upon termination, whereas 8% planned to archive for a predetermined amount of time. 14% would make their terminated data available to other registries.
Communication activities of the registries occurred through multiple channels. Registries that maintained a Website tended to have it available at all times. The publication of newsletters tended to be monthly or bimonthly. One example is the Canadian Neuromuscular Disease Registry (www.CNDR.org), which produces a bimonthly newsletter, available in English and French, highlighting registry composition, study enquiries, scientific discoveries, and upcoming conferences. Overwhelmingly, registries reported their efforts to publish in scientific journals and communication at scientific meeting as being as “often as possible” with several publications linked directly to TREAT-NMD. Examples of publications that cite TREAT-NMD include [Griggs et al., 2013; Nakamura et al., 2013; Hollingsworth et al., 2012; Nakamura et al., 2011; Scully et al., 2013; Straub et al., 2012].
The numbers of staff employed by registries was variable but the majority of registries reported between one and four administrative staff working directly on the registries. Sixty percent of registry employees were part-time or were not primarily employed by the registry. Many of the registries also employed part-time medical staff. Only 11% (four of 33) of registries reported employing dedicated, full time clinical personnel.
Roughly two of three of the registries had a governing board (Fig. 6), with the majority of the board being made up of internal registry staff (33%). External experts and patient organization representatives were also represented on the governing boards (29% and 27%, respectively). Patient representatives were often represented and made up 11% of the governing boards.
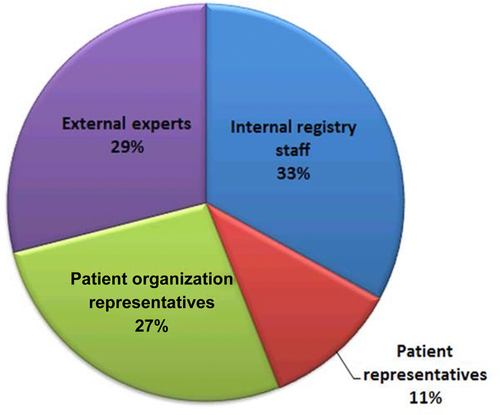
Overall, the main function of the governing board was to oversee ethical and legal issues and to facilitate data access and use by internal and external researchers (38% and 36%). Financial/administrative issues, and coordination of all parties involved in the registries was also an important function of the governing body (19%).
Utilization of the Registries
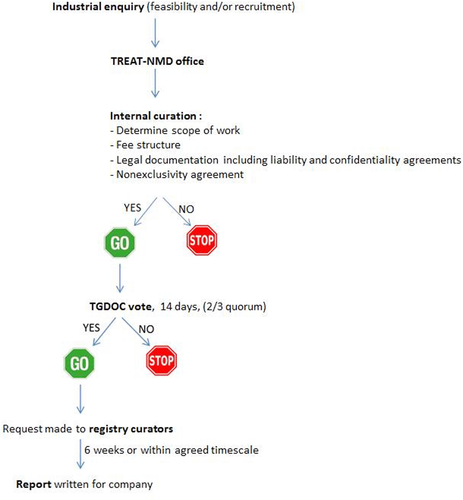
Third party access to the data in the registries is governed by the TREAT-NMD oversight committee (TGDOC). To obtain access to data, a third party approaches the TREAT-NMD global registry with a request for information contained within the registry. The oversight committee reviews the request and votes whether or not the request is in line with the charter and DMD patient interests. A vote of two-third is required to achieve quorum and therefore approval. If approval occurs, then the global registry approaches the national registries and obtains the data requested. The information is then put into a report, which is then given to the third party company (Fig. 7).
In order to further facilitate clinical trial development, the TREAT-NMD Network established a Care and Trial Site Registry (CTSR) in December 2007. The TREAT-NMD CTSR is the largest and most comprehensive database of neuromuscular centers in the world. In addition, CARE-NMD was set up as a 3-year EU-funded project to improve access to best-practice care for DMD across seven EU countries—Bulgaria, the Czech Republic, Denmark, Germany, Hungary, Poland, and the UK [http://www.care-nmd.eu/, ]. CARE-NMD has used patient registries in all participating countries as a powerful tool to perform healthcare research in a specific rare disease. In contrast to common diseases, patient registries are often the only resource in rare disease, as ICD coding systems and other traditional health research methods are not applicable to rare diseases. Through the registries with a very high response rate CARE-NMD has collected care and quality of life information from more than 1,000 Duchenne patients in Europe and the findings are due to be published in 2013.
Registries were asked a number of specific questions regarding their uses in academia and industry. Information was collected on the number of national and international studies registries had been used for.
At the national level, the registries had been used for a total of 115 studies, some registries having only been involved in one or two studies, whereas others were involved in >10. Overall, 52% of registries were used for feasibility studies, split between academic (23%), industry (5%), and internal (24%). Forty-eight percent of registries were used for recruitment, split between academic (26%), industry (12%), and internal (10%).
At the international level, the registries had been used in at least 10 studies (Table 1). Sixty-four percent had been used for feasibility studies (90% of which were industry enquiries) and 36% had been used for recruitment purposes (93% of which were industrial recruitment).
| Year | Feasibility enquiry | Recruitment enquiry | Other (including trial site enquiries) |
|---|---|---|---|
| 2009 | 1 | 2 | |
| 2010 | 5 | ||
| 2011 | 1 | 1 | |
| 2012 | 1 |
- Table showing how the registries have been used for a number of industrial enquiries (both feasibility and recruitment) from 2009 to 2012.
There seemed to be a clear split between the uses of the registries at the national and international levels. Nationally, the registries were used mainly for academic and internal purposes, such as presentations at national conferences or applications for further funding. At the international level, there was a clear trend in the registries being involved in industrial collaborations.
Industrial liaison
Table 1 summarizes the enquiries made to the DMD registries from industry (2009–2012).
Although each industrial enquiry was unique, there were many similarities seen in the types of information requested by industry. Age, country of residence, and molecular diagnosis were requested 100% of the time. More than 85% of enquiries also requested patient ambulatory, ventilation, and cardiomyopathy status. Fifty percent of enquiries also requested trial site information from the trial site registry (Table 2). To date there have been more feasibility enquiries than recruitment enquiries.
| Question asked in enquiry | Percent requested |
|---|---|
| Patient demographics (e.g., age, country) | 100 |
| Genetic diagnosis | 100 |
| Ambulatory status | ≥85 |
| Ventilation status | ≥85 |
| Cardiomyopathy status | ≥85 |
| Information from trial site registry | 50 |
- Table showing the types of information most commonly requested from the registries by industry.
Examples of enquiries to the registries from industry include
In 2009, a feasibility enquiry was made to the Global DMD Patient Registry for a phase I clinical program looking at exon skipping technology (exon 51). National registries from 18 countries were in a position to provide the requested data. A total of 2307 patients were retrieved from the TREAT-NMD global registry and identified as being appropriate for exon 51 skipping based on their mutation profile.
In 2010, three feasibility enquiries were made to the Global DMD Patient Registry by different companies, two studies looking at exon skipping (exon 51 alone or multiple exon skipping 43, 44, 45, 46, 50, 52, 53, and 55), and one study looking at nonsense mutations. National registries from up to 27 countries were in a position to provide the requested data. More than 9,000 patients were identified as being appropriate for these studies.
Two further feasibility enquiries were initiated in 2010 looking at randomized, placebo-controlled drug evaluation studies.
In 2011, a recruitment enquiry to the Global DMD Patient Registry was initiated for a clinical trial looking at skipping exon 51. National registries representing seven countries were in a position to provide the requested information. A total of 50 DMD patients were identified as being appropriate for this study.
A multinational (Germany, Italy, UK, and US) health economics study titled: “Multi-National, Cross Sectional, Observational Study of Patient and Caregiver Burden of DMD” was carried out in 2012. The study aimed to understand and improve the medical care of DMD, identifying the needs of caregivers, and facilitating future studies of DMD, including evaluations of new medicines (publication in preparation).
Discussion
Patient registries for rare diseases exist to improve feasibility, planning, and recruitment for clinical trials. The overall patient experience is also enhanced. This might be achieved simply by making a newly diagnosed patient feel part of a community, or it might occur through the facilitation and promotion of the latest medical and scientific advances. The common thread in all rare diseases is the small patient population. Such small cohorts have, in the past, meant little or no interest from pharmaceutical companies. Patient registries have allowed us to greatly increase patient populations simply by sharing agreed and harmonized patient data from individual countries around the world. The establishment of harmonized national patient registries and ultimately a global patient registry for DMD has created a centralized access point for information. This in turn has served to increase interest from TREAT-NMD industrial partners. Clinical trial feasibility and recruitment studies have increased dramatically over the last 3–4 years leading to the development of potential treatment strategies for DMD, such as exon skipping [Aartsma-Rus et al., 2009]. The registries have also allowed for critical analysis of current treatment protocols like the use of corticosteroids [Hoffman et al., 2012]. The registries have also led to improvements in patient care. An example is the work of CARE-NMD. CARE-NMD has used patient registries in variety of countries as a tool to perform healthcare research and to influence policy. Successful registries cooperate with appropriate clinical networks, healthcare services, research groups, patient advocacy groups, and industrial partners. National registries cooperate with genetic databases such as Leiden University in the Netherlands (www.dmd.nl) by providing information on genetic variants. This will be further facilitated through new platforms such as RD-Connect (http://rd-connect.eu/). The key to successful industry/patient registry interaction is quality control of the data within the registry. TREAT-NMD has achieved this through an emphasis on the appointment and training of registry curators. Registry curators provide a regulatory “checkpoint” between data being submitted to a registry and it being made available to the wider scientific community. This aims to minimise errors in the data. This is critical when the data submitted to the registries is entered by several different sources (clinicians, patients, patient organizations, genetics units). Registry curators can also be “data recruiters” by soliciting data from other countries. As the global registry for DMD further develops and matures, curation and surveillance will become increasingly important, not only for maintaining quality assurance but also for critical analysis of these large and well-defined data sets allowing for the potential identification and development of novel and emerging technologies (e.g., omics research). Monitoring the latest advances in scientific research and bioinformatics will ensure that the registries remain high-impact and current.
The registries have been utilized extensively by industry for clinical trial activities. The number of returning industrial companies serves to further highlight the importance of the registries and the information that they provide.
The impact of the national registries will be further enhanced through the continued inclusion in the global DMD registry (http://www.treat-nmd.eu/resources/patient-registries/overview/). This federated style of registry development will ultimately provide a “one stop shop” for DMD patient information enquiries and clinical trial activities. Many of the national registries have already uploaded their information to the centralized global registry and it will be prudent to encourage emerging and planned national registries to also participate in the global registry.
Looking ahead, we foresee a need for setting up and developing surveillance registries. Such registries will provide industry with a system that is able to manage regulatory post-marketing commitments. This, for example, will allow for the monitoring of drug safety and efficacy.
Companies who are required to implement and maintain surveillance registries for their own products spend millions of dollars every year [Kaye, 2010]. A centrally managed registry platform will be independent, credible, and cost saving, and will link the TREAT-NMD global registry to individual surveillance modules.
These registries will be sustained by the stakeholders of the DMD community and our industrial partners will pay to use the platform.
However, issues do exist; currently, not all of the national registries have transferred their data to the global registry. The current mandatory data set is unlikely to be sufficient for all purposes and will need to be reviewed. Currently, the registries have no intellectual property protection and no sustainability assurance. Securing on-going funding for the registries remains critically important. Funding issues do exist and some registries have had to make use of short-term funding while alternative sources of funding were identified. Further collaboration with industry (clinical trial participation) and the continued support of patient advocacy groups remains vital for sustainability. The registries are not legal entities and rely heavily on the supporting framework of academic institutions. These issues will need to be addressed and resolved for the future of the registries to be assured. It is clear that the global and national DMD patient registries provide an unparalleled resource for patient information, clinical and academic research, and best standards of care assurance. Patients, their families, clinical professionals, and the wider scientific and industrial communities all benefit from the existence of patient registries. The conception and development of these registries has brought about increased clinical trial opportunities translating directly to significant improvements in patient care and treatment options.
Acknowledgments
The authors would like to acknowledge the families of those living with DMD who have been instrumental in the formation of the DMD national registries. We also acknowledge former and current members of the TREAT-NMD office at the Institute of Genetic Medicine in Newcastle specifically Rachel Thompson. We also extend our thanks to the members of the TREAT-NMD executive committee: Hanns Lochmuller, Annemieke Aartsma-Rus, Anna Ambrosini, Filippo Buccella, Kevin Flanigan, Eric Hoffman, Janbernd Kirschner, Eugenio Mercuri, Ichizo Nishino, Kathy North, Jes Rahbek, and Thomas Sejersen. We also acknowledge the members of the current TGDOC: Jan Verschuuren (Chair), Hugh Dawkins (Chair elect), Anna Ambrosini, Svetlana Artemieva, Alexander N. Baranov, Farhad Bayat, Christophe Béroud, Ria Broekgaarden, Filippo Buccella, Craig Campbell, Nick Catlin, Monica Ensini, Pat Furlong, Kevin Flanigan, Ole Gredal, Lauren Hache, Serap İnal, Jacqueline Jackson, Pierre-Yves Jeannet, Anna Kaminska, A. Ayse Karaduman, Veronika Karcagi, En Kimura, Janbernd Kirschner, Jaana Lähdetie, Hanns Lochmüller, Vitaliy Matyushenko, Vedrana Milic-Rasic, Violeta Mihaylova, Marie-Christine Ouillade, Ian Murphy, Miriam Rodrigues, Rosario dos Santos, Pascale Saugier-Veber, Inge Schwersenz, Thomas Sejersen, Rasha El Sherif, Eduardo Tizzano, Isabela Tudorache, Sylvie Tuffery-Giraud, Jen Wang, Simon Woods, W. Ludo van der Pol, Peter Van den Bergh, Petr Vondráček.
Disclosure statements: Professor Hanns Lochmuller was elected chair of the TREAT-NMD Alliance in April 2012 and is the previous chair of the oversight committee. Professor Lochmuller has a financial interest/arrangement with Pfizer, Ultragenyx, and GlaxoSmithKline (Research grant investigator).




